Synthesis of Some New bis-(p-Fluorophenyl)amides of the Thieno[3,2-b]thiophene, Thieno[3,2-b]furan and 1,2-bis{5-[2-(2-Thienyl)ethenyl]2-thienyl}ethene Series
Abstract
:Introduction
Results and Discussion
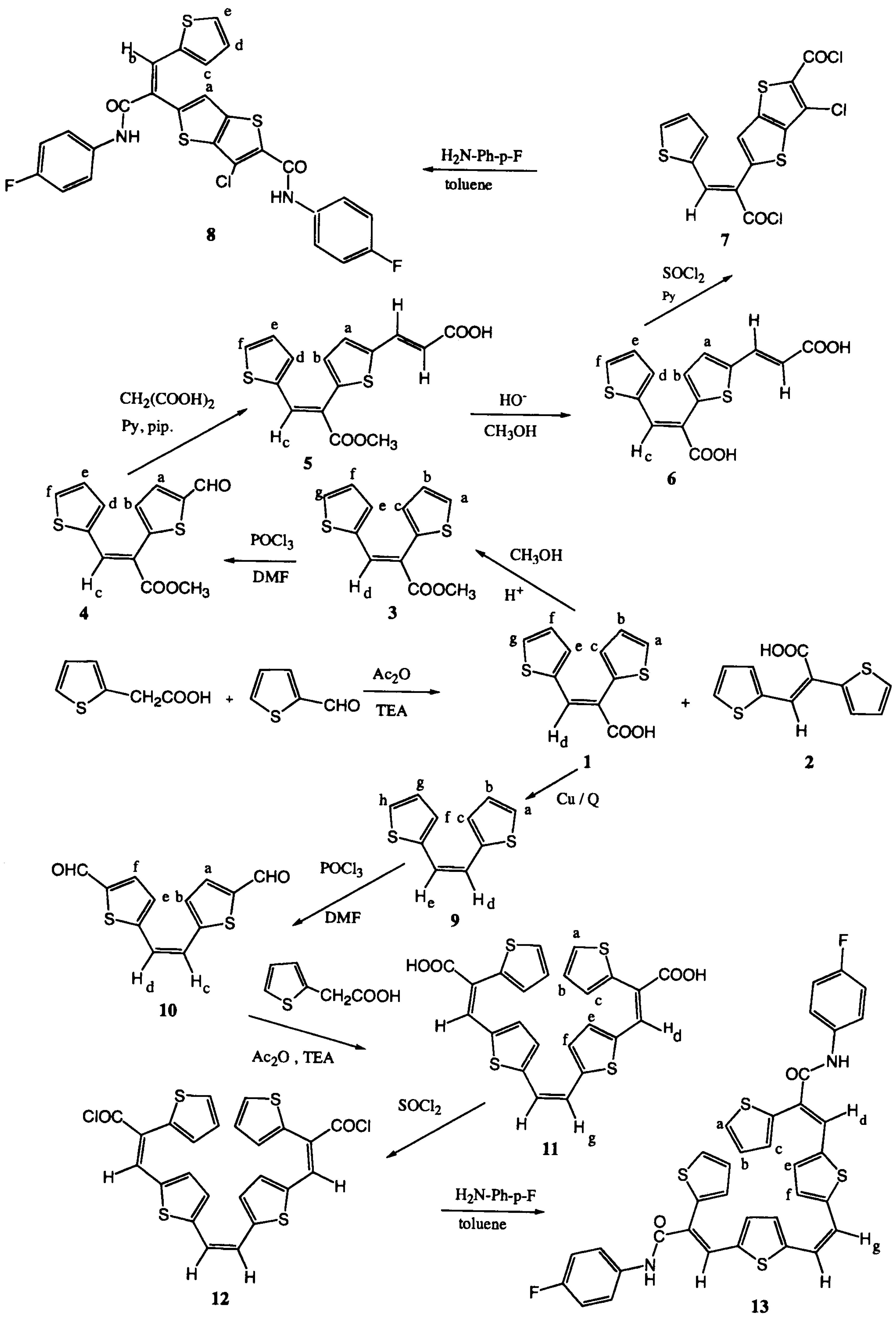
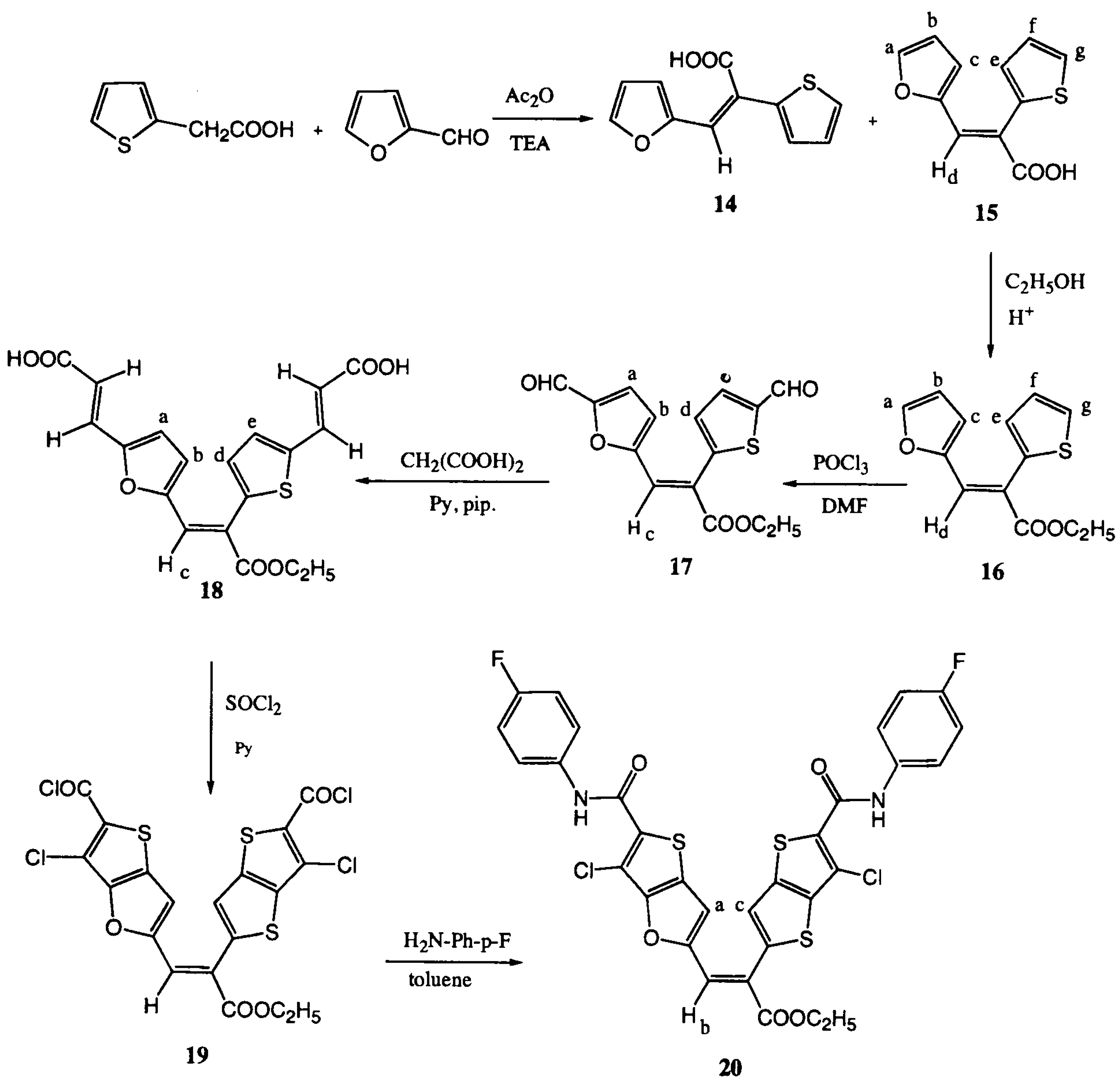
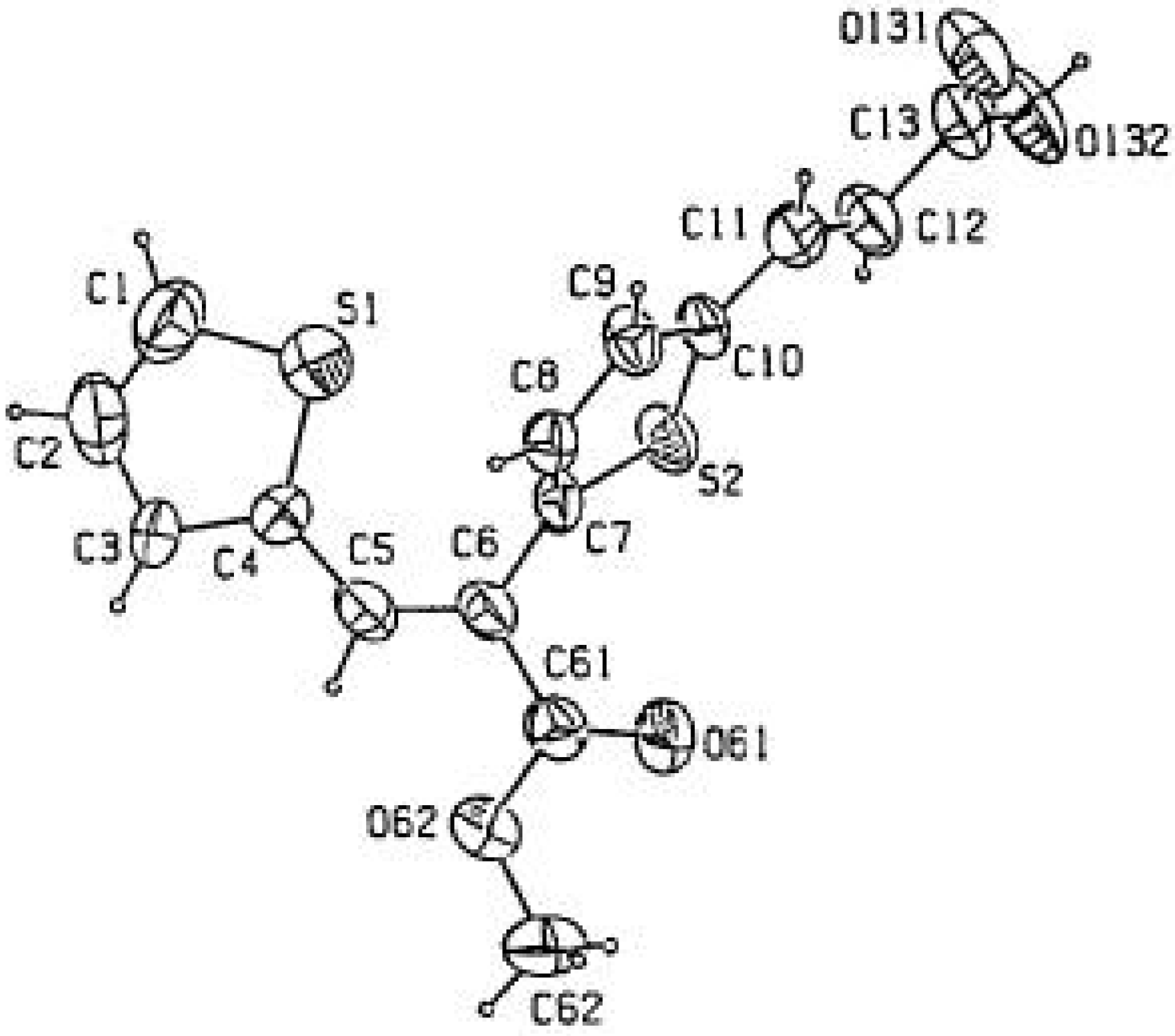
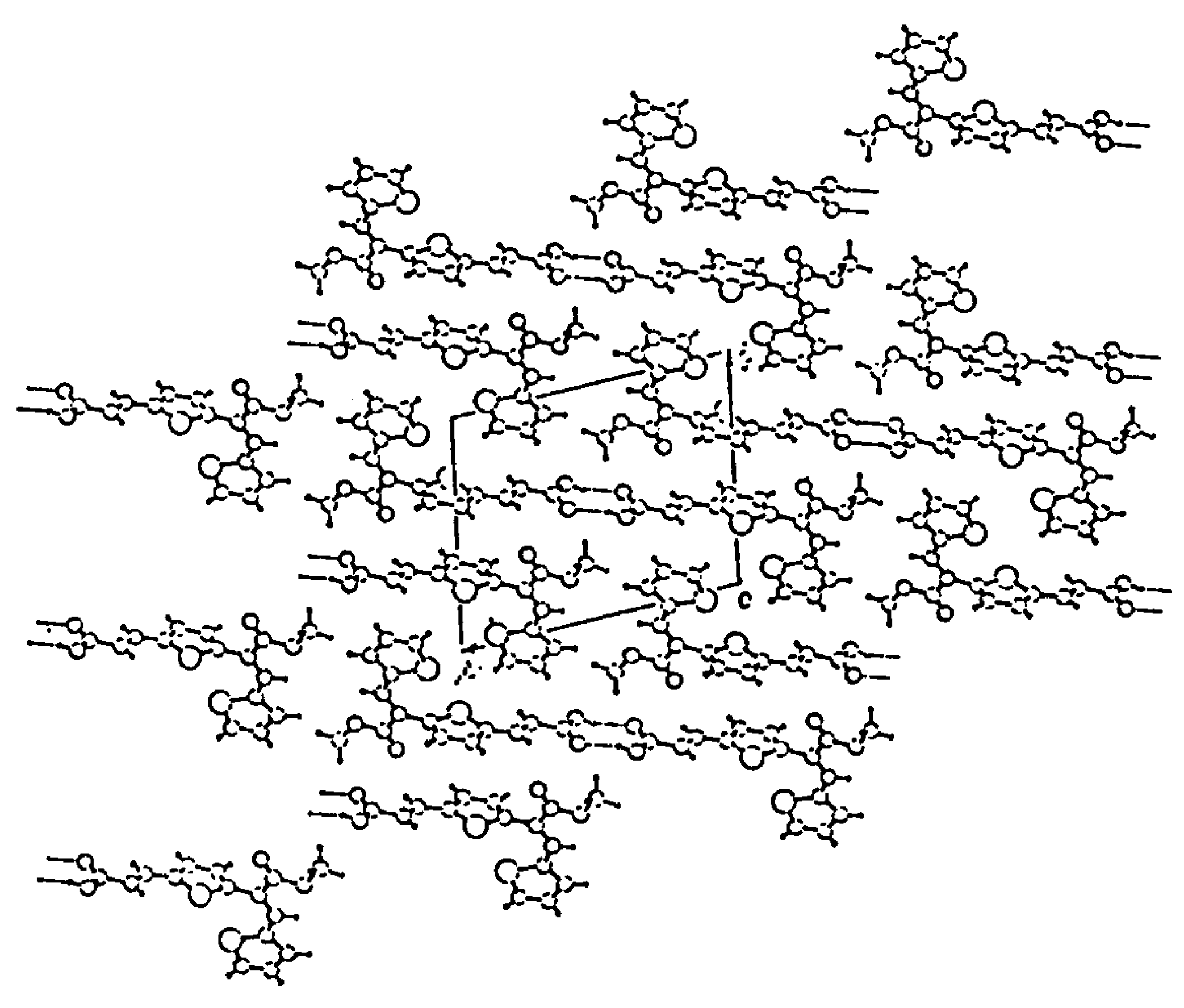
Experimental
General
(E)- and (Z)-2,3-di-(2-thienyl)acrylic acids (1) and (2) [23].
Methyl (Z)-2,3-di-(2-thienyl)acrylate (3) [23, 26].
Methyl (Z)-2-(5-formyl-2-thienyl)-3-(2-thienyl)acrylate (4) [23]
3-{5-[1-methoxycarbonyl-2-(2-thienyl)ethenyl]-2-thienyl}acrylic acid (5) [18]
3-{5-[2-(2-thienyl)-1-carboxy]ethenyl}-2-(2-thienyl)acrylic acid (6)
3-Chloro-5-[1-chlorocarbonyl-2-(2-thienyl)ethenyl]thieno[3,2-b]thiophene-2-carbonylchloride (7)
3-Chloro-5-[1-(p-fluorophenylcarbamoyl)-2-(2-thienyl)ethenyl]thieno[3,2-b]thiophene-2-carboxy-p-fluoroanilide (8)
(Z)-1,2-di-(2-thienyl)ethene (9)
(Z)-1,2-di-[5-formyl-2-(2-thienyl)]ethene (10)
(Z)-1,2-bis{5-[2-carboxy-2-(2-thienyl)ethenyl]-2-thienyl}ethene (11)
(Z)-{5,5'-di-[2-(2-thienyl)-2-chlorocarbonylethenyl]}-1,2-di-(2-thienyl)ethene (12)
(Z)-1,2-bis{5-[2-(p-fluorophenylcarbamoyl)-2-(2-thienyl)ethenyl]-2-thienyl}ethene (13)
(Z)- and (E)-3-(2-furyl)-2-(2-thienyl)acrylic acids (14) and (15).
Ethyl (E)-3-(2-furyl)-2-(2-thienyl)acrylate (16).
Ethyl (Z)-3-[5-formyl-(2-furyl)]-2-[5-formyl-(2-thienyl)]acrylate (17)
Ethyl (Z)-3-[5-(2-carboxy)ethenyl-(2-furyl)]-2-[5-(2-carboxyethenyl)-(2-thienyl)]acrylate (18)
3-Chloro-5-{2-ethoxycarbonyl-2-[3'-chloro-2'-chlorocarbonyl-5'-thieno[3,2-b]thienyl]ethenyl} thieno[3,2-b]furan-carbonylchloride (19)
6-Chloro-2-{2-[3-chloro-2-(p-fluorophenylcarbamoyl)-5-thieno[3,2-b]thienyl]-2-{ethoxycarbonyl-ethenyl}thieno[3,2-b]furan-5-carbox-p-fluoroanilide (20)
Acknowledgements
References
- Fuller, L.S.; Iddon, B.; Smith, K.A. Thiophenes. Part 3. On the ring opening reaction of 3-lithiated and 3,6-dilithiated[3,2-b]thiophenes; new routes to polyfunctionalized thiophenes and enedyines. J. Chem. Soc. Perkin Trans. 1 1999, 1273–1277. [Google Scholar]
- Fuller, L.S.; Iddon, B.; Smith, K.A. Synthesis of polyfunctionalized thiophenes and enedyines via ring-opening reactions of 3-lithiated thieno[2,3-b] (and [3,2-b]) thiophenes, 3,4-dilithiated thieno[2,3-b]tiophenes and 3,6-lithiated thieno[3,2-b]tiophenes. Chem. Commun. 1997, 2355–2356. [Google Scholar]
- Fuller, L.S.; Iddon, B.; Smith, K.A. Thiophenes. 2. Synthesis, metallation and bromine-lithium exchange reactions of thieno[3,2-b]tiophene and polybromo derivatives. J Chem. Soc. Perkin Trans. 1 1997, 3465–3470. [Google Scholar]
- Whitfield, F.B.; Mottram, D.S. Heterocyclic volatiles formed by heating cysteine or hydrogen sulfide with 4-hydroxy-5-methyl-3(2H)-furanone at pH 6.5. J. Agr. Food Chem. 2001, 49, 816–822. [Google Scholar]
- Whitfield, F.B.; Mottram, D.S. Investigation of the reaction between 4-hydroxy-5-methyl-3(2H)-furanone and cysteine or hydrogen sulfide at pH 4.5. J. Agr. Food Chem. 1999, 47, 1626–1634. [Google Scholar]
- Yasuke, S.; Kurita, J.; Tsuchiya, T. Synthesis of novel group 15 and 16 thieno[2,3-b]-, thieno[3,4-b]- and thieno[3,2-b]-heteroles. Heterocycles 1997, 45, 1891–1894. [Google Scholar]
- Hawkins, D.W.; Iddon, B.; Longthorne, D.S.; Rosyk, P.J. Synthesis of thieno[2,3-b]-, -[3,2-b]- and -[3,4-b]thiophenes and thieno[3’,2’/4,5]-, -[2’,3’/4,5]- and -[3’,4’/4,5]-thieno[3,2-d]pyrimidin- 7(6)H)-ones starting from thiophene. J. Chem. Soc. Perkin Trans. 1 1994, 2735–2743. [Google Scholar]
- Choi, K.S.; Sawada, K.; Dong, H.; Hoshino, M.; Nakayama, J. A one-pot synthesis of substituted thieno[3,2-b]thiophenes and selenolo[3,2-b]selenophenes. Heterocycles 1994, 38, 143–149. [Google Scholar]
- Nakayama, J.; Dong, H.; Sawada, K.; Ishii, A.; Kumakura, S. Synthesis and characterization of dimers, trimers, and tetramers of 3,6-dimethylthieno[3,2b]thiophene and 3,6-dimethylselenolo-[3,2-b]selenophene. Tetrahedron 1996, 52, 471–488. [Google Scholar]
- Capozzi, G.; De Sio, F.; Manichetti, S.; Nativi, C.; Pacini, P.L. Phthalimidesulfenyl chloride .7. Synthesis of 2-substituted 3-chlorobenzo[b]thiophenes and related heteroaromatics. Synthesis 1994, 521–525. [Google Scholar]
- Otsubo, T.; Kono, Y.; Hozo, N.; Miyamoto, H.; Aso, Y.; Ogura, F.; Tanaka, T.; Sawada, M. Syntheses, structures, and properties of 2,3,6,7-tetrathiabenzo[1,3-cd:4,6-c'd']dipentalene and its methyl, ethyl, methylthio, and ethylthio derivatives - novel fused polynuclear heteroarenes. Bull. Chem. Soc. Jpn. 1993, 66, 2033–204. [Google Scholar]
- Schroth, W.; Hintzsche, E.; Jordan, H.; Jende, T.; Spitzner, R.; Thondorf, I. 1,2-Dithiines and precursors. 17. Synthesis and properties of thieno anelated 1,2-dithiins- structural influence on colour. Tetrahedron 1997, 53, 7509–7528. [Google Scholar]
- Fernández Fernández, M.I; Hotten, T.M.; Tupper, D.E. Thiophene carboxamide derivatives preparation. Span. Pat. Appl. ES 2,008,635. 1988. [Chem. Abstr., 1991, 114, 207008b]. [Google Scholar] Fernández Fernández, M.I.; Hotten, T.M.; Tupper, D.E. Amino-thiophene derivatives preparation. Span. Pat. Appl. ES 2,008,634. 1989. [Chem. Abstr., 1991, 114, 207009c]. [Google Scholar] Fernández Fernández, M.I.; Hotten, T.M.; Tupper, D.E. Thiophene carboxamide derivatives preparation. Span. Pat. Appl., ES 2,008,630. 1989. [Chem. Abstr., 1991, 114, 207010w]. [Google Scholar] Fernández Fernández, M.I.; Hotten, T.M.; Tupper, D.E. Thiophene carboxamide. Span. Pat. Appl. ES 2,008,631. 1989. [Chem. Abstr., 1991, 114, 207011x]. [Google Scholar] Fernández Fernández, M.I.; Hotten, T.M.; Tupper, D.E. Thiophene-carboxamide derivatives preparation. Span. Pat. Appl. ES 2,008,632. 1989. [Chem. Abstr., 1991, 114, 207012y]. [Google Scholar] Fernández Fernández, M.I.; Hotten, T. M.; Tupper, D. E. Amino-thiophene derivatives preparation. Span. Pat Appl., ES 2,008,633. 1989. [Chem. Abstr., 1991, 114, 207013z]. [Google Scholar] Fernández Fernández, M.I.; Hotten, T.M.; Tupper, D.E. Thiophene carboxamide derivatives preparation. Span. Pat. Appl., ES 2,008,636. 1989. [Chem. Abstr., 1991, 115, 71382e]. [Google Scholar]
- Meunster, P.; Steiner, G.; Freund, W.; Wuerzer, B.; Westphalen, K.O. Carboxamides and their use as herbicides. Ger. Offen. Appl., DE 3,933,573. 1991. [Chem. Abstr., 1991, 115, 71383f]. [Google Scholar]
- Boschelli, D.H.; Kramer, J.B.; Connor, D.T.; Lesch, M.E.; Schrier, D.J.; Ferin, M.A.; Wright, C.D. 3-Alkoxybenzo[b]thiophene-2-carboxamides as inhibitors of neutrophil endothelial cell adhesion. J. Med. Chem. 1994, 37, 717–718. [Google Scholar]
- Karminski-Zamola, G.M.; Pavličić, D.; Bajić, M.; Blažević, N. The synthesis of new heteropolycyclic quinolone by twofold photocyclization: methoxycarbonylnaphtho[2’’,1’’:2’,3’-b]- thieno[4’,5’:2,3]thieno[5,4-c]quinolin-6-(5H)-one. Heterocycles 1991, 32, 2323–2327. [Google Scholar]
- Dogan, J.; Karminski-Zamola, G.; Boykin, D.W. Photosynthesis of heterocyclic quinolones. Heterocycles 1995, 41, 1659–1666. [Google Scholar]
- Malešević, M.; Karminski-Zamola, G.; Bajić, M.; Boykin, D.W. Photosynthesis of heteropolycyclic diquinolones, twofold photodehydrohalogenation reaction of benzo[1,2-b:4,5- b’]dithiophene and dithieno[3,2-b:2’,3’-d]thiophenedicarboxanilides. Heterocycles 1995, 41, 2691–2699. [Google Scholar]
- Dogan Koružnjak, J.; Slade, N.; Zamola, B.; Pavelić, K.; Karminski-Zamola, G. Synthesis, photochemical synthesis and antitumor evaluation of novel derivatives of thieno[3’,2’: 4,5]thieno[2,3-c]quinolones. Chem. Pharm. Bull. 2002, 50, 656–660. [Google Scholar]
- Hopkins, H.P.; Ming, Y.; Wilson, W.D.; Boykin, D.W. Intercalation binding of 6-substituted naphthothiopheneamides to DNA: enthalpy and entropy components. Biopolymers 1991, 31, 1105–1114. [Google Scholar]
- Boykin, D.W.; Kumar, A.; Hall, J.E.; Bender, B.C.; Tidwell, R.R. Anti-pneumocystis activity of bis-amidoximes and bis-O-alkylamidoximes prodrugs. Bioorg. & Med. Chem. Lett. 1996, 6, 3017–3020. [Google Scholar]
- Clark, G.R.; Boykin, D.W.; Czarny, A.; Neidle, S. Structure of a bis-amidinium derivative of Hoechst 33258 complexed to dodecanucleotide D(CGCGAATTCGCG)2 - The role of hydrogen bonding in minor groove drug DNA recognition. Nucleic Acids Res. 1997, 25, 1510–1515. [Google Scholar]
- Badger, G.M.; Elix, J.A.; Lewis, G.E. The synthesis of [18]annulene trisulphide. Aust. J. Chem. 1965, 18, 70–89. [Google Scholar]
- Ried, W.; Oremek, G; Ocakcioglu, B. Synthese von sustituierten benzo[b]thiophenen. Liebigs Ann. Chem. 1980, 1424–1427. [Google Scholar]
- Karminski-Zamola, G.M.; Jakopčić, K. The preparation of some 1-(2-furyl)-2-arylethylenes. J. Heterocycl. Chem. 1981, 18, 193–1936. [Google Scholar]
- Bajić, M.; Karminski-Zamola, G.M.; Blažević, N. Twofold photochemical dehydrocyclization reaction of substituted 2,5-distyrylthiophenes and 2,5-distyrylfuranes. Croat. Chem. Acta 1992, 65, 835–846. [Google Scholar]
- Karminski-Zamola, G.M.; Fišer-Jakić, L; Jakopčić, K. Photochemistry of furans. Photochemical transformations of some substituted 2-phenyl-3-furylacrylic acids. Tetrahedron 1982, 38, 1329–1336. [Google Scholar]
- CCDC 199584 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk). Unit cell parameters: a 5.661(9) b 10.656(16) c 12.879(12); α 101.14(9); β 100.28(9); γ 92.78(10); space group P-1.
- Moutheard, J.P.; Dubois, J.C. Preparation de dithienyl-2-ethene-1,2- de dithienyl-2-pyrimidine-2,4 et de dithienyl-2-pyridazone-3,6. J. Heterocycl. Chem. 1985, 22, 719–720. [Google Scholar]
- Sample Availability: Samples of compounds 5 and 13 are available from authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Pavlicic, D.; Koružnjak, J.D.; Banic-Tomišic, Z.; Karminski-Zamola, G. Synthesis of Some New bis-(p-Fluorophenyl)amides of the Thieno[3,2-b]thiophene, Thieno[3,2-b]furan and 1,2-bis{5-[2-(2-Thienyl)ethenyl]2-thienyl}ethene Series. Molecules 2002, 7, 871-884. https://doi.org/10.3390/71200871
Pavlicic D, Koružnjak JD, Banic-Tomišic Z, Karminski-Zamola G. Synthesis of Some New bis-(p-Fluorophenyl)amides of the Thieno[3,2-b]thiophene, Thieno[3,2-b]furan and 1,2-bis{5-[2-(2-Thienyl)ethenyl]2-thienyl}ethene Series. Molecules. 2002; 7(12):871-884. https://doi.org/10.3390/71200871
Chicago/Turabian StylePavlicic, Davorka, Jasna Dogan Koružnjak, Zrinka Banic-Tomišic, and Grace Karminski-Zamola. 2002. "Synthesis of Some New bis-(p-Fluorophenyl)amides of the Thieno[3,2-b]thiophene, Thieno[3,2-b]furan and 1,2-bis{5-[2-(2-Thienyl)ethenyl]2-thienyl}ethene Series" Molecules 7, no. 12: 871-884. https://doi.org/10.3390/71200871
APA StylePavlicic, D., Koružnjak, J. D., Banic-Tomišic, Z., & Karminski-Zamola, G. (2002). Synthesis of Some New bis-(p-Fluorophenyl)amides of the Thieno[3,2-b]thiophene, Thieno[3,2-b]furan and 1,2-bis{5-[2-(2-Thienyl)ethenyl]2-thienyl}ethene Series. Molecules, 7(12), 871-884. https://doi.org/10.3390/71200871



