Characterisation of Neuropeptide Y Receptor Subtypes by Synthetic NPY Analogues and by Anti-receptor Antibodies
Abstract
:Introduction
Results and Discussion
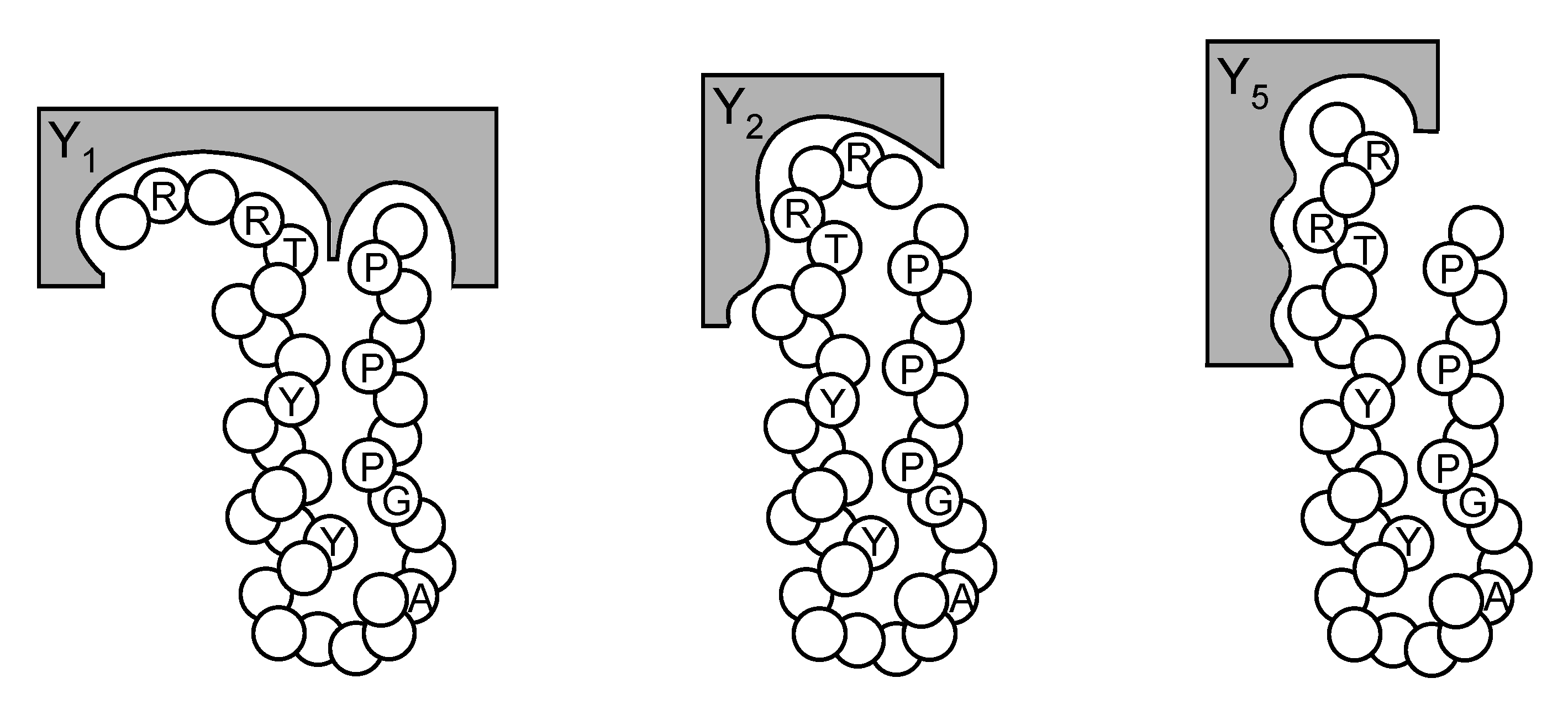
Synthesis of the Analogues and Receptor Segments
| Name | Sequence | Masscalc [Da] | Massexp [Da] | Position in the receptor |
| Y1 E2/2 | Q I L T D E P F Q N V S L A A F K D K | 2163.5 | 2165 | 76-94 (rat) |
| Y2 E2/1 | I F R E Y S L I E I I P D F E I V A F | 2313.7 | 2314 | 75-93a (human) |
| Y5 E2/2 | L L S S R Y L C V E S W P S D S Y R I A F | 2491.9 | 2491 | 186-206 (human) |
| Y5 E3 | H V V T D F N D N L I S N R H F K L V | 2267.6 | 2268 | 278-296 (human) |
Characterisation of the Sera
Binding Potency of Full Length NPY Analogues
| Peptide | Masscalc. [Da] | Massexp [Da] |
| pNPY | 4253.7 | 4253 |
| [A1]-pNPY | 4161.7 | 4162 |
| [A2]-pNPY | 4227.7 | 4224 |
| [A5]-pNPY | 4227.7 | 4228 |
| [A8]-pNPY | 4227.7 | 4225 |
| [A11]-pNPY | 4209.7 | 4210 |
| [A13]-pNPY | 4227.7 | 4226 |
| [A19]-pNPY | 4168.6 | 4169 |
| [A20]-pNPY | 4161.7 | 4160 |
| [A21]-pNPY | 4161.7 | 4160 |
| [A25]-pNPY | 4168.7 | 4167 |
| [A27]-pNPY | 4161.7 | 4159 |
| [A32]-pNPY | 4223.8 | 4221 |
| [A33]-pNPY | 4168.7 | 4167 |
| [A34]-pNPY | 4196.7 | 4195 |
| [A35]-pNPY | 4168.7 | 4167 |
| [A36]-pNPY | 4161.7 | 4158 |
| [L34]-pNPY | 4238.8 | 4238 |
| [D-P34]-pNPY | 4222.8 | 4222 |
| [Ahx5-24]-NPY | 2220.6 | 2219 |
| [Ahx5-24, P34]-NPY | 2189.6 | 2190 |
| [Ahx8-20]-NPY | 2981.4 | 2980 |
| [Ahx8-19]-NPY | 3144.6 | 3144 |
| [Ahx9-20]-NPY | 3078.6 | 3078 |
| [Ahx9-17]-NPY | 3469.0 | 3470 |
| hPP | 4181.8 | 4181 |
| [Ahx5-24]-hPP | 2161.6 | 2162 |
| [Ahx5-20]-hPP | 2532.0 | 2531 |
| [Y5-20]-hPP | 2582.0 | 2582 |
| Peptide | IC50 | |||||||
| hY1 [nM] | IC50 (Pep) IC50 (NPY) | hY2 [nM] | IC50 (Pep) IC50 (NPY) | hY4 [nM] | IC50 (Pep) IC50 (NPY) | hY5 [nM] | IC50 (Pep) IC50 (NPY) | |
| pNPY | 0.23 | (1) | 0.04 | (1) | 5.5 | (1) | 0.8 | (1) |
| [A1]-pNPY | 21 | (91) | 0.2 | (5) | 5.8 | (1.1) | 2.2 | (2.8) |
| [A2]-pNPY | 114 | (496) | 0.3 | (8) | 7.8 | (1.4) | 5.5 | (7) |
| [A5]-pNPY | 228 | (991) | 24 | (600) | 25 | (4.5) | 32 | (40) |
| [A8]-pNPY | 32 | (139) | 0.7 | (18) | 60 | (11) | 55 | (69) |
| [A11]-pNPY | 8.0 | (35) | 0.2 | (5) | 3.1 | (0.6) | 0.5 | (0.6) |
| [A13]-pNPY | 7.5 | (33) | 0.1 | (3) | 37 | (6.7) | 17 | (21) |
| [A19]-pNPY | 282 | (1226) | 1.6 | (40) | 4.1 | (0.7) | 1.4 | (1.8) |
| [A20]-pNPY | 71 | (309) | 1.2 | (30) | 161 | (29) | 19 | (24) |
| [A21]-pNPY | 5.5 | (24) | 0.2 | (5) | 66 | (12) | 32 | (40) |
| [A25]-pNPY | 11 | (48) | 0.7 | (18) | 201 | (37) | 80 | (100) |
| [A27]-pNPY | 250 | (1087) | 1.4 | (35) | 340 | (62) | 370 | (463) |
| [A32]-pNPY | 723 | (3143) | 45 | (1125) | 380 | (69) | 7.7 | (9.5) |
| [A33]-pNPY | >1000 | (>4348) | 54 | (1350) | >1000 | (>182) | 94 | (118) |
| [A34]-pNPY | 94 | (409) | 6.0 | (150) | 7.4 | (1.3) | 1.3 | (1.6) |
| [A35]-pNPY | >1000 | (>4348) | >1000 | (>25000) | >1000 | (>182) | >1000 | (>1250) |
| [A36]-pNPY | 970 | (4217) | 48 | (1200) | 141 | (26) | 68 | (85) |
| [L34]-pNPY | 0.3 | (0.05) | 1.8 | (2.3) | ||||
| [D-P34]-pNPY | 266 | (1157) | 271 | (49) | 156 | (195) | ||
| [Ahx5-24]-NPY | >1000 | (>4348) | 1.4 | (35) | 600 | (109) | 795 | (994) |
| [Ahx5-24, P34]-NPY | >1000 | (>4348) | 514 | (93) | >10000 | (>12500) | ||
| [Ahx8-20]-NPY | 28 | (122) | 67 | (12) | 31 | (39) | ||
| [Ahx8-19]-NPY | 46 | (200) | 95 | (17) | 19 | (24) | ||
| [Ahx9-20]-NPY | 74 | (322) | 108 | (20) | 29 | (36) | ||
| [Ahx9-17]-NPY | 13 | (57) | 45 | (8) | 11 | (14) | ||
| hPP | >1000 | (>4348) | >1000 | (>25000) | 0.04 | (0.007) | 24 | (30) |
| [Ahx5-24]-hPP | >500 | (>2174) | >1000 | (>25000) | 144 | (26) | >1000 | (>1250) |
| [Ahx5-20]-hPP | >1500 | (>6522) | >1000 | (>25000) | 216 | (39) | >7000 | (>8750) |
| [Y5-20]-hPP | >500 | (>2174) | >1000 | (>25000) | 27 | (5) | >5000 | (>6250) |
Binding Potency of Centrally Truncated NPY Analogues
Immunofluorescence on Cells expressing Y-Receptor Subtypes
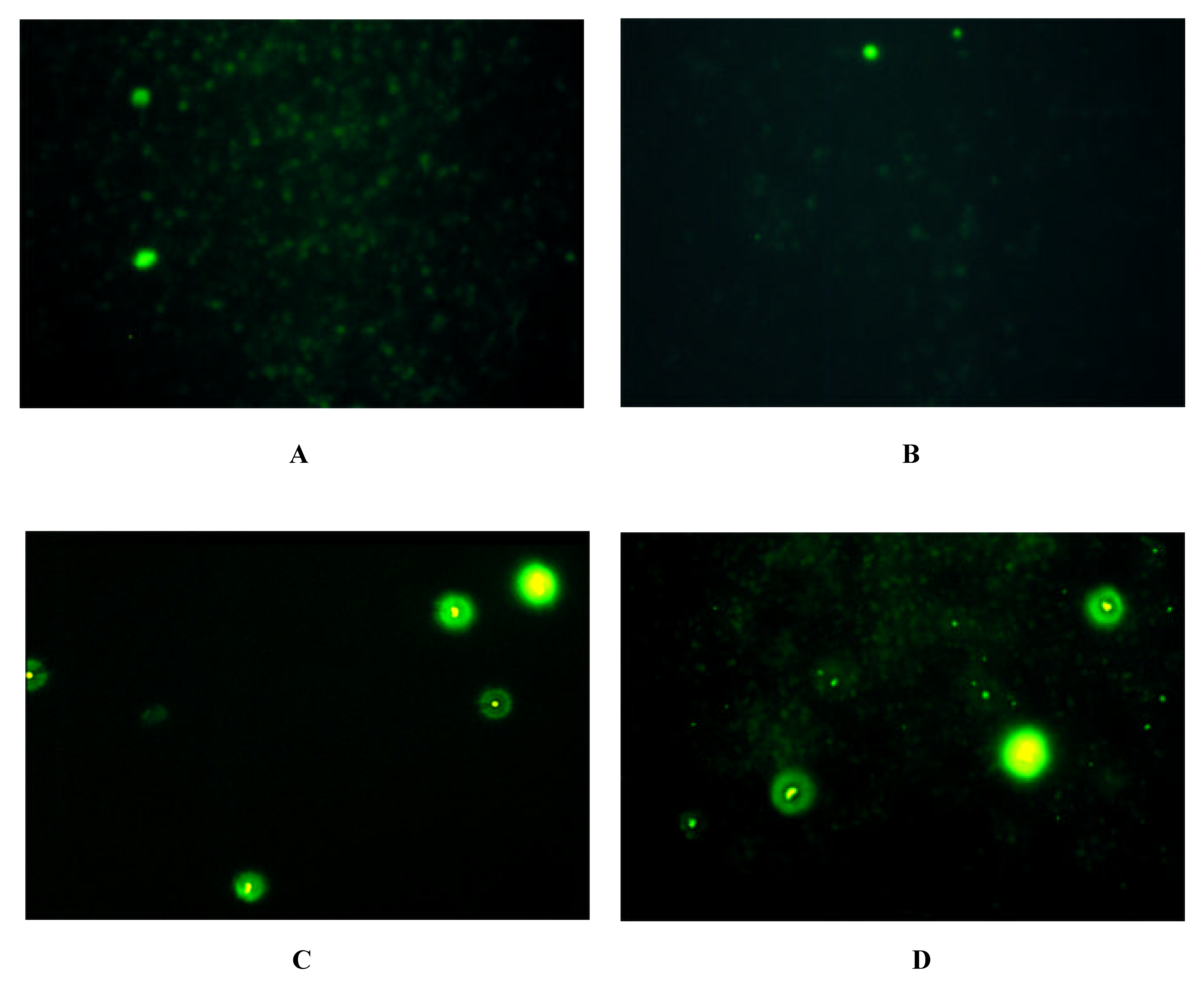
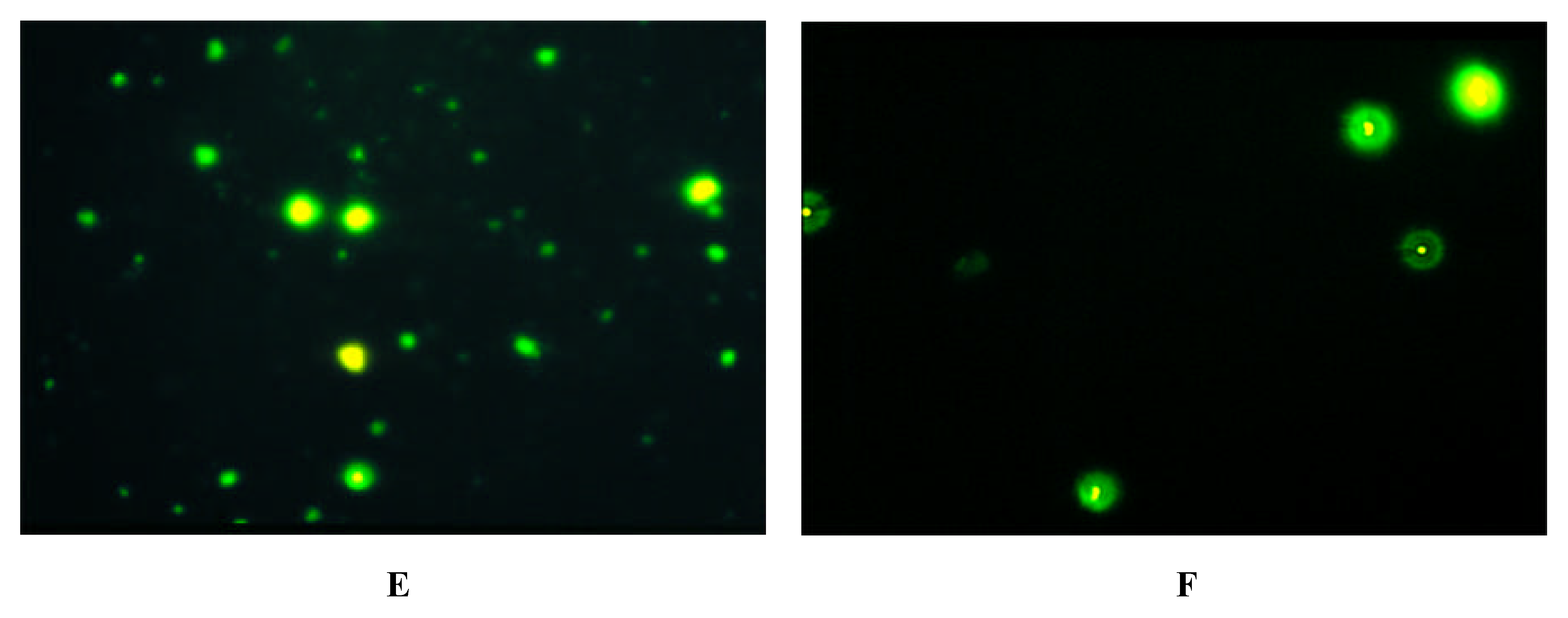
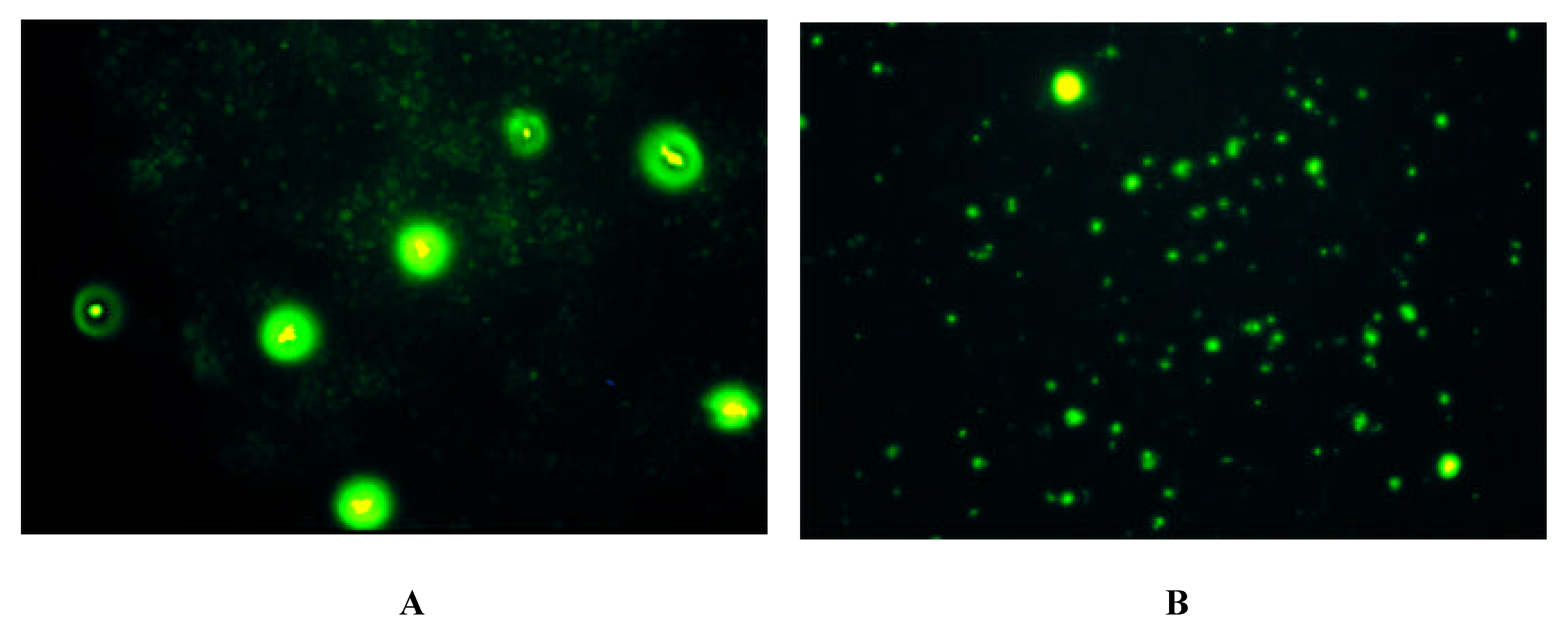
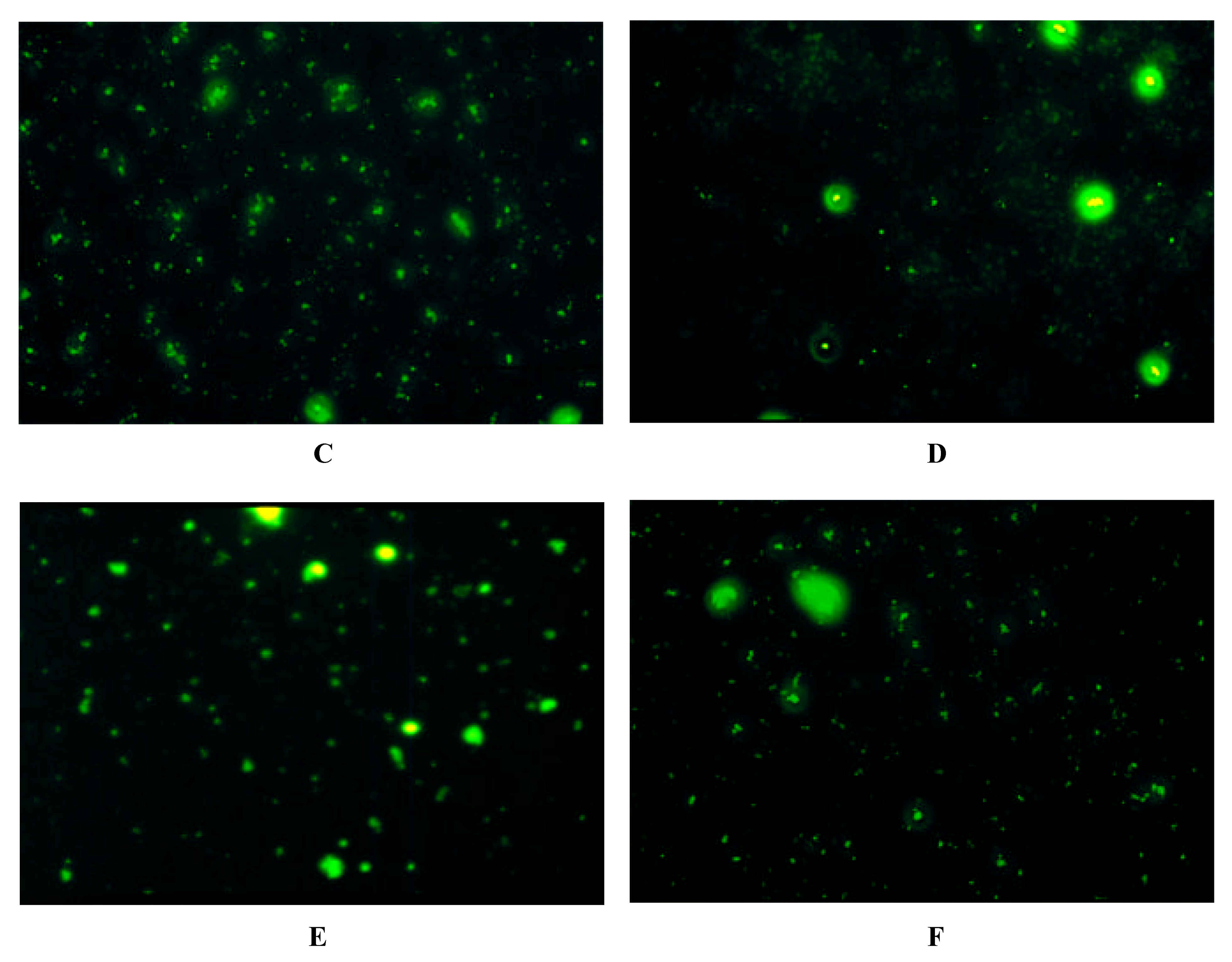
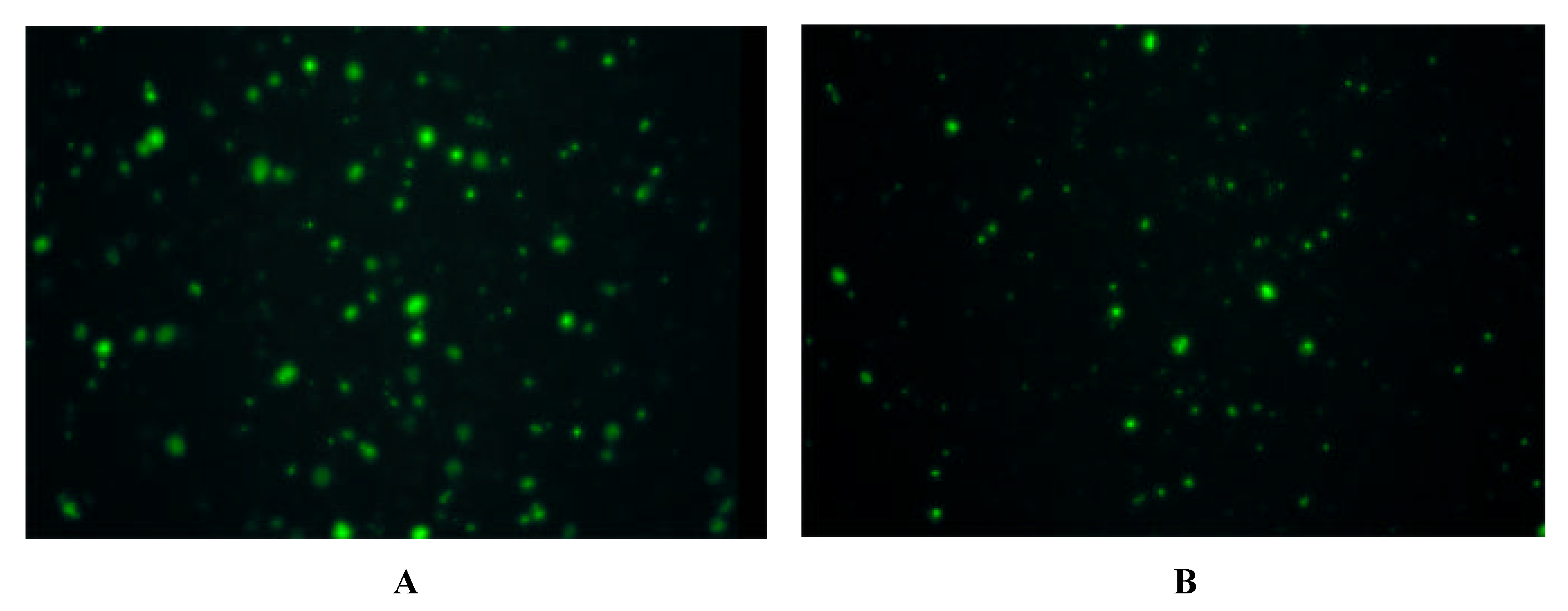
| antibody | Y1-receptor SK-N-MC | Y2-receptor SMS-KAN | Y5-receptor BHK |
| Y1 E2/2 | + | - | - |
| Y2 E2/1 | - | + + | - |
| Y5 E2/2 | - | + + + | + + + |
| Y5 E3 | - | + + + | + + + |
Discussion
Conclusions
Abbreviations
Acknowledgements
Experimental
Synthesis and Characterisation of the NPY Analogues and Segments of Y-Receptors
Preparation of the Conjugates and Immunisation
Titer Determination of the Receptor Segment Peptides
Cell Culture
Binding Potency of NPY Analogues
Immunofluorescence
References
- Tatemoto, K.; Carlquist, M.; Mutt, V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 1982, 296, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Inui, A. Neuropeptide Y feeding receptors: are multiple subtypes involved? Trends Pharm. Sci. 1999, 20, 43–46. [Google Scholar] [CrossRef]
- Kalra, S. P.; Crowley, W. R. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front. Neuroendocrinol. 1992, 13, 1–46. [Google Scholar] [PubMed]
- McDonald, J. K.; Lumpkin, M. D.; Samson, W. K.; McCann, S. M. Neuropeptide Y affects secretion of luteinizing hormone and growth hormone in ovariectomized rats. Proc. Natl. Acad. Sci. U.S.A. 1985, 82, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Moltz, J. H.; McDonald, J. K. Neuropeptide Y: direct and indirect action on insulin secretion in the rat. Peptides 1985, 6, 1155–1159. [Google Scholar] [CrossRef]
- Wahlestedt, C.; Yanaihara, N.; Håkanson, R. Evidence for different pre- and postjunctional receptors for neuropeptide Y and related peptides. Regul. Pept. 1986, 13, 307–318. [Google Scholar] [CrossRef]
- Flood, J. F.; Hernandez, E. N.; Morley, J. E. Modulation of memory processing by neuropeptide Y. Brain Res. 1987, 421, 280–290. [Google Scholar] [CrossRef]
- Jolicoeur, F. B.; Michaud, J. N.; Rivest, R.; Menard, D.; Gaudin, D.; Fournier, A.; St-Pierre, S. Neurobehavioral profile of neuropeptide Y. Brain. Res. Bull. 1991, 26, 265–268. [Google Scholar] [CrossRef]
- Jolicoeur, F. B.; Michaud, J. N.; Menard, D.; Fournier, A. In vivo structure activity study supports the existence of heterogeneous neuropeptide Y receptors. Brain. Res. Bull. 1991, 26, 309–311. [Google Scholar] [CrossRef]
- Esteban, J.; Chover, A. J.; Sanchez, P. A.; Mico, J. A.; Gibert-Rahola, J. Central administration of neuropeptide Y induces hypothermia in mice. Possible interaction with central noradrenergic systems. Life Sci. 1989, 45, 2395–2400. [Google Scholar] [CrossRef]
- Clark, J. T.; Kalra, P. S.; Kalra, S. P. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Obes. Res. 1997, 5, 275–283. [Google Scholar] [CrossRef]
- Calza, L.; Giardino, L.; Zanni, M.; Velardo, A.; Parchi, P.; Marrama, P. Daily changes of neuropeptide Y-like immunoreactivity in the suprachiasmatic nucleus of the rat. Regul. Pept. 1990, 27, 127–137. [Google Scholar] [CrossRef]
- McAuley, M. A.; Chen, X.; Westfall, T. C. Central cardiovascular actions of neuropeptide Y. In The biology of neuropeptide Y and related peptides; Colmers, W. F., Wahlestedt, C., Eds.; Humana Press Inc.: Totowa, 1993; pp. 389–418. [Google Scholar]
- Heilig, M. Neuropeptide Y in relation to behavior and psychiatric disorders: some animal and clinical observations. In The biology of neuropeptide Y and related peptides; Colmers, W. F., Wahlestedt, C., Eds.; Humana Press Inc.: Totowa, 1993; pp. 511–555. [Google Scholar]
- Thiele, T. E.; Marsh, D. J.; Marie, L. S.; Bernstein, I. L.; Palmiter, R. D. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature 1998, 396, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Michel, M. C.; Beck-Sickinger, A. G.; Cox, H.; Doods, H. N.; Herzog, H.; Larhammar, D.; Quirion, R.; Schwartz, T.; Westfall, T. XVI. International union of pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998, 50, 143–150. [Google Scholar]
- Krause, J.; Eva, C.; Seeburg, P. H.; Sprengel, R. Neuropeptide Y1 subtype pharmacology of a recombinantly expressed neuropeptide receptor. Mol. Pharmacol. 1992, 41, 817–821. [Google Scholar] [PubMed]
- Larhammar, D.; Blomqvist, A. G.; Yee, F.; Jazin, E.; Yoo, H.; Wahlestedt, C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. J. Biol. Chem. 1992, 267, 10935–10938. [Google Scholar]
- Herzog, H.; Hort, Y. J.; Ball, H. J.; Hayes, G.; Shine, J.; Selbie, L. A. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 5794–5798. [Google Scholar] [CrossRef]
- Rose, P. M.; Fernandes, P.; Lynch, J. S.; Frazier, S. T.; Fisher, S. M.; Kodukula, K.; Kienzle, B.; Seethala, R. Cloning and functional expression of a cDNA encoding a human type 2 neuropeptide Y receptor. J. Biol. Chem. 1995, 270, 29038. [Google Scholar] [CrossRef]
- Gerald, C.; Walker, M. W.; Vaysse, P. J.; He, C.; Branchek, T. A.; Weinshank, R. L. Expression cloning and pharmacological characterization of a human hippocampal neuropeptide Y/peptide YY Y2 receptor subtype. J. Biol. Chem. 1995, 270, 26758–26761. [Google Scholar] [CrossRef]
- Gehlert, D. R.; Beavers, L. S.; Johnson, D.; Gackenheimer, S. L.; Schober, D. A.; Gadski, R. A. Expression cloning of a human brain neuropeptide Y Y2 receptor. Mol. Pharmacol. 1996, 49, 224–228. [Google Scholar]
- Lundell, I.; Blomqvist, A. G.; Berglund, M. M.; Schober, D. A.; Johnson, D.; Statnick, M. A.; Gadski, R. A.; Gehlert, D. R.; Larhammar, D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J. Biol. Chem. 1995, 270, 29123–29128. [Google Scholar] [PubMed]
- Bard, J. A.; Walker, M. W.; Branchek, T. A.; Weinshank, R. L. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J. Biol. Chem. 1995, 270, 26762–26765. [Google Scholar] [CrossRef] [PubMed]
- Gerald, C.; Walker, M. W.; Criscione, L.; Gustafson, E. L.; Batzl-Hartmann, C.; Smith, K. E.; Vaysse, P.; Durkin, M. M.; Laz, T. M.; Linemeyer, D. L.; Schaffhauser, A. O.; Whitebread, S.; Hofbauer, K. G.; Taber, R. I.; Branchek, T. A.; Weinshank, R. L. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature 1996, 382, 168–171. [Google Scholar]
- Weinberg, D. H.; Sirinathsinghji, D. J.; Tan, C. P.; Shiao, L. L.; Morin, N.; Rigby, M. R.; Heavens, R. H.; Rapoport, D. R.; Bayne, M. L.; Cascieri, M. A.; Strader, C. D.; Linemeyer, D. L.; MacNeil, D. J. Cloning and expression of a novel neuropeptide Y receptor. J. Biol. Chem. 1996, 271, 16435–16438. [Google Scholar]
- Beck-Sickinger, A. G. Structural characterization and binding sites of G-protein coupled receptors. Drug Discov. Today 1996, 1, 502–513. [Google Scholar] [CrossRef]
- Ingenhoven, N.; Beck-Sickinger, A. G. Fluorescent labelled analogues of neuropeptide Y for the characterization of cells expressing NPY receptor subtypes. J. Rec. & Signal Transd. Res. 1997, 17, 407–418. [Google Scholar]
- Beck-Sickinger, A. G.; Jung, G. Structure-activity relationships of neuropeptide Y analogues with respect to Y1 and Y2 receptors. Biopolymers 1995, 37, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Rist, B.; Wieland, H. A.; Willim, K. D.; Beck-Sickinger, A. G. A rational approach for the development of reduced-size analogues of neuropeptide Y with high affinity to the Y1 receptor. J. Pept. Sci. 1995, 1, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Jahns, R.; Siegmund, C.; Jahns, V.; Reilander, H.; Maidhof, A.; Muller-Esterl, W.; Lohse, M. J.; Boege, F. Probing human beta 1- and beta 2-adrenoceptors with domain-specific fusion protein antibodies. Eur. J. Pharm. 1996, 316, 111–121. [Google Scholar] [CrossRef]
- Wall, S. J.; Yasuda, R. P.; Hory, F.; Flagg, S.; Martin, B. M.; Ginns, E. I.; Wolfe, B. B. Production of antisera selective for m1 muscarinic receptors using fusion proteins: distribution of m1 receptors in rat brain. Mol. Pharmacol. 1991, 39, 643–649. [Google Scholar]
- Muller-Newen, G.; Kohne, C.; Keul, R.; Hemmann, U.; Muller-Esterl, W.; Wijdenes, J.; Brakenhoff, J. P.; Hart, M. H.; Heinrich, P. C. Purification and characterization of the soluble interleukin-6 receptor from human plasma and identification of an isoform generated through alternative splicing. Eur. J. Biochem. 1996, 236, 837–842. [Google Scholar] [CrossRef]
- Abd Alla, S.; Godovac-Zimmermann, J.; Braun, A.; Roscher, A. A.; Muller-Esterl, W.; Quitterer, U. Structure of the bradykinin B2 receptors' amino terminus. Biochemistry 1996, 35, 7514–7519. [Google Scholar] [CrossRef] [PubMed]
- Van den Pol, A. N.; Romano, C.; Ghosh, P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J. Comp. Neurol. 1995, 362, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Westphal, R. S.; Backstrom, J. R.; Sanders-Bush, E. Increased basal phosphorylation of the constitutively active serotonin 2C receptor accompanies agonist-mediated desensitization. Mol. Pharmacol. 1995, 48, 200–205. [Google Scholar]
- Palmer, T. M.; Gettys, T. W.; Jacobson, K. A.; Stiles, G. L. Desensitization of the canine A2a adenosine receptor: delineation of multiple processes. Mol. Pharmacol. 1994, 45, 1082–1094. [Google Scholar]
- Ingenhoven, N.; Eckard, C. P.; Gehlert, D. R.; Beck-Sickinger, A. G. Molecular characterization of the human neuropeptide Y Y2-receptor. Biochemistry 1999, 38, 6897–6902. [Google Scholar] [CrossRef] [PubMed]
- Rist, B.; Ingenhoven, N.; Scapozza, L.; Schnorrenberg, G.; Gaida, W.; Wieland, H. A.; Beck- Sickinger, A. G. The bioactive conformation of neuropeptide Y analogues at the human Y2- receptor. Eur. J. Biochem. 1997, 247, 1019–1028. [Google Scholar] [CrossRef]
- Beck-Sickinger, A. G.; Wieland, H. A.; Wittneben, H.; Willim, K. D.; Rudolf, K.; Jung, G. Complete L-alanine scan of neuropeptide Y reveals ligands binding to Y1 and Y2 receptors with distinguished conformations. Eur. J. Biochem. 1994, 225, 947–958. [Google Scholar] [CrossRef]
- Allen, J.; Novotny, J.; Martin, J.; Heinrich, G. Molecular structure of mammalian neuropeptide Y: analysis by molecular cloning and computer-aided comparison with crystal structure of avian homologue. Proc. Nat. Acad. Sci. U.S. A. 1987, 84, 2532–2536. [Google Scholar] [CrossRef]
- Beck, A. G.; Jung, G.; Gaida, W.; Köppen, H.; Lang, R.; Schnorrenberg, G. Highly potent and small neuropeptide Y agonist obtained by linking NPY 1-4 via spacer to a-helical NPY 25-36. FEBS Lett. 1989, 244, 119–122. [Google Scholar] [CrossRef]
- Rist, B.; Entzeroth, M.; Beck-Sickinger, A. G. From micromolar to nanomolar affinity: a systematic approach to identify the binding site of CGRP at the human calcitonin gene-related peptide 1 receptor. J. Med. Chem. 1998, 41, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wieland, H. A.; Eckard, C. P.; Doods, H. N.; Beck-Sickinger, A. G. Probing of the neuropeptide Y- Y-1-receptors interaction with anti-receptor antibodies. Eur. J. Biochem. 1998, 255, 595–603. [Google Scholar] [CrossRef]
- Eckard, C. P.; Beck-Sickinger, A. G. Anti-receptor antibodies to characterize G-protein coupled receptors, Curr. Med. Chem. 2000, 7, 897–910. [Google Scholar]
- Jacques, D.; Tong, Y.; Dumont, Y.; Shen, S. H.; Quirion, R. Expression of the neuropeptide Y Y1 receptor mRNA in the human brain: an in situ hybridization study. Neuroreport 1996, 7, 1053–1056. [Google Scholar] [CrossRef]
- Sample Availability: Contact authors
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Eckard, C.P.; Cabrele, C.; Wieland, H.A.; Beck-Sickinger, A.G. Characterisation of Neuropeptide Y Receptor Subtypes by Synthetic NPY Analogues and by Anti-receptor Antibodies. Molecules 2001, 6, 448-467. https://doi.org/10.3390/60500448
Eckard CP, Cabrele C, Wieland HA, Beck-Sickinger AG. Characterisation of Neuropeptide Y Receptor Subtypes by Synthetic NPY Analogues and by Anti-receptor Antibodies. Molecules. 2001; 6(5):448-467. https://doi.org/10.3390/60500448
Chicago/Turabian StyleEckard, Christophe P., Chiara Cabrele, Heike A. Wieland, and Annette G. Beck-Sickinger. 2001. "Characterisation of Neuropeptide Y Receptor Subtypes by Synthetic NPY Analogues and by Anti-receptor Antibodies" Molecules 6, no. 5: 448-467. https://doi.org/10.3390/60500448
APA StyleEckard, C. P., Cabrele, C., Wieland, H. A., & Beck-Sickinger, A. G. (2001). Characterisation of Neuropeptide Y Receptor Subtypes by Synthetic NPY Analogues and by Anti-receptor Antibodies. Molecules, 6(5), 448-467. https://doi.org/10.3390/60500448




