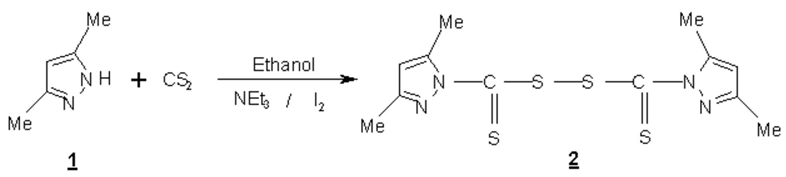
Bis[(3,5-dimethyl pyrazol)-1-yl Thiocarbonyl)] Disulfide
References
- Jones, R.G.; Hanret, M.J.; Lauglin, K.M. J. Org. Chem. 1954, 19, 1428.
- Haque, S.A.; Clouet, G. Makromol. Chem. Phys. 1994, 195, 315–327.
- Reiser, A. Photoreactive Polymer. The Science and Technology of Resist; Wiley: New York, 1986; p. 26. [Google Scholar]
- El Idrissi, A.; Tebbji, K.; Radi, S. Molecules. 2001, 6, M232. [Google Scholar]
Sample Availability: Available from the authors and from MDPI. |
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/.
Share and Cite
El Idrissi, A.; Tebbji, K.; Radi, S. Bis[(3,5-dimethyl pyrazol)-1-yl Thiocarbonyl)] Disulfide. Molecules 2001, 6, M233. https://doi.org/10.3390/M233
El Idrissi A, Tebbji K, Radi S. Bis[(3,5-dimethyl pyrazol)-1-yl Thiocarbonyl)] Disulfide. Molecules. 2001; 6(12):M233. https://doi.org/10.3390/M233
Chicago/Turabian StyleEl Idrissi, Abderrahman, Karim Tebbji, and Smaail Radi. 2001. "Bis[(3,5-dimethyl pyrazol)-1-yl Thiocarbonyl)] Disulfide" Molecules 6, no. 12: M233. https://doi.org/10.3390/M233



