Abstract
Utilization of 2-arylhydrazonopropanals for the synthesis of 2-arylhydrazonoimino propanones, 1,2,4-trizolo[4,3-a]pyrimidines, pyridopyridazine hydrazones, 3-oxaloalkanonitriles and 1,2,3-trizole derivatives by conventional heating and under microwave irradiation is described. Structural assignments are based on spectroscopic data and confirmed in some cases by X-ray crystallography.
Introduction
The utility of microwaves in heterocyclic synthesis is now receiving considerable attention [1,2,3,4] and enaminones have also been recently extensively utilized as precursors for the synthesis of heteroaromatics. As a part of an ongoing project, aiming to explore potential utility of microwaves as an energy source for heterocyclic synthesis, we report here on synthesis of iminoarylhydrazonopropanone, azolopyrimidine and 3-oxaloalkanonitrile derivatives of potential interest as pharmaceuticals and photochromic dyes [5,6,7], and then we investigate the possibility of conducting these reactions under microwave irradiation in addition to the standard thermal conditions.
Results and Discussion
The chemistry of 2-arylhydrazonopropanals has received considerable interest in the last few years [7,8,9,10,11,12,13,14]. As part of an ongoing project in our laboratory aimed at exploring the potential utility of microwave irradiation as a source of heat for producing polyfunctionally substituted heteroaromatics, we report here on reactivity of compounds 1a-f toward amines and some active methylene nitriles in the absence of solvent under MW irradiation in a domestic microwave oven. The yield of products obtained under the microwave heating technique (MW) and the time taken for complete the reaction are compared with those obtained by conventional heating (Δ). Thus, 1a-f were heated with a variety of heterocyclic amines 2a-c, hydrazine 7a and phenylhydrazine 7b with both conventional heating (Δ) and in a microwave oven yielding the corresponding condensation products 3a-f, 8a-d (Scheme 1)
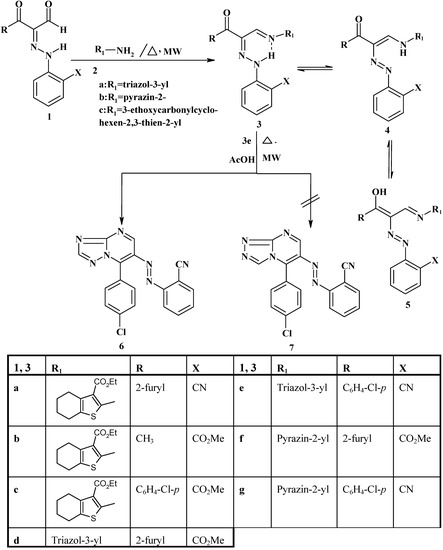
Scheme 1.
It is noteworthy that the microwave reactions were brought to completion in a very short time compared to conventional heating. Several tautomeric forms (cf. 4, 5), seemed possible for the condensation products 3a-f. An X-ray diffraction single crystal structure [15] of a representative example of the reaction products with both amines (3b) and with hydrazines (8d), established that they exist in the hydrazone form (Figure 1, Figure 2). Compound 3b (Figure 1) is fixed in a sterically crowded configuration to permit efficient hydrogen bonding.
Refluxing 3e in AcOH afforded a product that may be formulated as 6 or its isomer 7. 1H-NMR indicated the presence of single triazole signal at δ= 9.1 ppm shifted to lower field by almost δ= 1.0 ppm compared to the single triazole signal in the parent aminotriazole. Consequently the 1,2,4-triazolo[4,3-a]pyrimidine structure 6 was assigned to this reaction product (Scheme 1).
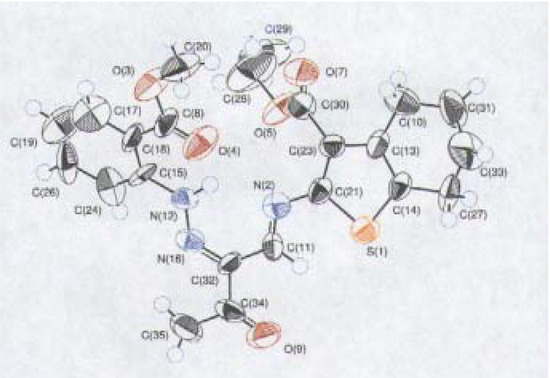
Figure 1.
X-ray structure of compound 3b.
Reaction of 1a,c,d,e with hydrazine hydrate 7a and phenylhydrazine 7b afforded 8a-d both by conventional heating (Δ) and MW irradiation. On treatment of 8a-d with pyridine using both conventional heating (Δ) and MW irradiation, 9a-d were only obtained by conventional heating (Δ) and not by MW irradiation under a variety of conditions (Scheme 2).
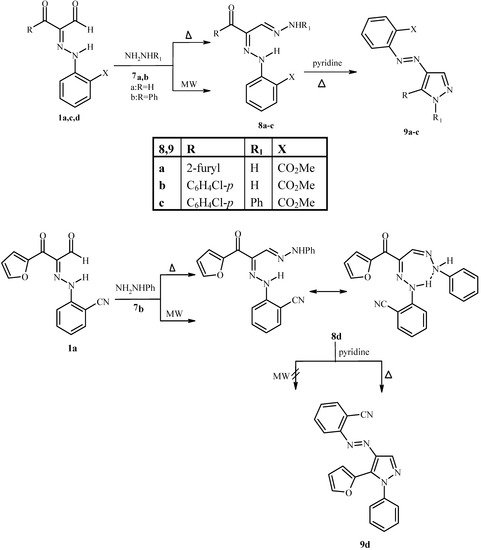
Scheme 2.
Product 8d was recrystallized and its structure was solved by X-ray diffraction (Figure 2).
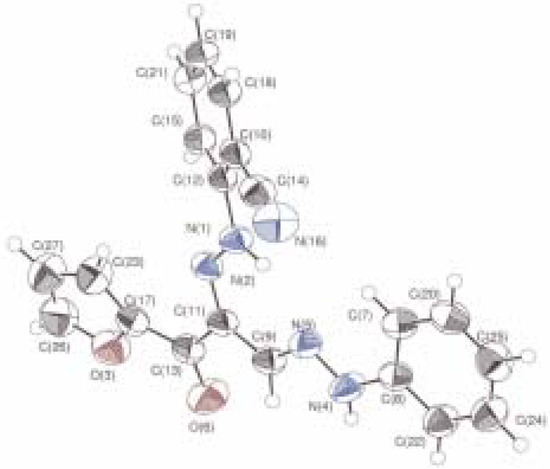
Figure 2.
Molecular structure of 8d with the atom labeling scheme.
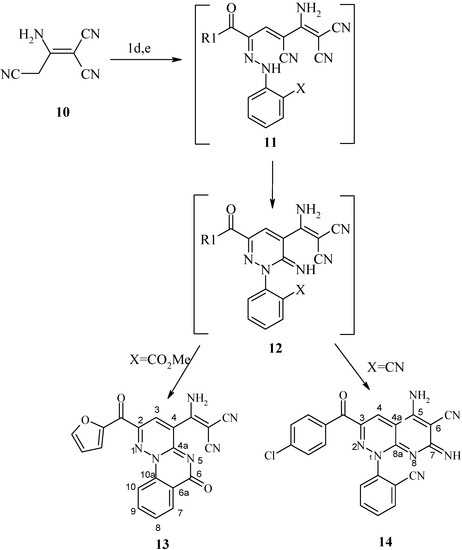
Scheme 3.
The reaction of 1a and 1c with 2-amino-1,1,3-tricyanopropene (10) yielded the products of condensation, pyridopyridazines 13 and 14 respectively (Scheme 3). Structures 13, 14 were established based on spectral data (IR, 13C-NMR). Formation of 13, 14 are assumed to proceed via intermediates 11 and 12. Similar reactions under microwave irradiation were unsuccessful. Treatment of 1c with phenylhydrazine by conventional heating (Δ) afforded the oxoalkanonitrile 15 most likely via hydrolysis of the formed phenylhydrazone intermediate 8c, while 8c could be also obtained via treatment of 1 with phenylhydrazine under MW irradiation.
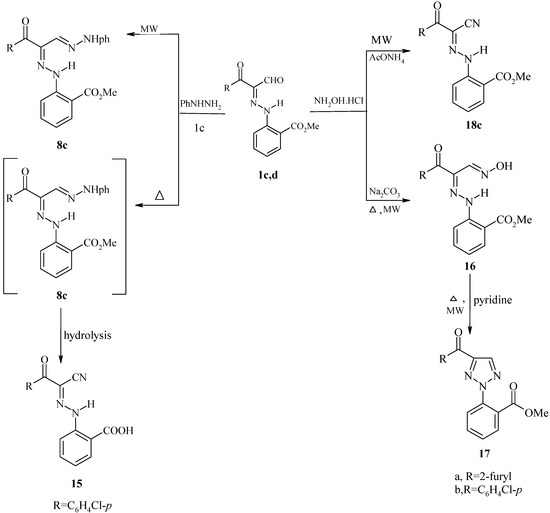
Scheme 4.
Reaction of 1c,d with hydroxylamine hydrochloride in the presence of sodium carbonate under MW irradiation and conventional heating (Δ) afforded the oxime 16, which converted into the triazole 17 on refluxing in pyridine, while reaction of 1 with hydroxylamine hydrochloride in the presence of ammonium acetate under MW irradiation only resulted in the formation of the oxoalkanonitrile 18. In a previous paper it was reported that treatment of 2-arylhydrazonopropanals with hydroxylamine hydrochloride by conventional heating (Δ) afforded the 3-oxoalkanonitrile [16] (Scheme 4).
Experimental
General
Melting points were measured on a Gallenkamp Electrothermal melting point apparatus and are uncorrected. The IR spectra were measured on a Nicolet Magna 520 FT IR Spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded in deuterated dimethylsulfoxide (DMSO-d6) or deuterated chloroform (CDCl3) at 200 MHz on a Varian Gemini NMR spectrometer and a Bruker DPX 400 MHz spectrometer using tetramethylsilane (TMS) as an internal reference and results are expressed as δ values. Mass spectra were performed on a Shimadzu GCMS-QP 1000 Ex mass spectrometer at 70 eV. Microwave irradiation was carried out using a commercial microwave oven (SGO 390 W). Elemental analyses were carried out at the Microanalytical Center at Cairo University. All X-ray diffraction diagrams and caculations were performed using Maxus (Bruker Nonius, Delft & Macscienece, Japan).
General Procedure for the Preparation of 1a-i [8]:
A cold solution of aryldiazonium salt (0.1 mol) was prepared by adding a solution of NaNO2 (1.5g in 10 mL H2O) to a cold solution of aryl amine (0.1 mol) in concentrated HCl (5 mL) with stirring as described earlier. The resulting solution of the aryldiazonium salt was then added to a cold solution of enaminones 2 in EtOH (50 mL) containing sodium acetate (0.1 mol). The mixture was stirred at r.t for 1h and the solid product formed was collected by filtration and crystallized from ethanol.
3-(4-Chlorophenyl)-2-(2-methoxycarbonylphenylhydrazono)-3-oxopropanal (1c): Yellow crystals from ethanol; m.p.189°C; yield 80%; IR (shows complex spectra due to H-bond between O and NH): 3022 (CH aromatic), 1711 (C=O ester), 1650 (C=O aldehyde), 1638 (C=O ketone) and 1586 (C=N) cm-1; 1H-NMR: δ= 4.05 (s, 3H, CH3), 7.20-8.09 (m, 8H, Ar-H), 10.25 (s, 1H, CHO) and 15.69 (s, 1H, disappeared after D2O exchange, NH) ppm; 13C-NMR: δ= 52.90 (COOCH3), 116.19, 116.40, 118.98, 128.36, 133.33, 138.75 (C6H4-CO2M-o), 124.98, 131.59, 131.89, 134.74, 135.50 (C6H4-Cl-p), 143.54 (C=N-N), 166.87 (COOCH3), 188.23 (C=O) and 190.49 (CHO) ppm; MS: M+ (344); Anal. Calcd. for C17H13N2O4Cl (344.76): C, 59.23; H, 3.80; N, 8.13; Found: C, 59.33; H, 3.82; N, 8.15.
General Procedure for the Preparation of 3a-g:
Method A: A mixture of compounds 1a-e (0.1 mol) and amines (0.1 mol) was refluxed in ethanol or dioxane (30 mL) for 2h, left to cool to room temperature and the solid was collected and crystallized from ethanol.
Method B: A mixture of compounds 1a-e (0.1 mol) and amine (0.1 mol) and drops of ethanol were placed in the microwave oven and irradiated at 390 w for 5-15 min, left to cool to room temperature and the solid was collected and crystallized from ethanol.
2-{2-[(2-Cyanophenyl)-hydrazono]-3-furan-2-yl-3-oxo-propylideneamino}-4,5,6,7-tetrahydrobenzo-[b]thiophene-3-carboxylic acid ethyl ester (3a). Dark red crystals from ethanol; m.p. 174°C; yield 93% (method A), 95% (method B); IR: 3120 (NH), 3030 (CH aromatic), 2932 (CH aliphatic), 2224 (CN), 1698 (C=O ester) and 1619 (C=O ketone) cm-1; 1H-NMR: δ= 1.30 (t, 3H, CH3CH2), 1.79, 2.79 (m, 8H, cyclohexene), 4.32 (q, 2H, CH2CH3), 6.57 (m, 1H, furyl H-4), 7.59, 7.76 (m, 2H,furyl H-3, H-5), 7.22, 7.63 (m, 4H, Ar-H), 8.69 (s, 1H, CH, olefinic) and 14.99 (s, 1H, NH) ppm; 13C-NMR: δ= 14.25 (CH3CH2O), 22.60, 22.86, 25.71, 26.53 (cyclohexene carbons), 60.71 (CH3CH2O), 116.81 (CN), 102.51, 125.06, 146.92, 147.05 (thienyl carbons), 122.23, 122.27, 148.32 (furyl carbons), 118.82, 124.84, 133.36, 133.82, 133.95, 135.99 (C6H4CN-o), 150.60 (C=N-N), 152.93 (HC=N), 163.39 (CO2CH2CH3) and 177.21 (C=O) ppm; MS: M+ (474); Anal. Calcd. for C25H22N4O4S (474.54): C, 63.28; H, 4.67; N, 11.81; Found: C, 63.38; H, 4.60; N 11.89.
2-{2-[(2-Methoxycarbonyl-phenyl)-hydrazono]-3-oxo-butylideneamino}-4,5,6,7-tetrahydrobenzo[b]-thiophene-3-carboxylic acid ethyl ester (3b): Dark orange crystals from ethanol; m.p. 181°C; yield 90% (method A), 99% (method B); IR: 2944 (CH aliphatic), 1695 (C=O ester) and 1600 (C=O ketone), 1508 (C=N) cm-1; 1H-NMR: δ= 1.07 (t, 3H, CH3CH2), 1.81, 2.75 (m, 8H, cyclohexene-H), 2.55 (s, 3H, CH3), 3.85 (s, 3H, CH3O), 4.03 (q, 2H, CH2CH3), 7.13-8.02 (m, 4H, Ar-H), 8.69 (s, 1H, CH olefinic) and 15.58 (s, 1H, NH) ppm; 13C-NMR: δ= 13.96 (CH3CH2O), 22.56 (CH3CO), 23.04, 24.90, 25.51, 25.86 (cyclohexene carbons), 52.58 (COOCH3), 116.16, 116.74, 123.63, 124.83, 131.38, 132.53 (C6H4CO2Me-o), 134.22, 136.28, 144.52, 149.62 (thienyl carbons), 149.64 (C=N-N), 153.85 (HC=N), 163.89 (CO2CH2CH3), 166.50 (COOCH3) and 197.55 (C=O) ppm; MS: M+ (455); Anal. Calcd. for C23H25N3O5S (455.54): C, 60.64; H, 5.53, N, 9.22; Found: C, 60.70; H, 5.59, N, 9.15.
2-{3-(4-Chlorophenyl)-2-[(2-cyanophenyl)-hydrazono]-3-oxo-propylideneamino}-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid ethyl ester (3c): Orange crystals from ethanol/dioxane (2:1); m.p. 189°C; yield 70% (method A), 98% (method B); IR: 3394(NH), 3000(CH aromatic), 2941(CH aliphatic), 1707 (ester C=O) and 1635 (ketone C=O) cm-1; MS: M+ (551); Anal. Calcd. for C28H26N3O5ClS (552.05): C, 60.92; H, 4.75; N, 7.61; Found: C, 60.87; H, 4.79; N, 7.70.
2(N’-{2-Furan-2-yl)-2-oxo-1-[(4H-[1,2,4]triazol-3-ylimino)-methyl]-ethylidene}-hydrazino)benzoic acid methyl ester (3d): Brown crystals from ethanol/dioxane; m.p. 260°C; yield 30% (method A), 40% (method B); IR (shows complex spectra due to H-bond between O in carbonyl ester group and NH hydrazone): 3490 (NH triazole), 3110 (CH aromatic), 2998 (CH aliphatic), 1697 (shows weak C=O ester), 1611(C=O ketone) and 1499 (N=N) cm-1; 1H-NMR (d6-DMSO) δ= 4.04 (s, 3H, CH3O), 6.80 (m, 1H, furyl H-4), 7.3, 7.7 (m, 2H, Ar H-4, H-5), 7.5, 7.9 (d, 2H, furyl H-3, H-5), 8.02, 8.04 (d, 2H, Ar H-3, H-6), 8.12 (s, 1H, CH olefinic), 8.54 (s, 1H, 1,2,4-trizole NH), 9.35 (s, 1H, 1,2,4-trizole H-5) and 15.26 (s, 1H, NH hydrazone) ppm; 13C-NMR: δ= 53.59 (COOCH3), 113.25, 122.62, 148.58, 150.63 (furyl carbons), 116.90 ,117.18, 124.86, 131.98, 133.96, 135.41 (C6H4-CO2Me-o ), 143.80 (C=N-N), 147.5 (1,2,4-trizole carbons), 155.64 (N=CH-C), 166.45 (COOCH3) and 177.64 (C=O) ppm; MS: M+ (366) Anal. Calcd. for C17H14N6O4 (366.34):C, 55.74; H, 3.85; N, 22.94; Found: C, 55.80; H, 3.89; N, 22.99.
2(N’-{2-(4-Chlorophenyl)-2-oxo-1-[(4H-[1,2,4]triazol-3-ylimino)-methyl]-ethylidene}-hydrazino)-benozonitrile (3e): Light orange crystals from dioxane; m.p. 240°C; yield 30% (method A), 35% (method B); IR: 3293 (2NH), 2227 (C≡N), 1649 (C=O), 1588 (C=N) and 1499 (N=N) cm-1; 1H-NMR δ= 7.36-7.98 (m, 8H, Ar-H), 8.68 (s, 1H, CH olefinic), 9.35 (s, 1H, 1,2,4-triazole H-5), 14.30 (s, 1H, 1,2,4-triazole NH) and 14.82 (s, 1H, NH hydrazone) ppm; 13C-NMR: δ= 116.62 (CN), 116.06, 116.09, 126.75, 126.78, 128.62, 128.77 (C6H4CN-o), 128.85, 132.63, 133.96, 135.43 (C6H4Cl-p), 136.42 (1,2,4-triazole carbons), 137.47 (C=N-N), 150.97 (HC=N) and 190.42 (C=O) ppm.; MS: M+ (377); Anal. Calcd. for C18H12N7OCl (377.80): C, 57.23; H, 3.20; N, 25.95; Found: C, 57.26; H, 57.26; N, 25.90.
2-{N'-[2-Furan-2-yl-2-oxo-1-(pyrazin-2-yliminomethyl)-ethylidene]-hydrazino}-benzoic acid methyl ester (3f): Light brown crystals from ethanol/dioxane (2:1); m.p. 210°C; yield 70% (method A), 90% (method B); IR (complex due to H-bond between O and NH): 3043 (CH aromatic), 2990 (CH aliphatic), 1698 (C=O ester), 1626 (C=O ketone) and 1583 (C=N) cm-1; 1H-NMR: δ=3.97 (s, 3H, CH3O), 6.83 (m, 1H furyl H-4), 7.34 (m, 1H, Ar H-3), 7.60-7.61 (d, 2H, furyl H-3, H-5), 7.80 (m, 1H, Ar H-6), 7.97, 8.08 (d, 2H, Ar H-4, H-5), 8.67 (d, 2H, pyrazine H-5, H-6), 9.15 (s, 1H, CH olefinic), 9.53 (s, 1H, pyrazine H-3) and 15.93 (s, 1H, NH) ppm; MS: M+ (377); Anal. Calcd. for C19H15N5O4 (377.36): C, 60.48; H, 4.01; N, 18.56; Found: C, 60.50; H, 4.25; N, 18.59.
2-{N'-[2-(4-Chlorophenyl)-2-oxo-1-(pyrazin-2-yliminomethyl)-ethylidene]-hydrazino}-benzonitrile (3g): Orange crystals from ethanol/dioxane (2:1); m.p. 255°C; yield 73% (method A), 78% (method B); IR: 3750 (NH), 3086 (CH aromatic), 2222 (CN), 1649 (C=O) and 1590 (C=N)cm-1; 1H-NMR: δ= 7.32-7.90 (m, 8H, Ar-H), 8.45, 8.51 (m, 2H, H-5, H-6 pyrazine), 9.04 (s, 1H, CH olefinic), 9.45 (s, 1H, H-3 pyrazine) and 14.79 (s, 1H, NH) ppm; .MS: M+ (388); Anal. Calcd. for C20H13N6OCl (388.82): C, 61.78; H, 3.37; N, 21.61; Found: C, 61.70; H, 3.40; N, 21.20.
General Procedure for the Preparation of 6:
Method A: Compound 3e (0.1 mol) was refluxed in acetic acid for 2h, then left to cool at room temperature and treated with ethanol. The solid product, so formed, was collected by filtration and crystallized from ethanol.
Method B: Compound 3e (0.1 mol) and a few drops of acetic acid were placed in the microwave oven and irradiated at 390 W for 7-20 min, then left to cool to room temperature and the solid was collected and crystallized from ethanol.
7-(4-Chlorophenyl)-6-(2-cyanophenylazo)-1,2,4-triazolo[1,5-a]pyrimidine (6): Orange crystals from ethanol; m.p. 258°C; yield 25% (method A), 30% (method B); IR: 3090 (CH aromatic), 2235 (C≡N), 1594 (C=N), 1485 (N=N) cm-1; 1H-NMR: δ= 7.46-8.02 (m, 8H, Ar-H), 9.01, 9.62 (2s, 1H, H-2, H-5); MS: M+ (359); Anal. Calcd. for C18H10N7Cl (359.78): C, 60.09; H, 2.80; N, 27.25; Found: C, 60.24; H, 2.89; N, 27.30.
General Procedure for the Preparation of 8a-d:
Method A: A mixture of compound 1a,c,d or e (0.1 mol) and hydrazine monohydrate or phenyl hydrazine (0.12 mol) was refluxed in ethanol or dioxane (30 mL) for 2 h., then left to cool to room temperature and the solid was collected and crystallized from ethanol.
Method B: Compound 1a, c, d or e (0.1 mol) and hydrazine monohydrate or phenyl hydrazine (0.12 mol), and a few drops of ethanol were placed in the microwave oven and irradiated at 390 W for 1-10 min , then left to cool to room temperature and the solid was collected and crystallized from ethanol.
1-(2-Furyl)-3-hydrazono-2-(2-methoxycarbonylphenylhydrazono)-1-propanone (8a): Brown crystals from ethanol; m.p. 168°C; yield 60% (method A); IR: 3421, 3306 (NH2), 3230 (NH), 1702 (C=O ester), 1617 (C=O ketone) and 1540 (C=N) cm-1; MS: M+ 314; Anal. Calcd. for C15H14N4O4 (314.30): C, 57.32; H, 4.49; N, 17.83; Found: C, 57.40; H, 4.50; N, 17.89.
1-(4-Chlorophenyl)-3-hydrazono-2-(2-methoxycarbonylphenylhydrazono)-1-propanone (8b): Yellow crystals from ethanol: m.p. 204°C; yield 66% (method A), 90% (method B); IR: 3397, 3286 (NH2), 3220 (NH), 3002 (CH aromatic), 2954 (CH aliphatic), 1699 (C=O ester), 1633 (C=O ketone) and 1580 (C=N) cm-1; 1H-NMR: δ= 3.95 (s, 3H, CH3O), 7.54-7.92 (m, 10H, Ar-H and NH2), 8.12 (s, 1H, CH olefinic) and 14.27 (s, 1H, NH) ppm; MS: M+ (358); Anal. Calcd. for C17H15N4O3Cl (358.79): C, 56.91; H, 4.21; N, 15.62; Found: C, 56.99; H, 4.25; N, 15.59.
1-(4-Chlorophenyl)-2-(2-methoxycarbonylphenylhydrazono)-3-phenylhydrazono-1-propanone (8c): Orange crystals from ethanol/dioxane(2:1): m.p. 244°C; yield 99% (method B); IR: 3273, 3178 (NH), 3178 (2 NH), 3065 (CH aromatic), 1706 (C=O ester) and 1630 (C=O ketone) cm-1; 1H-NMR: δ= 3.87 (s, 3H, CH3O), 7.28-7.95 (m, 13H, Ar-H), 8.31 (s, 1H, CH olefinic), 10.87 (s, 1H, disappeared after D2O exchange, NH phenylhydrazone) and 13.56 (s, 1H, disappeared after D2O exchange, NH hydrazone); MS: M+ (434); Anal. Calcd. for C23H19N4O3Cl (434.89): C, 63.52; H, 4.40; N, 12.88; Found: C, 63.60; H, 4.49; N, 12.79.
2-(2-Cyanophenylhydrazono)-1-(2-furyl)-3-phenylhydrazono-1-propanone (8d): Orange crystals from ethanol: m.p. 229°C; yield 78% (method A), 80% (method B); IR: 3260, 3196 (2 NH), 3092 (CH aromatic), 2215 (C≡N), 1603 (C=O) and 1537 (C=N) cm-1; 1H-NMR: δ= 6.79-6.80 (m, 1H, furyl H-4), 7.26-7.63 (m, 9H, Ar-H), 7.79 (d, 1H, furyl H-3, H-5), 8.29 (s, 1H, CH olefinic), 11.06 (s, 1H, NH phenyl hydrazone) and 12.71(s, 1H, NH hydrazone); MS: M+ (357); Anal. Calcd. for C20H15N5O2 (357.37): C, 67.22; H, 4.23; N, 19.60; Found: C, 67.32; H, 4.30; N, 19.58.
General Procedure for the Preparation of 9a, d.
Method A: A solution of each of compound 8a-d (0.1 mol) in pyridine (10 mL) was refluxed for 2h., then left to cool to room temperature and the solid was collected and crystallized from ethanol.
Method B: Compounds 8a-d (0.1 mol) and some drops of pyridine were placed in the microwave oven and irradiated at 390 W for 30 min., then left to cool to room temperature and the solid was collected and crystallized from ethanol.
3-(2-Furyl) 4-(2-methoxycarbonylphenylazo)-pyrazole (9a): Red crystals from ethanol; m.p. 160°C; yield 50% (method A); IR: 3343 (NH), 3100 (CH aromatic), 2990 (CH alifatic), 1699 (C=O ester) and 1461 (N=N) cm-1; MS: M+ (296); Anal. Calcd. for C15H12N4O3 (296.29): C, 60.81; H, 4.08; N, 18.91; Found: C, 60.89; H, 4.20; N, 18.99.
3-(4-Chlorophenyl) 4-(2-methoxycarbonylphenylazo) pyrazole (9b): Orange crystals from ethanol: m.p. 189°C; yield 50% (method A); IR: 3507 (NH), 3123 (CH aromatic), 2881 (CH aliphatic), 1745 (C=O ester) and 1498 (N=N) cm-1; 1H-NMR: δ= 3.83 (s, 3H, CH3O), 7.49-8.24 (m, 8H, Ar-H), 8.48 (s, 1H, pyrazole H-5) and 9.10 (s, 1H, NH pyrazole) ppm;.MS: M+ (340); Anal. Calcd. for C17H13N4O2Cl (340.77): C, 59.92; H, 3.85; N, 16.44; Found: C, 59.80; H, 3.90; N, 16.55.
3-(4-Chlorophenyl)-4-(2-methoxycarbonylphenylazo)-1-phenylpyrazole (9c): Light brown crystals from methanol: m.p. 242°C; yield 50% (method A); 1H-NMR: δ= 3.98 (s, 3H, CH3), 7.36-8.18 (m, 13H, Ar-H) and 8.92 (s, 1H, pyrazole H-5) ppm; MS: M+ (416); Anal. Calcd. for C23H17N4O2Cl (416.87): C, 66.27; H, 4.11; N, 13.44; Found: C, 66.35; H, 4.29; N, 13.33.
4-(2-Cyanophenylazo)-3-(2-furyl)-1-phenylpyrazole (9d): Orange crystals from ethanol; m.p. 137°C; yield 50% (method A). IR: 3066 (CH aromatic), 2232 (C≡N) and 1495 (N=N) cm-1; MS: M+ (339); Anal. Calcd. for C20H13N5O (339.36): C, 70.79; H, 3.86; N, 20.64; Found: C, 70.67; H, 3.90, N, 20.69.
General Procedure for the Preparation of 13, 14
Method A: A solution of compound 1a,c (0.1 mol) in ethanol (30 mL) was treated with 2-amino-1-propene-1,1,3-tricarbonitrile (0.1mol) in the presence of few drops of piperidine and refluxed for 3h. The reaction product was washed with ethanol and dried, then recrystallized from ethanol.
Method B: Compound 1a,c (0.1 mol) and 2-amino-1-propene-1,1,3-tricarbonitrile (0.1mol) in the presence of a few drops of piperidine were placed in the microwave oven and irradiated at 390 W for 5-10 min., then left to cool to room temperature and the solid was collected and crystallized from ethanol.
3-Amino-1-cyano-2-(2-furoyl-6-oxopyridazino[2,3-c]quinqzoline-4-yl) acrylonitrile (13): Brown crystals from methanol; m.p. >300°C; yield 40%; IR: 3344 (NH2), 2218, 2202 (2 C≡N) and 1615 (C=O) cm-1; 1H-NMR: δ= 5.49 (s, 2H, NH2), 6.61 (m, 1H, furyl H-4), 6.65, 7.56 (m, 4H, Ar H), 7.23, 7.72 (d, 2H, furyl H-3, H-5) and 8.51 (s, 1H, H-4) ppm; 13C-NMR: δ= 117.2 (C≡N), 54.1 (C=C-CN), 186.6 (NH2-C=C), 113.25, 121.62, 148.58, 150.63 (furyl carbons), 155.0 (C-2), 123.0 (C-3), 138.0 (C-4), 164.0 (C-4a), 123.3 (C-6a), 130.5 (C-7), 119.0 (C-8), 135.6 (C-9), 115.6 (C-10), 147.9 (C-10a), 177.66 (ring C=O) and 190.0 ppm (C=O); MS: M+ (382); Anal. Calcd. for C20H10N6O3 (382.34): C, 62.83; H, 2.64; N, 21.98; Found: C, 62.80; H, 2.70; N, 21.95.
5-Amino-3-(4-chlorobenzoyl)-6-cyano-(2-cyanophenyl)-1,7-dihydro-7-iminopyrido [2,3-c] pyridazine (14): Green crystals from ethanol; m.p. 273°C; yield 30%; IR: 3460 (NH2), 3377 (NH), 3174 (CH aromatic), 2215, 2203 (2 C≡N) and 1632 (C=O) cm-1; 1H-NMR: δ= 5.29 (s, 2H, NH2), 6.64-7.75 (m, 9H, Ar-H and NH) and 8.42 (s, 1H, H-4) ppm; 13C-NMR: δ= 116.2, 117.2 (2 C≡N), 99.6; 115.9, 119.2, 132.8, 133.6, 150.2 (C6H4-CN), 128.85, 132.63, 133.96, 135.40 (C6H4Cl-p), 155.0 (C-3), 123.5 (C-4), 138.0 (C-4a), 172 (C-5), 99.0 (C-6), 164.0 (C-7), 166.1 (C-8a) and 189.64 ppm (C=O); MS: (M++2) (427); Anal. Calcd. for C22H12N7OCl (425.84): C, 62.05; H, 2.84; N, 23.02; Found: C, 62.40; H, 2.95; N, 23.25.
2-{N-[2-(4-Chlorophenyl)-1-cyano-2-oxo-ethylidene]-hydrazino}-benzoic acid (15): Green crystals from ethanol/ dioxane (2:1); m.p.238°C; yield 35%; IR: 3449 (OH), 3305 (NH), 3057 (CH aromatic), 2220 (C≡N) and 1647 (C=O) cm-1; MS (M+-2) (325); Anal. Calcd. for C16H10N3O3Cl (327.73): C, 58.64; H, 3.08; N, 12.82; Found: C, 58.70; H, 3.25; N, 12.77.
General Procedure for the Preparation of 16
Method A: A warm solution of hydroxylamine hydrochloride (0.1 mol) and sodium carbonate (0.12 mol) in water (10 mL) was added to a stirred solution of the arylhydrazonopropanals 1c,d (0.1 mol) in ethanol (10 mL). The reaction mixture was stirred at room temperature for one hour. The oximes soon separated as semisolid crystals that were solidified by cooling in crushed ice. The solid product so formed was collected by filtration and crystallized from ethanol.
Method B: Compound 1c,d (0.1 mol), hydroxylamine hydrochloride (0.1 mol), sodium carbonate (0.12 mol) and a few drops of ethanol were placed in the microwave oven and irradiated at 390 W for 5-10 min, then left to cool to room temperature and the solid was collected and crystallized from ethanol.
3-(2-Furoyl)-2-(2-methoxycarbonylphenylhydrazono)-3-oxo-propanal-1-oxime (16a): Yellow crystal from ethanol; m.p.199°C; yield 74% (method A), 82% (method B); IR (shows complex spectra due to H-bond between O ketone and NH): 3311 (br OH), 3075 (CH aromatic), 2957 (CH aliphatic), 1691 (C=O ester), 1630 (C=O ketone) and 1580 (C=N) cm-1; 1H-NMR: δ= 3.98 (s, 3H, CH3O), 6.80 (m, 1H, furyl H-4), 7.29-7.77 (m, 2H, Ar H-4, H-5), 7.53, 7.90 (d, 2H, furyl H-3 and H-5), 8.02, 8.13 (d, 2H, Ar H-3, H-6), 9.63 (s, 1H, CH olefinic), 12.12 (s, 1H, NH) and 15.39 (s, 1H, OH) ppm; MS: M+ (315); Anal. Calcd. for C15H13N3O5 (315.29 ): C, 57.14; H, 4.16; N, 13.33; Found: C, 57.20; H, 4.10; N, 13.45.
3-(4-Chlorophenyl)-2-(2-methoxycarbonylphenylhydrazono)-3-oxo-propanal-1-oxime (16b): Yellow crystals from dioxane; m.p.186°C; yield 74% (method A); IR: 3426 (br OH), 3300 (NH), 3020 (CH aromatic), 1713 (C=O ester), 1635 (C=O ketone) and 1586 (C=N) cm-1; 1H-NMR: δ= 4.04(s, 3H, CH3O), 7.25-8.08 (m, 8H, Ar-H), 8.62 (s, 1H, CH olefinic), 10.25 (s, 1H, disappeared after D2O exchange NH) and 15.69 (s, 1H, disappeared after D2O exchange, OH) ppm; 13C-NMR: δ= 52.90 (COOCH3), 128.35, 131.89, 134.74, 138.75 (C6H4-Cl-p), 116.18, 116.39, 124.98, 131.59, 133.32, 135.50 (C6H4-CO2Me-o), 143.54 (C=N-OH), 166.87 (C=N-N), 188.22 (COOCH3) and 190.49 (C=O) ppm;. MS: (M+-1) (358); Anal. Calcd. for C17H14N3O4 Cl (359.77 ): C, 56.76; H, 3.92; N, 11.68; Found: C, 56.80; H, 3.89; N, 11.63.
General Procedure for the Preparation of 17
Method A: Compound 16a (0.1mol) was refluxed in pyridine (10 mL) for 1h, then left to cool at room temperature and treated with ethanol. The solid product, so formed, was collected by filtration and crystallized from ethanol.
Method B: Compound 16a (0.1 mol) and drops of pyridine were placed in the microwave oven and irradiated at 390 W for 15-28 min, then left to cool to room temperature and the solid was collected and crystallized from ethanol.
4-(2-Furoyl)-2-(2-methoxycarbonylphenyl)-1,2,3-triazole (17a): Yellow crystals from ethanol; m.p.138°C; yield 56% (method A), 66% (method B); IR: 3074 (CH aromatic), 1691 (C=O ester), 1630 (C=O ketone) and 1580 (C=N) cm-1; MS: (M++1) (298); Anal. Calcd. for C15H11N3O4 (297.27): C, 60.61; H, 3.73; N, 14.14; Found: C, 60.70; H, 3.69; N, 14.26.
3-(4-Chlorophenyl)-3-oxo-2-(2-methoxycarbonylphenylhydrazono)propionitrile (18): Compound 1c (0.1 mol), hydroxylamine hydrochloride (0.1mol) and ammonium acetate (0.1 mol) and a few drops of ethanol were placed in the microwave oven and irradiated at 390 W for 30 min, then left to cool to room temperature, and the solid was collected and crystallized from ethanol, giving brown crystals; m.p.175°C; yield 40%; IR: 3230 (NH), 3070 (CH aromatic), 2222 (C≡N) and 1715 (C=O ester), 1640 (C=O ketone) cm-1; MS: (M++1) (342); Anal. Calcd. for C17H12N3O3Cl (341.76): C, 59.75; H, 3.54; N, 12.30; Found: C, 59.85; H, 3.50; N, 12.40.
References and Notes
- Caddick, S. Tetrahedron 1995, 51, 10403.
- Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacqnault, P.; Mathe, D. Synthesis 1998, 1213.
- Perreux, L.; Loupy, A. Tetrahedron 2001, 57, 9199.
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Tetrahedron 2001, 57, 9225.
- Al-Zaydi, K. M. Molecules 2003, 8, 541.
- Al-Naggar, A. A.; Abdel-Khalik, M. M.; Elnagdi, M. H. J. Chem. Res. 1999, (S). 648, (M). 2801.
- Al-Zaydi, K. M. J. Saudi Chem. Soc. 2002, 477.
- Al-Zaydi, K. M.; Hafez, E. A.; Elnagdi, M. H. J. Chem. Res. 2000, (S). 4, (M). 510.
- Bulow, C.; Spengler, W. Chem. Ber. 1925, 58, 1375.
- Mustafa, A.; Asker, W.; Harhash, A.A.; Messiha, N.A.; Elnagdi, M.H. Tetrahedron 1965, 21, 217.
- El-Sakka, T.A. J. Chem. Res.(S) 1996, 434.
- Elnagdi, M.H.; Kandeel, E.M.; Sadek, K.U. Z. Naturforch B. Anorg. Chem. Org. Chem. 1979, 34B, 275.
- Al-Omran, F.; Abdel-Khalik, M.M.; Abuel-Khair, A.; Elnagdi, M. H. Synthesis 1997, 91.
- Behbehani, H.; Abdel-Khalik, M.M.; Elnagdi, M. H. OPPI 1999, 31, 551.
- Crystallographic data for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary puplications nos. CCDC 227009 & 227010. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
- Abdel-Khalik, M.M.; Agamy, S.M.; Elnagdi, M.H. Z. Naturforsch 2000, 55b, 1211.
- Sample availability: Available from the corresponding author
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
