Electrochemical Anion Recognition By Novel Ferrocenyl Imidazole Systems
Abstract
:Introduction
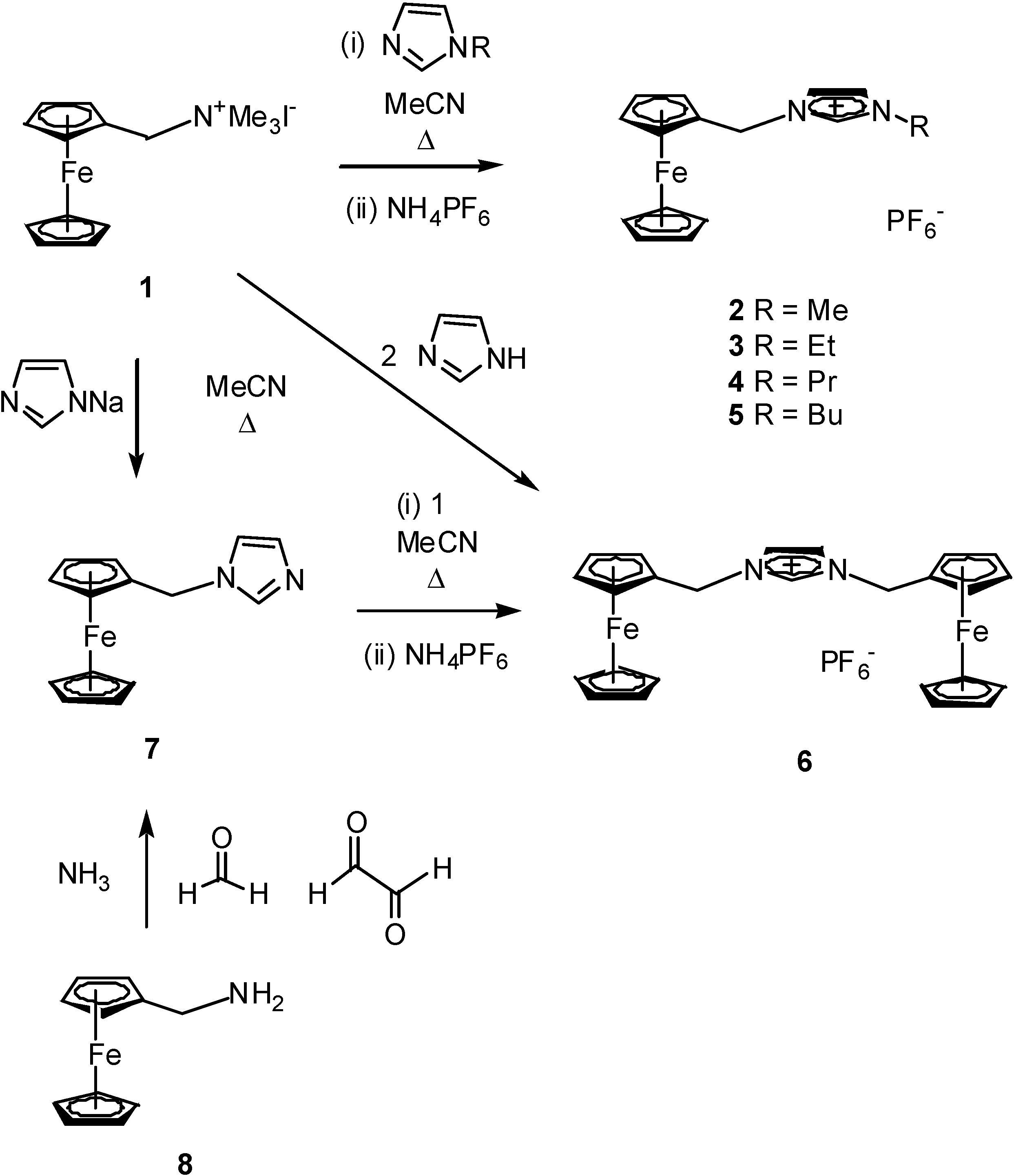
Results and Discussion
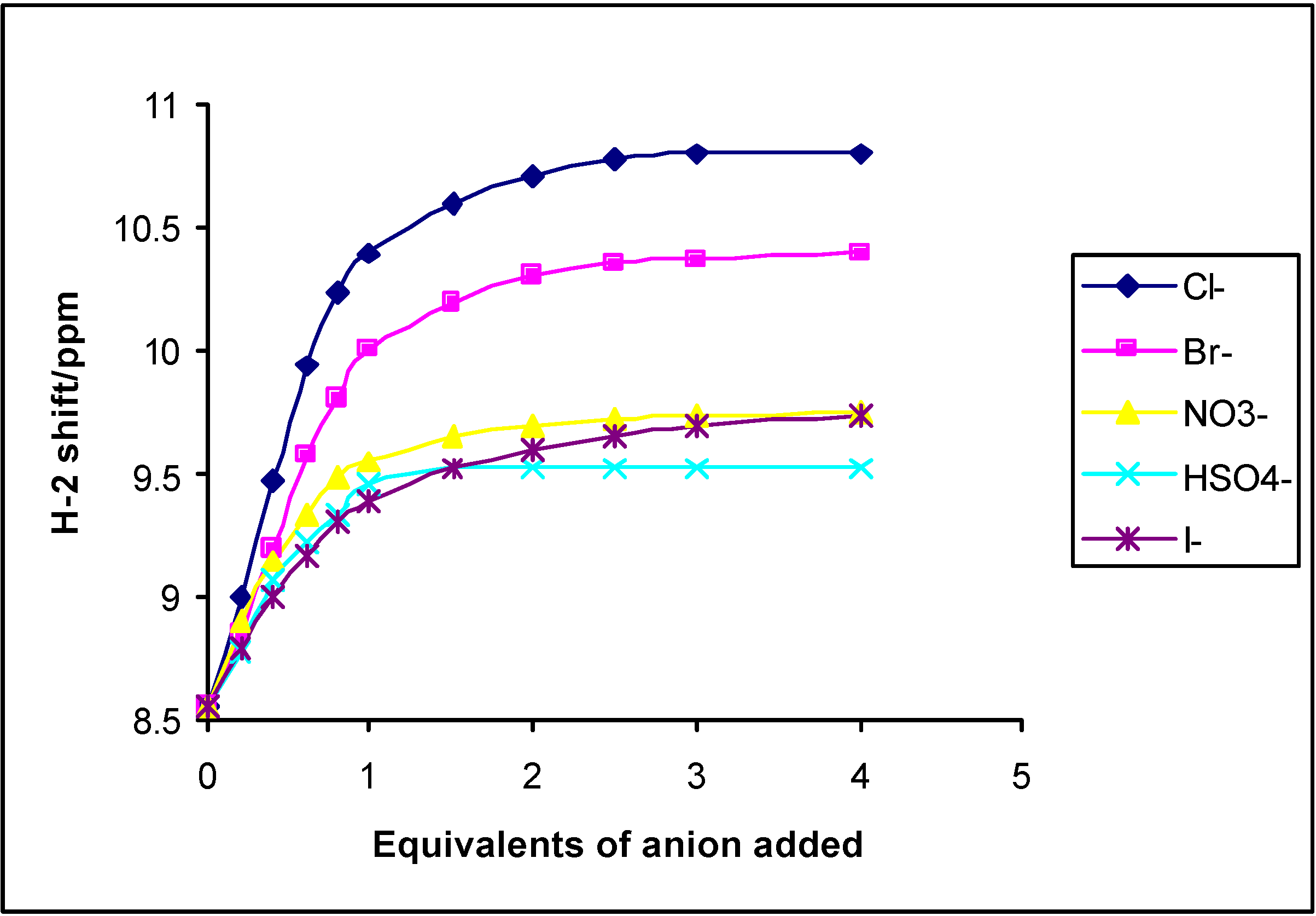
1H-NMR titrations
Electrochemical anion recognition
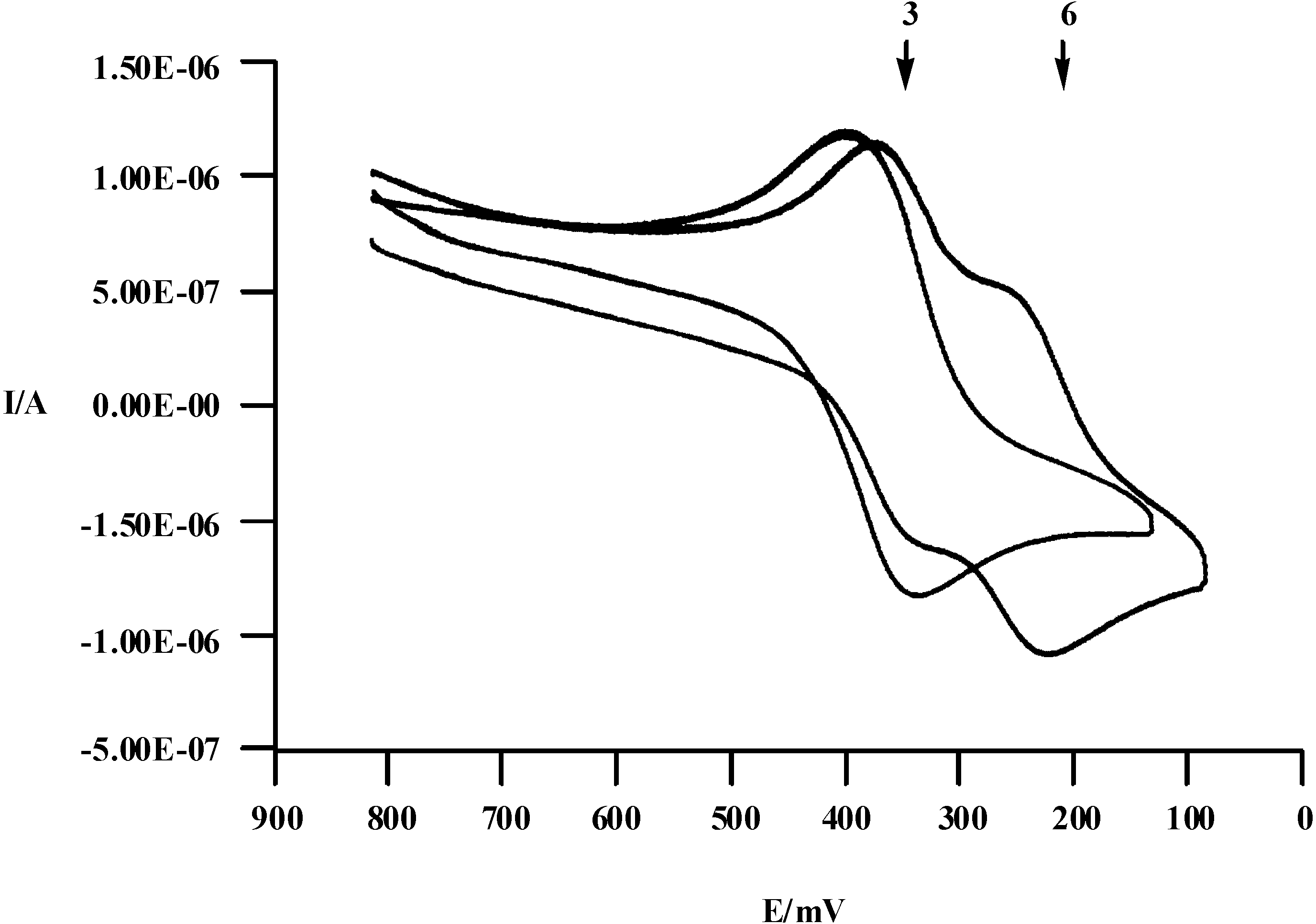
| Compound | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| Epa (free) / mV | 274 | 288 | 311 | 293 | 301 |
| Epc (free) / mV | 148 | 173 | 168 | 190 | 176 |
| ΔEpa (Cl-)a / mV | -24 | -10 | -39 | -3 | -23 |
| ΔEpa (Br-)a / mV | 105 | 71 | 142 | 112 | 194 |
| ΔEpa (NO3-)a / mV | -23 | -22 | -35 | -10 | -58 |
| ΔEpa (HSO4-) / mV | -118 | -83 | -97 | -82 | -70 |
| |Epa - Epc|b / mV | 126 | 115 | 143 | 103 | 125 |
| ipa / ipcb | 0.986 | 0.981 | 1.021 | 1.002 | 1.267 |
Conclusions
Experimental
General
Syntheses of ferrocenylimidazolium salts
References
- Lang, L. G.; Riordon, J. F.; Vallee, B. L. Biochemistry 1974, 13, 4361.
- Beer, P. D.; Hesek, D.; Hadocova, J.; Stokes, S. E. J. Chem. Soc., Chem. Commun. 1992, 270.Beer, P. D.; Hazlewood, C.; Hesek, D.; Hadocova, J.; Stokes, S. E. J. Chem. Soc., Dalton Trans. 1993, 1327.Beer, P. D.; Chen, Z.; Goulden, A. J.; Graydon, A.; Stokes, S. E.; Wear, T. J. Chem. Soc., Chem. Commun. 1993, 1834.Beer, P. D.; Chen, Z.; Goulden, A. J.; Grieve, A.; Hesek, D.; Szemes, F.; Wear, T. J. Chem. Soc., Chem. Commun. 1994, 1269.Beer, P. D.; Drew, M. G. B.; Hesek, D.; Jagessar, R. J. Chem. Soc., Chem. Commun. 1995, 1187.Beer, P. D.; Hesek, D.; Kingston, J. E.; Smith, D. K.; Stokes, S. E.; Drew, M. G. B. Organometallics 1995, 14, 3288.Beer, P. D.; Drew, M. G. B.; Grayton, A. R.; Smith, D. K.; Stokes, S. E. J. Chem. Soc., Dalton Trans. 1995, 403.Beer, P. D.; Drew, M. G. B.; Hodacova, J.; Stokes, S. E. J. Chem. Soc., Dalton Trans. 1995, 3447.Beer, P. D. Chem. Commun. 1996, 689.
- Dieter, K. M.; Dymek, C. J.; Heimer, N. E.; Rovang, J. W.; Wilkes, J. S. J. Am. Chem. Soc. 1988, 110, 2722.
- Dymek, C. J.; Stewart, J. J. P. Inorg. Chem. 1989, 28, 1472.
- Lapshin, S. A.; Yu. Chervinskii, A.; Litvinento, L. M.; Dadali, V. A.; Kapkan, L. M.; Vdovichenko, A. N. Zh. Org. Khim. 1985, 21, 357.
- Avent, A. G.; Chaloner, P. A.; Day, M. P.; Seddon, K. R.; Welton, T. J. Chem. Soc., Dalton Trans. 1994, 3405.
- Elaiwi, A.; Hitchcock, P. B.; Seddon, K. R.; Srinivasan, N.; Tan, Y.; Welton, T.; Zora, J. A. J. Chem. Soc., Dalton Trans. 1995, 3467.
- Sato, K.; Arai, S.; Yamagishi, T. Tetrahedron Lett. 1999, 40, 5219.
- Howarth, J.; Hanlon, K.; Fayne, D.; McCormac, P. Tetrahedron Lett. 1997, 17, 3097.
- Howarth, J.; Al-Hashimy, N. A. Tetrahedron Lett. 2001, 42, 5777. [Google Scholar]
- Howarth, J.; Thomas, J-L.; Hanlon, K.; McGuirk, D. Synthetic Commun. 2000, 30, 1865.
- Bublitz, D. E. J. Organomet. Chem. 1970, 2, 225.
- Grimshaw, J.; Trocha-Grimshaw, J. J. Chem. Soc., Perkin Trans. 2 1991, 751.
- Arduengo, A. J. US Patent 5,077,414, 1991.
- Howarth, J.; Hanlon, K. Tetrahedron Lett. 2001, 42, 751.
- Sample Availability: Available from the authors. Other analogues of monoferrocenyl substituted imidazolium salts, see reference [15] for structures, are also available.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Thomas, J.-L.; Howarth, J.; Kennedy, A.M. Electrochemical Anion Recognition By Novel Ferrocenyl Imidazole Systems. Molecules 2002, 7, 861-866. https://doi.org/10.3390/71200861
Thomas J-L, Howarth J, Kennedy AM. Electrochemical Anion Recognition By Novel Ferrocenyl Imidazole Systems. Molecules. 2002; 7(12):861-866. https://doi.org/10.3390/71200861
Chicago/Turabian StyleThomas, Jean-Luc, Joshua Howarth, and Anthony M. Kennedy. 2002. "Electrochemical Anion Recognition By Novel Ferrocenyl Imidazole Systems" Molecules 7, no. 12: 861-866. https://doi.org/10.3390/71200861
APA StyleThomas, J.-L., Howarth, J., & Kennedy, A. M. (2002). Electrochemical Anion Recognition By Novel Ferrocenyl Imidazole Systems. Molecules, 7(12), 861-866. https://doi.org/10.3390/71200861




