Introduction
Three compounds with antimicrobial activity have been isolated from the egg masses of the Australian muricid
Dicathais orbita [
2]. These were identified as tyrindoleninone (
3) and tyriverdin (
4), precursors of the ancient dye Tyrian Purple (6,6’-dibromoindigotin,
5), as well as the oxidative artifact, 6-bromoisatin (
6;
Scheme 1). The production of Tyrian Purple from precursors in the hypobranchial gland of the adult mollusc has been demonstrated from a number of other species of muricids [
3,
4,
5]. Consequently, the same compounds could provide antimicrobial protection for the egg masses of other species of Muricidae. Antimicrobial activity has been reported in the egg masses of 34 species of marine molluscs, including 6 species of Muricidae [
1].
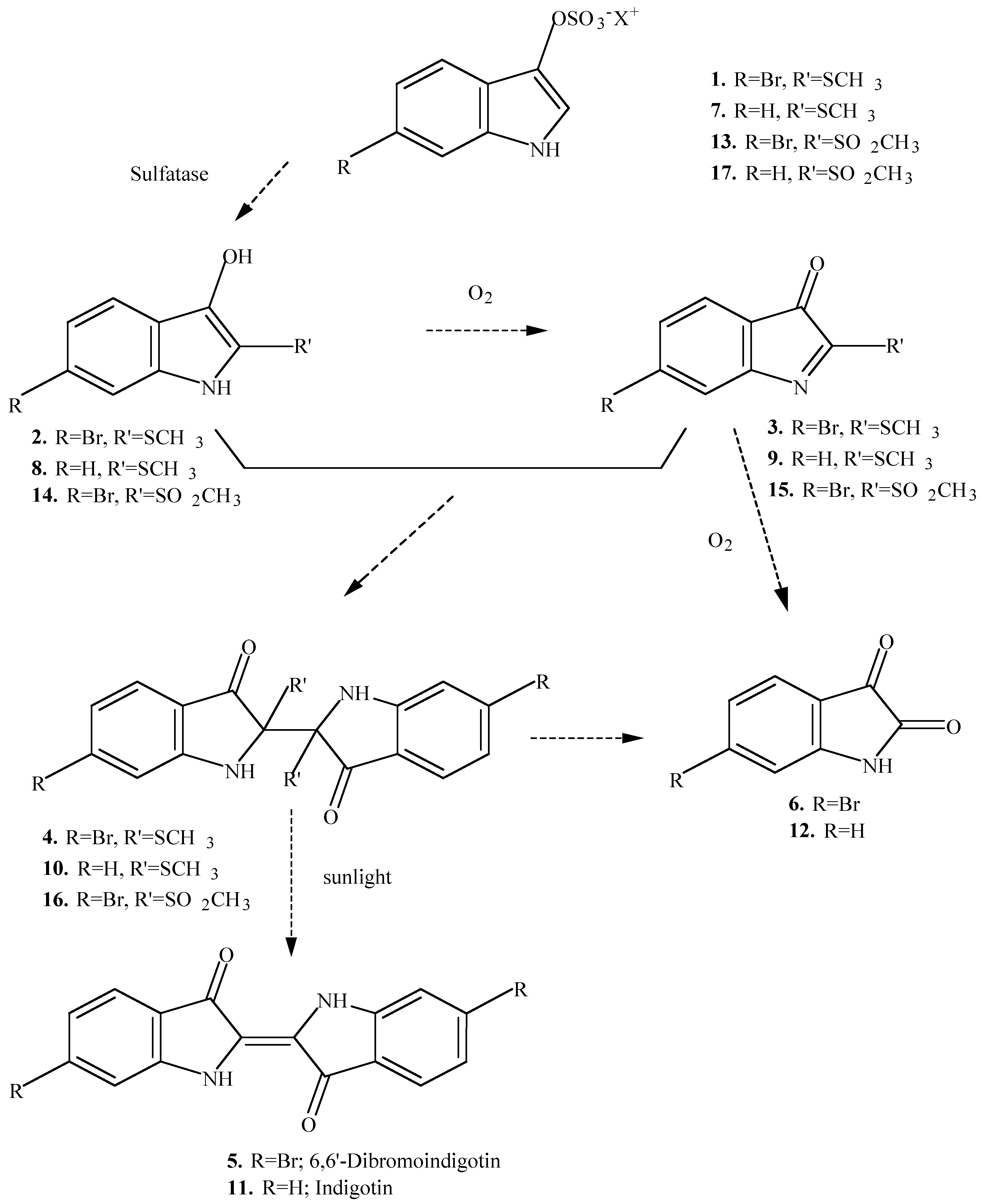
Investigations on the hypobranchial glands of the muricidae have revealed that the number and nature of precursors involved in the production of Tyrian Purple differs between species [
3,
4,
6,
7]. Only one ultimate precursor, tyrindoxyl sulfate (6-bromo-2-methylmercaptoindoxyl-3-sulfate,
1;
Scheme 1), has been isolated from the hypobranchial gland of
Dicathais orbita [
8]. However, Fouquet and Bielig [
5] have reported four chromogens from the glands of the Mediterranean muricid
Trunculariopsis (Murex) trunculus (
7,
13 Scheme 1;
18,
20 Scheme 2). Chromogen
13 was thought to be the sole precursor to Tyrian Purple in the glands of
Ceratostoma (Murex) erinaceum and two other Mediterranean muricids [
4]. However, Baker [
3] has suggested that these species contain two purple precursors, including the additional chromogen
17 in
Purpura haemastoma.
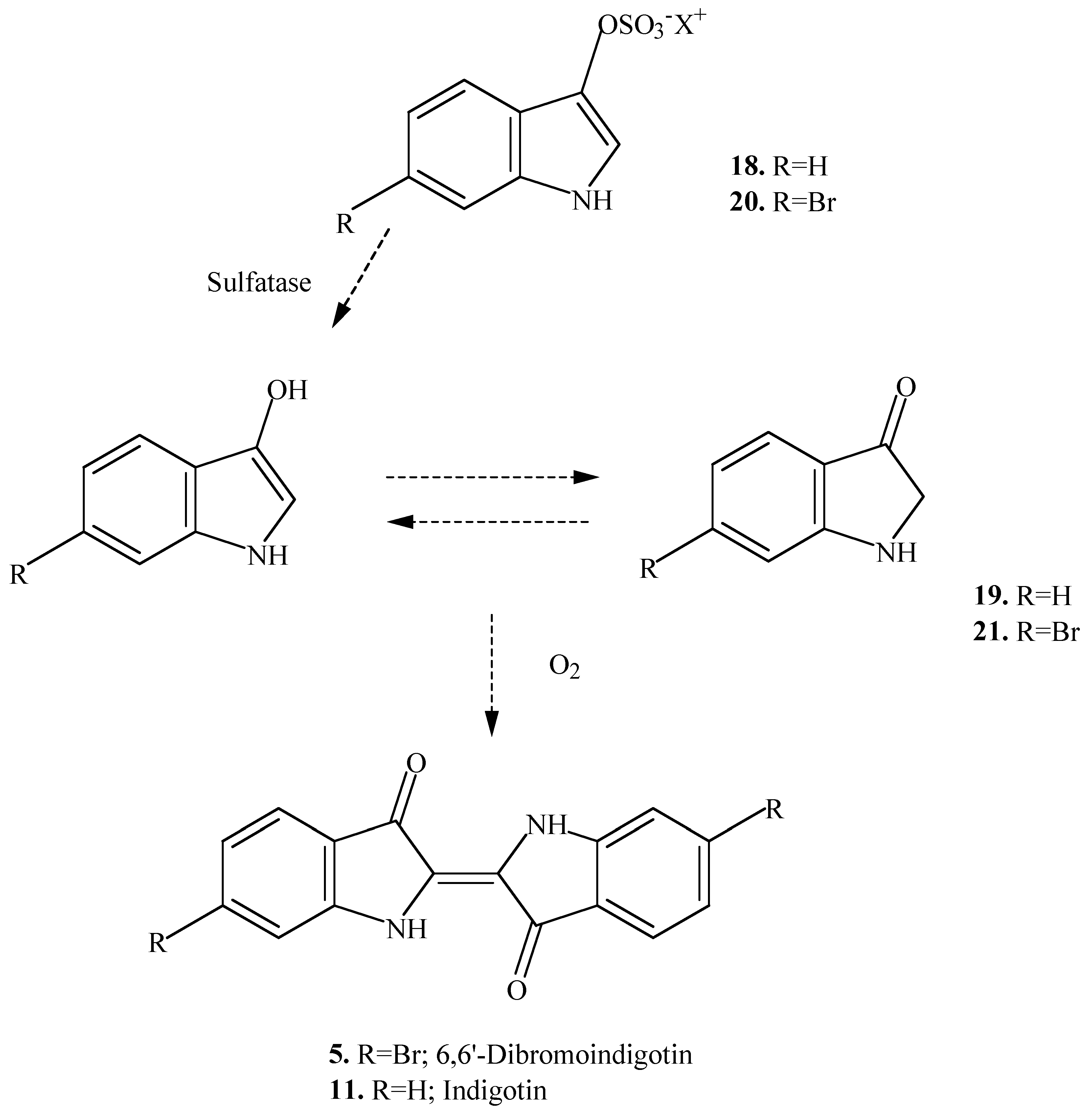
The different chromogens found in the hypobranchial glands of muricid molluscs react by two different pathways to produce a suite of intermediate precursors to the final indigoid dyes [
4]. Tyrindoxyl sulfate
1 and the chromogens
7 and
13 react aerobically with sulfatase in the dark to give the corresponding indoxyls (
2,
8 and
14), and their oxidation products (
3,
9 and
15), and then tyriverdin (
4) or tyriverdin type products
10 and
16 (
Scheme 1). These undergo photolytic cleavage in sunlight to give the corresponding indigoid dyes
5 and
11. The chromogens
18 and
20 were found to react anaerobically in the dark with a sulfatase enzyme to yield indoxyl (
19) and 6-bromoindoxyl (
21) respectively (
Scheme 2). These then react aerobically in the dark to afford the same dyes (
5 and
11). Consequently, it is possible that some of these other indole intermediates could be found in the egg masses of different species of muricids. The production of indigotin (
11) in addition to Tyrian Purple (6,6’-dibromoindigotin;
5) has been reported from the hypobranchial glands of only one muricid,
Trunculariopsis (Murex) trunculus [
9,
10]. In addition to these two dyes,
T. trunculus was found to produce a suite of other purple products [
3], including 6-bromoindigotin, a cross reaction product of the indoxyls and 6-bromoindoxyls [
10].
The objective of this study was to determine if the same types of compounds were likely to be responsible for antimicrobial activity in the egg masses of different species of marine mollusc. Secondary metabolites are typically characterized by their heterogeneity and restricted distribution, occurring in only some groups or species [
11]. GC/MS was used to screen egg masses of 23 molluscs for the presence of volatile indole derivatives.
Results and Discussion
Brominated metabolites were present as minor components in the organic extracts from the egg masses of six species of Muricidae, including the biologically active precursors of Tyrian Purple (
3 and
4) in all or some of the species (
Table 1). However, GC/MS analyses provided no evidence for the presence of brominated compounds in extracts from the egg masses of the remaining 17 species of marine molluscs. These include molluscs from 12 different families, two of which are in the same infraorder as the Muricidae (
Mitra carbonaria and
Conus paperliferus; Neogastropoda). Some previous reports have suggested that molluscs in the genus
Mitra produce Tyrian Purple [
11,
12], although this has not been supported by any experimental data. The egg mass of the Australian species
M. carbonaria was not found to contain any precursors to Tyrian Purple during this study. It appears likely that the precursors of Tyrian Purple are unique to the Muricidae and may even be a characteristic feature of their egg masses.
The egg capsules of all muricids were found to contain tyrindoleninone (
3;
Table 1), which is the major antimicrobial metabolite isolated from the fresh egg mass of
Dicathais orbita [
2]. The highly bacteriostatic compound tyriverdin (
4), and the mildly antimicrobial oxidation product 6-bromoisatin (
6), were also isolated from the egg masses of several muricids (
Table 1). Consequently, it is likely that these three compounds are responsible for much of the antimicrobial activity that was observed in the egg masses of these muricids [
1]. The compounds responsible for the observed activity in the egg masses of molluscs from other families are not presently known.
A number of additional brominated indole derivatives were observed in the muricid egg masses (
Table 1). These include 6-bromoindoxyl (
21; retention time 37.5 min, M
+∙ m/z 211, 213
79Br,
81Br and major fragments at
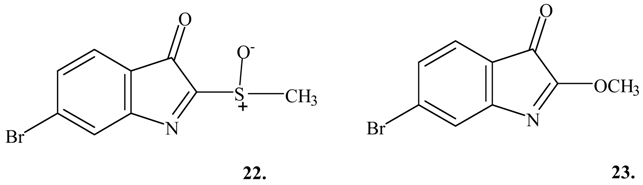
m/z 102, 104 and 132), 6-bromo-2-methylsulfinyl-3
H-indol-3-one (
22; retention time 41.6 min, M
+∙ m/z 271, 273
79Br,
81Br and major fragments at
m/z 224, 226 and
m/z 168, 170) and 6-bromo-2-methoxy-3
H-indol-3-one (
23; retention time 32.2 min, M
+∙ m/z 239, 241
79Br,
81Br and major fragments at
m/z 224, 226 and
m/z 168, 170). There was insufficient data to assign structures to a further five brominated compounds (
Table 1), although these were identified as brominated indoles based on similarities in their mass spectral fragmentation patterns.
The presence of 6-bromoindoxyl (
21) was unexpected in the egg mass of
D. orbita. This compound is an intermediate precursor of Tyrian Purple and is formed in turn from the precursor
20 (
Scheme 2), which has only been found in the hypobranchial glands of
Trunculariopsis trunculus [
3,
6,
8]. The 6-bromoindoxyl (
21) could be a decomposition product from tyrindoxyl (
2), while
22 and
23 are most likely to be oxidation and decomposition products from tyrindoleninone (
3). Freeze-drying of the egg mass appears to increase the concentration of 6-bromo-2-methoxy-3
H-indol-3-one (
23) after chloroform/methanol extraction, relative to tyrindoleninone (
3,
Table 1), suggesting that this compound could also be an artifact in the freeze dried egg extract from
Ceratostoma erinaceum.
Table 1.
Volatile indole derivatives found in the egg masses of six muricid molluscs using gas chromatography/ mass spectrometry. R.T. refers to the retention time in the gas chromatograph (GC). The percent abundance of each compound is calculated from the total relative intensities of all volatile compounds found in each extract (presented as a mean with standard error where duplicate samples were examined). The extracts from four Australian species, Agnewia tritoniformis (A.t.), Dicathais orbita (D.o.), Lepsiella reticularis (L.r.), Morula marginalba (M.m.), as well as two Mediterranean muricids Ceratostoma erinaceum (C.e.) and Trunculariopsis trunculus were examined. The eggs were either extracted fresh (F) or after freeze drying (FD).
Table 1.
Volatile indole derivatives found in the egg masses of six muricid molluscs using gas chromatography/ mass spectrometry. R.T. refers to the retention time in the gas chromatograph (GC). The percent abundance of each compound is calculated from the total relative intensities of all volatile compounds found in each extract (presented as a mean with standard error where duplicate samples were examined). The extracts from four Australian species, Agnewia tritoniformis (A.t.), Dicathais orbita (D.o.), Lepsiella reticularis (L.r.), Morula marginalba (M.m.), as well as two Mediterranean muricids Ceratostoma erinaceum (C.e.) and Trunculariopsis trunculus were examined. The eggs were either extracted fresh (F) or after freeze drying (FD).
| R.T. | Identity | Compound Number | Percent abundance (standard error) |
|---|
| A.t. F | D.o. F | D.o. FD | L.r. F | M.m. F | C.e. FD | T.t. FD |
|---|
| 13.1 | Methyl methanethiosulfonate | 24 | - | - | 3.6 (0.1) | - | - | 0.2 | 0.2 |
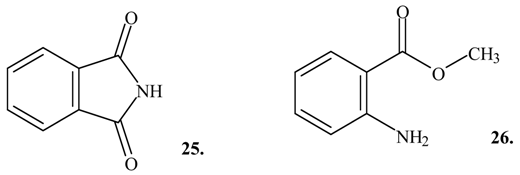
| 23.4 | 2-Aminobenzoic acid methyl ester | 26 | - | - | - | - | - | - | 0.1 |
| 27.7 | 1H-Isoindole-1,3(2H)-dione | 25 | - | - | - | - | - | 0.1 | - |
| 30.2 | Unidentified brominated indole | | - | - | - | - | - | 0.3 | 0.2 |
| 30.3 | 2-Methylthio-3H-indol-3-one | 9 | - | - | - | - | - | 0.6 | 0.6 |
| 32.4 | 6-Bromo-2-methoxy-3H- indol-3- one | 23 | - | 1.5 (1.5) | 13.5 (2.8) | - | - | 3.5 | 0.3 |
| 32.6 | Unidentified brominated indole | | - | - | - | - | - | 0.4 | 0.2 |
| 34.5 | Unidentified brominated indole | | - | - | 1.4 (1.4) | - | - | 0.2 | - |
| 34.7 | 1H-Indole-2,3-dione | 12 | - | - | - | - | - | - | 0.1 |
| 36.1 | Unidentified brominated indole | | - | 0.4 (0.4) | 0.2 (0.2) | - | - | - | - |
| 37.5 | 6-Bromoindoxyl | 21 | - | 0.9 (0.9) | 1.3 (1.3) | - | - | - | - |
| 38.0 | Tyrindoleninone | 3 | 5.1 | 24.1(10.5) | 11.5 (1.2) | 23.8 | 4.6 | 1.1 | 0.2 |
| 39.1 | Unidentified brominated indole | | - | 0.4 (0.4) | 0.2 (0.2) | - | 1.2 | - | - |
| 41.6 | 6-Bromo-2-methylsulfinyl-3H- indol-3-one | 22 | - | 1.2 (0.1) | - | - | - | 1.3 | - |
| 42.5 | 6-Bromoisatin | 6 | - | 3.5 (2.8) | 4.5 (2.8) | 0.7 | 3.9 | 2.5 | 0.1 |
| 45.0 | Tyriverdin | 4 | - | 0.2 (0.2) | 0.4 (0.4) | - | - | 1.1 | 0.1 |
![Molecules 06 00070 i001]()
The presence of 6-bromo-2-methylsulfonyl-3
H-indol-3-one (
15) was expected but not found in the egg masses of the two Mediterranean muricids (
Ceratostoma erinaceum and
Trunculariopsis trunculus). This compound, which arises from the chromogen
13, is intermediate in the formation of Tyrian purple (
Scheme 1); the indoxyl sulfate (
13) is known to occur in these two muricids [
3,
4,
6]. While 6-bromo-2-methylsulfonyl-3
H-indol-3-one (
15) was not positively identified in the egg masses it remains possible that it was present but failed to give a clear molecular ion (refer to
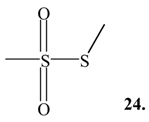
Table 1). It is also interesting to note that a significant quantity of methyl methanethiosulfonate (
24; retention time 13.1 min, M
+∙ m/z 126, major fragment ions
m/z 81, 63, 58 and 47) was detected in the eggs of
Trunculariopsis trunculus.
![Molecules 06 00070 i002]()
Several non-brominated indole derivatives were found as minor components in the extracts from the egg masses of the two Mediterranean muricids (
Table 1). Four of these compounds gave positive matches in the mass spectrum library to 2-methylthio-3
H-indol-3-one (
9; retention time 30.3 min, M
+∙m/z 177, major fragment ions at
m/z 162, 149, 143 and 104), 1
H-indole-2,3-dione (isatin,
12; retention time 34.7 min, M
+∙ m/z 147, major fragment ions at
m/z 119, 92, and 64), 1
H-isoindole-1,3 (2
H)-dione (phthalimide,
25; retention time 27.7 min, M
+∙ m/z 147, major fragment ions at
m/z 104, 76 and 50) and 2-aminobenzoic acid methyl ester (
26; retention time 23.4 min, M
+∙ m/z 151, major fragment ions at
m/z 119, 92 and 65). None of these compounds were observed in the solvent or environmental controls, although artifacts can not be completely excluded at this stage.
![Molecules 06 00070 i003]()
Indigotin (
11) has only previously been reported from
Trunculariopsis (Murex) trunculus [
9,
10] and therefore the precursor 2-methylthio-3
H-indol-3-one (
9,
Scheme 1) was only expected to occur in the egg mass of this species. However, this compound was also found in the egg mass of
Ceratostoma (Murex) erineceum (
Table 1), which is thought to contain only the brominated chromogen (
21,
Scheme 1) [
4] or possibly a second brominated chromogen (
17, Scheme 1) [
3]. Isatin (
12) was also found in the egg mass of
C. erinaceum. This compound could be expected to occur as an oxidation product from the precursors
9 and
10 (
Scheme 1). Consequently, it seems likely that
C. erinaceum may also contain the chromogen
7 (
Scheme 1), which has been reported from
T. trunculus [
6].
Experimental
The egg masses from 20 species of molluscs were collected along the Illawarra Coast and the egg masses from a further three species were collected from the Mediterranean Sea, Spain (
Table 2). Samples of these egg masses are represented in a photographic reference collection maintained by the Department of Biological Sciences at the University of Wollongong, NSW, Australia. The amount collected and relative abundance of each species are provided by Benkendorff [
1]. Most of the egg material was extracted immediately after collection. However, the Mediterranean specimens were freeze-dried prior to shipping into Australia. Consequently, some samples of Dicathais orbita egg masses were also freeze-dried for comparative purposes.
The egg capsules were macerated in a Waring blender and extracted in chloroform/methanol (1:1, v/v) by soaking for several hours, followed by decanting and replacing the solvent. This was repeated twice with the final soak being overnight. The extracts were then combined and evaporated to dryness in a rotary evaporator with water pump vacuum. A small volume of distilled water was added to the chloroform/methanol extracts to facilitate a chloroform-water/methanol separation before the solvent was evaporated. Solvent controls were prepared by evaporating down a mixture of chloroform/methanol (1:1, v/v; 200ml). Environmental controls included chloroform/methanol extracts of seawater and intertidal pebbles.
The volatile organic components in the egg masses were examined using a GC-17A (Shimadzu) gas chromatograph coupled to a QP-5000 (Shimadzu) mass spectrometer. The samples were run in splitless mode. In general, 50 μl of sample dissolved in dichloromethane (DCM) was injected, at an estimated concentration of 10 mg/ml. The injector temperature was set at 260°C. The oven temperature was held at 40°C for 2 min then ramped to 290°C at a rate of 4°C per minute. The final oven temperature was then held at 290°C for 10 min. The carrier gas was helium and the flow rate was 1.4 ml/min. The electron beam energy in the mass spectrometer was 70eV and the source temperature was 200°C. Blank runs were performed sporadically to check for contaminants on the column or in the injection loop, by injecting 50 μl of DCM.
Volatile compounds in the egg extracts were identified by their characteristic mass spectral fragmentation patterns. The fragmentation patterns were compared to known compounds contained in the mass spectrum library. Tyrindoleninone (
3), tyriverdin (
4) and 6-bromoisatin (
6) were identified according to their known retention times and fragmentation patterns, as determined under identical conditions in the GC/MS [
1,
2]. For tyrindoleninone the retention time was 38.0 min, M
+∙ m/z 255, 257
79Br,
81Br and with major fragments at
m/z 240, 242 and 133. For tyriverdin the retention time was 45- 46 min (ions at
m/z 418, 420, 422, as well as
m/z 257, 259 and
m/z 240, 242). 6-Bromoisatin has a retention time of 42.5 min with
m/z M
+∙ 225, 227 (
79Br,
81Br) and major fragment ions at
m/z 197 and 170.
Table 2.
Mollusc species selected for GC/MS examination of volatile organic chemicals in the egg mass. The reference numbers refer to samples lodged in the Department of Biological Sciences at the University of Wollongong, NSW, Australia.
Table 2.
Mollusc species selected for GC/MS examination of volatile organic chemicals in the egg mass. The reference numbers refer to samples lodged in the Department of Biological Sciences at the University of Wollongong, NSW, Australia.
| Order/ infraorder | Family | Species | Reference Number |
|---|
| NEOGASTROPODA | Muricidae | Dicathais orbita | M-em-001 |
| Agnewia tritoniformis | M-em-002 |
| Morula marginalba | M-em-003 |
| Lepsiella reticularis | M-em-007 |
| Trunculariopsis trunculus* | M-em-008 |
| Ceratostoma erinaceum* | M-em-009 |
| Mitridae | Mitra carbonaria | M-em-015 |
| Conidae | Conus paperliferus | M-em-017 |
| LITTORINIMORPHA | Littorinidae | Bembicium nanum | M-em-020 |
| Naticidae | Conuber c.f. sordidus | M-em-021 |
| Ranellidae | Cabestana spengleri | M-em-022 |
| BASOMMATOPHORA | Amphibolidae | Salinator fragilis | M-em-031 |
| Salinator solida | M-em-032 |
| Siphonariidae | Siphonaria denticulata | M-em-033 |
| Siphonaria zelandica | M-em-034 |
| Planorbidae | Isidorella hainesi | M-em-035 |
| ANASPIDAE | Aplysiidae | Aplysia juliana | M-em-038 |
| Aplysia sydneyensis | M-em-039 |
| Aplysia parvula | M-em-040 |
| Stylocheilus longicauda | M-em-042 |
| CEPHALASPIDA | Philinidae | Philine angasi | M-em-047 |
| Glaucidae | Spurilla neopolitana* | M-em-064 |
| TEUTHOIDAE | Loliginidae | Sepioteuthis australis | M-em-072 |