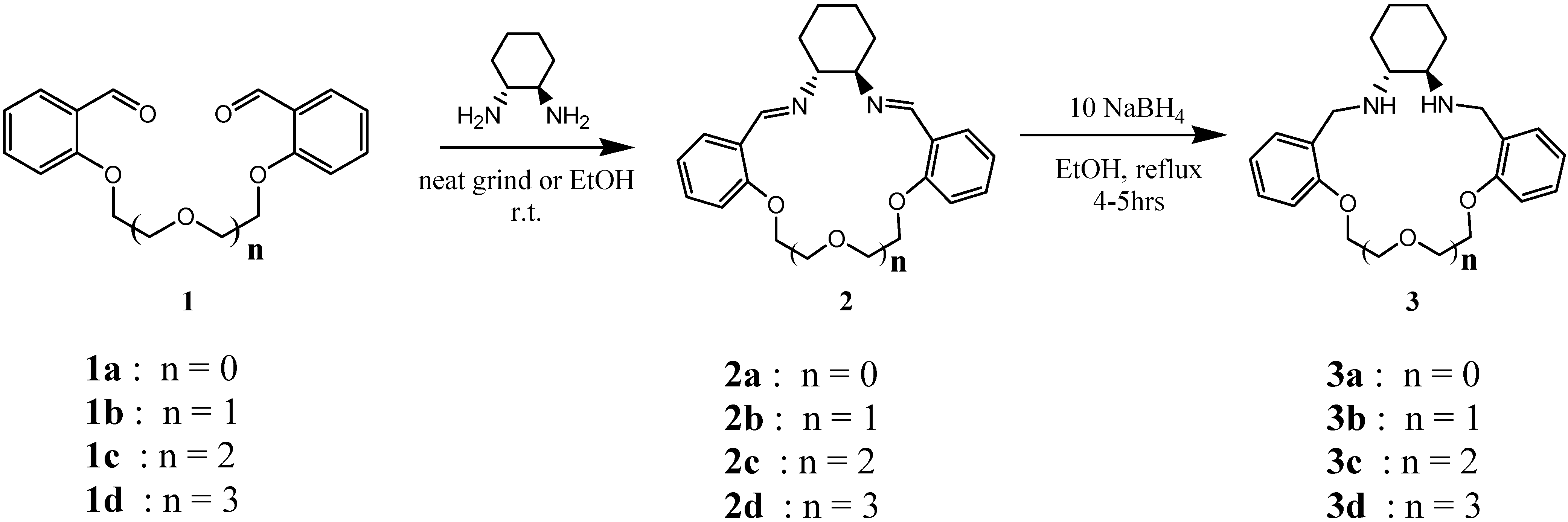
General Procedure for the Synthesis of Diamino Macrocycles
An equimolar quantity of the relevant dialdehyde 1 together with (1R,2R)-(-)–1,2-diamino-cyclohexane was ground using a mortar and pestle at room temperature for approximately 5 minutes. The resultant powder was washed with cold ethanol; in the case of 2c and 2d the corresponding dialdehyde precursors are oils at room temperature and, consequently, the condensation was carried out in absolute ethanol at room temperature. After 20-30 min the oily products began to separate from solution; after 4.5 h the supernatant was decanted yielding the desired Schiff base. The larger macrocyclic products are all pale yellow oils, (only 2a is a solid), and were purified by washing with hot ethanol. The respective Schiff bases were combined with 10 mol equiv. of NaBH4 in absolute ethanol. The reaction mixture was heated under reflux for 4-5 h, cooled and quenched with distilled water. Solvent was removed in vacuo and the product extracted into dichloromethane. The organic layer was washed twice with brine, dried with MgSO4 and concentrated to afford the products as pale yellow oils. Spectroscopic data is given for the free base. The dihydrochloride salts were obtained by dissolving the oils in dichloromethane and adding an excess HCl in ether. White or pale yellow gummy solids formed instantly which were triturated and washed with hexane and filtered off.
1,12-Diaza-3,4;9,10-dibenzo-13,14-cyclohexo-5,8-dioxacyclobutadecane (2a). Isolated as a yellow solid (89%); m.p. 124-126 ºC; 1H-NMR (CDCl3) δ: 1.41 (bs, 2H, 2 x CH), 1.74-1.98 (bm, 6H, 2 x CH & 2 x CH2), 3.36 (bs, 2H, 2 x -C-CH-N=), 3.85-3.90 (m, 2H, 2 x Ar-OCH), 4.05-4.11 (m, 2H, 2 x Ar-O-CH-), 6.77-6.89 (2d, 4H, Ar-CH), 7.26 (m, 2H, Ar-CH), 7.83 (d, 2H, Ar-CH), 8.52 (s, 2H, -N=CH); 13C-NMR (CDCl3) δ: 24.5, 32.9, 66.7 (-C-NH=), 73.7 (-C-O-C), 112.1 (Ar-C), 120.1 (Ar-C), 125.4 (Ar-C), 127.5 (Ar-C), 131.3 (Ar-C), 157.0 (Ar-C), 157.7 (-NH=C-); IR (cm-1): 3080 (aromatic C-H stretch), 2926 and 2855 (C-H methylene stretch), 1634 (C=N stretch), 1599, 1486 and 1450 (C C aromatic ring stretch), 1238 (aryl-O-CH2stretch), 1051 (in plane C-H bend), 735 (out of plane C-H bend), 428 (out of plane ring C C bend); ESI-MS for C22H24N2O2(MH+): Calc 349.2; Found 349.2; = -30.0 (c = 0.012, CH2Cl2).
1,15-Diaza-3,4;12,13-dibenzo-16,17-cyclohexo-5,8,11-trioxacyclohexaundecane (
2b). Isolated as a pale yellow gum (95%);
1H-NMR (CDCl
3) δ: 1.44 (bs, 2H, 2 x CH), 1.80 (bs, 6H, 2 x CH & 2 x CH
2), 3.39 (bs, 2H, 2 x -C-CH-N=), 3.74 (t, 4H, -CH
2-O-CH
2), 3.90-4.00 (m, 2H, 2 x Ar-O-CH-), 4.02-4.15 (m, 2H, 2 x Ar-O-CH-), 6.80 (d, 2H, Ar-CH), 6.86 (t, 2H, Ar-CH) 7.24 (2t, 2H, Ar-CH), 7.82 (2d, 2H, Ar-CH), 8.61 (s, 2H, -N=CH-);
13C-NMR (CDCl
3) δ: 21.2, 34.1, 68.4 (-C-NH=), 69.6 (-C-O-C), 71.2 (Ar-C-O-), 112.4 (Ar-C), 120.7 (Ar-C), 125.8 (Ar-C), 128.0 (Ar-C) 131.1 (Ar-C), 157.1 (Ar-C-), 157.9 (-NH=C-); IR (cm
-1): 3080 (aromatic C-H stretch), 2928 and 2855 (C-H methylene stretch), 1634 (C=N stretch), 1601, 1491 and 1450 (C
![Molecules 09 00513 i001]()
C aromatic ring stretch), 1243 (aryl-O-CH
2 stretch), 1055 (in plane C-H bend), 751 (out of plane C-H bend); ESI-MS for C
24H
28N
2O
3 (MH
+): Calc. 393.2; Found 393.1;
= -28.1 (c = 0.005, CH
2Cl
2).
1,18-Diaza-3,4;15,16-dibenzo-19,20-cyclohexo-5,8,11,14-tetraoxacycloctadodecane (
2c). Isolated as a viscous yellow oil (95%);
1H-NMR (CDCl
3) δ: 1.46 (bs, 2H, 2 x CH), 1.82 (bs, 6H, 2 x CH & 2 x CH
2), 3.4 (bs, 2H, 2 x -C-CH-N=), 3.70 (s, 4H, -O-CH
2-CH
2-O-), 3.75 (t, 4H, 2 x Ar-O-CH
2-C
H2-O-), 3.88-4.00 (m, 2H, 2 x Ar-O-CH), 4.02-4.13 (m, 2H, 2 x Ar-O-CH), 6.77 (d, 2H, Ar-CH), 6.86 (t, 2H, Ar-CH) 7.22 (2t, 2H, Ar-CH), 7.81 (2d, 2H, Ar-CH), 8.61 (s, 2H, -N=CH-);
13C-NMR (CDCl
3) δ: 24.5, 32.9, 67.8 (-C-NH=), 69.5 (Ar-O-C-
C-O-), 70.8 (O-C-C-O-), 73.9 (Ar-O-C-), 112.1 (Ar-C), 120.9 (Ar-C), 125.2 (Ar-C), 127.8 (Ar-C) 131.0 (Ar-C), 156.9 (Ar-C-), 158.0 (-NH=C-); IR (cm
-1): 3080 (aromatic C-H stretch), 2926 and 2855 (C-H methylene stretch), 1635 (C=N stretch), 1600, 1488 and 1450 (C
![Molecules 09 00513 i001]()
C aromatic ring stretch), 1247 (aryl-O-CH
2 stretch), 1056 (in plane C-H bend), 754 (out of plane C-H bend), 428 (out of plane ring C
![Molecules 09 00513 i001]()
C bend); ESI-MS for C
26H
32N
2O
4 (MH
+): Calc. 437.2; Found 437.3;
= -35.2 (c = 0.010, CH
2Cl
2).
1,21-Diaza-3,4;18,19-dibenzo-22,23-cyclohexo-5,8,11,14,17-pentaoxacyclodecatridecane (
2d). A viscous yellow oil (95%);
1H-NMR (CDCl
3) δ: 1.48 (bt, 2H, 2 x CH), 1.83 (bs, 6H, 2 x CH & 2 x CH
2), 3.41 (bs, 2H, 2 x -C-CH-N=), 3.68 (s, 8H, -O-CH
2-CH
2-O-CH
2-CH
2-O-) 3.74 (t, 4H, 2 x Ar-O-CH
2-
CH2-), 3.90-4.00 (m, 2H, 2 x Ar-O-CH), 4.02-4.12 (m, 2H, 2 x Ar-O-CH), 6.77 (d, 2H, Ar-CH), 6.86 (t, 2H, Ar-CH) 7.23 (2t, 2H, Ar-CH), 7.80 (2d, 2H, Ar-CH), 8.60 (s, 2H, -N=CH-);
13C-NMR (CDCl
3) δ: 24.4, 32.9, 67.8 (-C-NH=), 69.4 (Ar-O-C-
C-O-), 70.6 (Ar-O-C-C-O-
C-), 70.7 (Ar-O-C-C- O-C-
C-O-) 73.8 (Ar-O-C), 112.1 (Ar-C), 120.7 (Ar-C), 125.2 (Ar-C), 127.3 (Ar-C) 131.2 (Ar-C), 157.0 (Ar-C-), 157.8 (-NH=C-); IR (cm
-1): 3080 (aromatic C-H stretch), 2929 and 2855 (C-H methylene stretch), 1636 (C=N stretch), 1600, 1488 and 1449 (C
![Molecules 09 00513 i001]()
C aromatic ring stretch), 1254 (aryl-O-CH
2 stretch), 1045 (in plane C-H bend), 754 (out of plane C-H bend), 438 (out of plane ring C
![Molecules 09 00513 i001]()
C bend); ESI-MS for C
28H
36N
2O
5 (MH
+): Calcd 481.3; Found 481.5;
= -52.5 (c = 0.012, CH
2Cl
2).
1,12-Diamino-3,4;9,10-dibenzo-13,14-cyclohexo-5,8-dioxacyclobutadecane (
3a). Isolated as an orange oil (80%);
1H-NMR (CDCl
3) δ: 0.93 (bt, 2H, 2 x CH), 1.14 (bt, 2H, 2 x CH), 1.64 (bd, 2H, 2 x CH), 2.00 (bd, 2H, 2 x CH), 2.13 (bd, 2H, 2 x -CH-N-), 3.58 (d, 2H,
J = 13.2 Hz, 2 x –NH-C
H-CH
2-Ar-), 3.81 (d, 2H,
J = 13.5 Hz, –NH-C
H-CH
2-Ar-), 5.58 (s, 4H, -O-CH
2-CH
2-O-), 6.92 (bt, 2H, Ar-CH), 7.02-7.20 (m, 4H, Ar-CH), 7.25 (bd, 2H, Ar-CH);
13C-NMR (CDCl
3) δ: 24.9, 31.3, 45.6 (-NH-C-), 60.8 (-C-NH-), 90.6 (-O-C-O-), 111.8 (Ar-C), 122.1 (Ar-C), 127.8 (Ar-C), 129.5 (Ar-C) 130.1 (Ar-C), 154.7 (Ar-C); IR (cm
-1): 3080 (aromatic C-H stretch), 2926 and 2855 (C-H methylene stretch), 1634 (C=N stretch), 1599, 1486 and 1450 (C
![Molecules 09 00513 i001]()
N aromatic ring stretch), 1238 (aryl-O-CH
2 stretch), 1051 (in plane C-H bend), 735 (out of plane C-H bend), 428 (out of plane ring C
![Molecules 09 00513 i001]()
C bend); ESI-MS for C
22H
28N
2O
2 (MH
+): Calcd 353.2; Found 353.4; Anal. Calc. for C
22H
28N
2O
2 2HCl·H
2O: C, 59.6; H, 7.3; N, 6.3. Found: C, 59.4; H, 7.1; N, 6.1;
= -48.1 (c = 0.018, CH
3OH).
1,15-Diamino-3,4;12,13-dibenzo-16,17-cyclohexo-5,8,11-trioxacyclohexaundecane (
3b). Isolated as a pale orange oil (91%);
1H-NMR (CDCl
3) δ: 1.09 (bs, 4H, -CH
2-CH
2-), 1.62 (bs, 2H, 2 x CH), 2.07 (d, 2H, 2 x CH), 2.19 (bs, 2H, 2 x -C
H-NH-), 2.78 (bs, 2H, NH), 3.60 (d, 2H,
J = 13.5 Hz, 2 x -NH-C
H-), 3.67 (t, 4H, -CH
2-O-CH
2-), 3.92 (d, 2H,
J = 13.2 Hz, 2 x -NH-C
H-), 3.96 (t, 4H, 2 x Ar-O-CH
2), 6.76 (d, 2H, Ar-CH), 6.87 (t, 2H, Ar-CH), 7.15-7.28 (m, 4H, Ar-CH);
13C-NMR (CDCl
3) δ: 2425.0, 31.2, 45.9 (-NH-C-), 60.7 (-C-NH-), 67.5 (-C-O-C-), 69.8 (Ar-O-C-), 111.3 (Ar-C), 120.7 (Ar-C), 127.9 (Ar- C), 129.2 (Ar-C) 129.6 (Ar-C), 156.7 (Ar-C); IR (cm
-1): 3080 (aromatic C-H stretch), 2920 and 2855 (C-H methylene stretch), 1601, 1490 and 1450 (C
![Molecules 09 00513 i001]()
C aromatic ring stretch), 1240 (aryl-O-CH
2 stretch), 1052 (in plane C-H bend), 750 (out of plane C-H bend); ESI-MS for C
24H
32N
2O
3 (MH
+): Calcd 397.2; Found 397.3; Anal. Calc. for C
24H
32N
2O
3·2HCl·2H
2O: C, 57.0; H, 7.6, N, 5.5. Found C, 56.8; H, 7.6; N, 5.2;
= -51.2 (c = 0.012, CH
3OH).
1,18-Diamino-3,4;15,16-dibenzo-19,20-cyclohexo-5,8,11,14-tetraoxacyclooctadodecane (
3c). Isolated as a pale yellow oil (92%);
1H-NMR (CDCl
3) δ: 1.03 (bs, 2H, 2 x CH), 1.21 (bt, 2H, 2 x CH) 1.68 (bd, 2H, 2 x CH), 2.11-2.21 (m, 4H, 2 x CH & 2 x –C
H-NH-), 3.65 (d, 2H,
J = 13.5 Hz, 2 x -NH-C
H-), 3.61 (s, 4H, -O-CH
2-CH
2-O-), 3.70 (t, 4H, 2 x Ar-O-CH
2-C
H2-O-), 3.89 (d, 2H,
J = 13.5 Hz, 2 x –NH-C
H-), 4.02 (t, 4H, 2 x Ar-O-CH
2-) 6.77 (d, 2H, Ar-CH), 6.87 (t, 2H, Ar-CH), 7.13-7.25 (m, 4H, Ar-CH);
13C-NMR (CDCl
3) δ: 25.0, 31.3, 45.9 (-NH-C-), 60.7 (-C-NH-), 67.4 (Ar-O-C-
C-), 69.7 (-O-C-C-O-), 70.8 (Ar-O-C), 111.2 (Ar-C), 120.5 (Ar-C), 127.7 (Ar-C), 129.4 (Ar-C) 129.5 (Ar-C), 156.7 (Ar-C-); IR (cm
-1): 3080 (aromatic C-H stretch), 2936 and 2860 (C-H methylene stretch), 1603, 1497 and 1458 (C
![Molecules 09 00513 i001]()
C aromatic ring stretch), 1248 (aryl-O-CH
2 stretch), 1052 (in plane C-H bend), 754 (out of plane C-H bend); ESI-MS for C
26H
36N
2O
4 (MH
+): Calc 441.3; Found 441.5; Anal. Calc. for C
26H
36N
2O
4 2HCl: C, 60.8; H, 7.5; N, 5.5. Found: C, 60.8; H, 7.5; N, 5.3;
= -26.9 (c = 0.024, CH
3OH).
1,21-Diamino-3,4;12,13-dibenzo-18,19-cyclohexo-5,8,11,14,17-pentaoxacyclodecatridecane (
3d). A pale yellow oil (91%);
1H-NMR (CDCl
3) δ: 1.00-1.26 (m, 4H, -CH
2-CH
2-), 1.68 (bd, 2H, 2 x CH), 2.10 (d, 2H, 2 x CH), 2.23 (bs, 2H, 2 x –C
H-NH-), 2.33 (bs, 2H, NH), 3.60 (bs, 8H, -O-CH
2-CH
2-O-CH
2-CH
2-O-), 3.61 (d, 2H, J = 13.5 Hz, 2 x NH-C
H-), 3.69 (t, 4H, 2 x Ar-O-CH
2-C
H2-O) 3.92 (d, 2H,
J = 13.5 Hz, 2 x -NH-C
H-), 4.01 (t, 4H, 2 x Ar-O-CH
2-), 6.77 (d, 2H, Ar-CH), 6.88 (t, 2H, Ar-CH), 7.17-7.25 (m, 4H, Ar-CH);
13C-NMR (CDCl
3) δ: 24.9, 31.1, 45.9 (-NH-C-), 60.5 (-C-NH-), 67.3 (Ar-O-C-
C-O-), 69.5 (-O-C-C-O-C-C-O-), 70.5 (Ar-O-C-), 70.6 (Ar-O-C-) 111.1 (Ar-C), 120.4 (Ar-C), 127.7 (Ar-C), 129.0 (Ar-C) 129.5 (Ar-C), 156.6 (Ar-C-); IR (cm
-1): 3080 (aromatic C-H stretch), 2924 and 2860 (C-H methylene stretch), 1602, 1492 and 1458 (C
![Molecules 09 00513 i001]()
C aromatic ring stretch), 1259 (aryl-O-CH
2 stretch), 1052 (in plane C-H bend), 754 (out of plane C-H bend), 438 (out of plane ring C
![Molecules 09 00513 i001]()
C bend); ESI-MS for C
28H
40N
2O
5 (MH
+): Calc. 485.3; Found 485.5; Anal. Calc. for C
28H
40N
2O
5 2HCl·H
2O: C, 58.4; H, 7.7; N, 4.9. Found: C, 58.7; H, 8.0; N, 4.8;
= -25.7 (c = 0.019, CH
3OH).
 C aromatic ring stretch), 1243 (aryl-O-CH2 stretch), 1055 (in plane C-H bend), 751 (out of plane C-H bend); ESI-MS for C24H28N2O3 (MH+): Calc. 393.2; Found 393.1; = -28.1 (c = 0.005, CH2Cl2).
C aromatic ring stretch), 1243 (aryl-O-CH2 stretch), 1055 (in plane C-H bend), 751 (out of plane C-H bend); ESI-MS for C24H28N2O3 (MH+): Calc. 393.2; Found 393.1; = -28.1 (c = 0.005, CH2Cl2). C aromatic ring stretch), 1247 (aryl-O-CH2 stretch), 1056 (in plane C-H bend), 754 (out of plane C-H bend), 428 (out of plane ring C
C aromatic ring stretch), 1247 (aryl-O-CH2 stretch), 1056 (in plane C-H bend), 754 (out of plane C-H bend), 428 (out of plane ring C  C bend); ESI-MS for C26H32N2O4 (MH+): Calc. 437.2; Found 437.3; = -35.2 (c = 0.010, CH2Cl2).
C bend); ESI-MS for C26H32N2O4 (MH+): Calc. 437.2; Found 437.3; = -35.2 (c = 0.010, CH2Cl2). C aromatic ring stretch), 1254 (aryl-O-CH2 stretch), 1045 (in plane C-H bend), 754 (out of plane C-H bend), 438 (out of plane ring C
C aromatic ring stretch), 1254 (aryl-O-CH2 stretch), 1045 (in plane C-H bend), 754 (out of plane C-H bend), 438 (out of plane ring C  C bend); ESI-MS for C28H36N2O5 (MH+): Calcd 481.3; Found 481.5; = -52.5 (c = 0.012, CH2Cl2).
C bend); ESI-MS for C28H36N2O5 (MH+): Calcd 481.3; Found 481.5; = -52.5 (c = 0.012, CH2Cl2). N aromatic ring stretch), 1238 (aryl-O-CH2 stretch), 1051 (in plane C-H bend), 735 (out of plane C-H bend), 428 (out of plane ring C
N aromatic ring stretch), 1238 (aryl-O-CH2 stretch), 1051 (in plane C-H bend), 735 (out of plane C-H bend), 428 (out of plane ring C  C bend); ESI-MS for C22H28N2O2 (MH+): Calcd 353.2; Found 353.4; Anal. Calc. for C22H28N2O2 2HCl·H2O: C, 59.6; H, 7.3; N, 6.3. Found: C, 59.4; H, 7.1; N, 6.1; = -48.1 (c = 0.018, CH3OH).
C bend); ESI-MS for C22H28N2O2 (MH+): Calcd 353.2; Found 353.4; Anal. Calc. for C22H28N2O2 2HCl·H2O: C, 59.6; H, 7.3; N, 6.3. Found: C, 59.4; H, 7.1; N, 6.1; = -48.1 (c = 0.018, CH3OH). C aromatic ring stretch), 1240 (aryl-O-CH2 stretch), 1052 (in plane C-H bend), 750 (out of plane C-H bend); ESI-MS for C24H32N2O3 (MH+): Calcd 397.2; Found 397.3; Anal. Calc. for C24H32N2O3·2HCl·2H2O: C, 57.0; H, 7.6, N, 5.5. Found C, 56.8; H, 7.6; N, 5.2; = -51.2 (c = 0.012, CH3OH).
C aromatic ring stretch), 1240 (aryl-O-CH2 stretch), 1052 (in plane C-H bend), 750 (out of plane C-H bend); ESI-MS for C24H32N2O3 (MH+): Calcd 397.2; Found 397.3; Anal. Calc. for C24H32N2O3·2HCl·2H2O: C, 57.0; H, 7.6, N, 5.5. Found C, 56.8; H, 7.6; N, 5.2; = -51.2 (c = 0.012, CH3OH). C aromatic ring stretch), 1248 (aryl-O-CH2 stretch), 1052 (in plane C-H bend), 754 (out of plane C-H bend); ESI-MS for C26H36N2O4 (MH+): Calc 441.3; Found 441.5; Anal. Calc. for C26H36N2O4 2HCl: C, 60.8; H, 7.5; N, 5.5. Found: C, 60.8; H, 7.5; N, 5.3; = -26.9 (c = 0.024, CH3OH).
C aromatic ring stretch), 1248 (aryl-O-CH2 stretch), 1052 (in plane C-H bend), 754 (out of plane C-H bend); ESI-MS for C26H36N2O4 (MH+): Calc 441.3; Found 441.5; Anal. Calc. for C26H36N2O4 2HCl: C, 60.8; H, 7.5; N, 5.5. Found: C, 60.8; H, 7.5; N, 5.3; = -26.9 (c = 0.024, CH3OH). C aromatic ring stretch), 1259 (aryl-O-CH2 stretch), 1052 (in plane C-H bend), 754 (out of plane C-H bend), 438 (out of plane ring C
C aromatic ring stretch), 1259 (aryl-O-CH2 stretch), 1052 (in plane C-H bend), 754 (out of plane C-H bend), 438 (out of plane ring C  C bend); ESI-MS for C28H40N2O5 (MH+): Calc. 485.3; Found 485.5; Anal. Calc. for C28H40N2O5 2HCl·H2O: C, 58.4; H, 7.7; N, 4.9. Found: C, 58.7; H, 8.0; N, 4.8; = -25.7 (c = 0.019, CH3OH).
C bend); ESI-MS for C28H40N2O5 (MH+): Calc. 485.3; Found 485.5; Anal. Calc. for C28H40N2O5 2HCl·H2O: C, 58.4; H, 7.7; N, 4.9. Found: C, 58.7; H, 8.0; N, 4.8; = -25.7 (c = 0.019, CH3OH).



