Abstract
Deprotection of various phenols from their respective methoxymethyl ethers using an heteropolyacid catalyst was studied. The catalyst was the Wells-Dawson heteropolyacid, used both in bulk or supported on silica. Yields were high to quantitative after less than one hour reaction time and the catalyst was easily recoverable and reusable.
Introduction
Usually, the removal of methoxymethylene protective groups is carried out in liquid phase using mainly sulphuric or hydrochloric acids as catalysts [1,2,3]. However, the problems associated with the handling and disposal of the inorganic acids, and the environmental and potential hazards of the same have raised interest in the development of an alternative process using solid acid catalysts. The use of heteropolyoxoanions (HPAs) as catalysts is an interesting area that has widely been studied recently. The HPAs are composed of a close-packed framework of metal-oxygen octahedra, MOx (M=Mo6+, W6+) surrounding a central atom, X (Si4+, P5+). In particular, the Wells-Dawson acid structure (H6P2W18O62.aq.) consists of two identical “half units” (P2W9) linked through the oxygen atoms.
In this work, we present a deprotection study carried out using such a heteropolyacid (HPA) with Wells-Dawson structure in organic solvents. The Wells-Dawson acid (WD) was tested using both bulk solid samples and solid-supported ones using on large pore silica as the support.
Results and Discussion
The deprotection reaction was studied using compounds 1-9 as the substrates. Different reaction conditions were checked, e.g. temperature, time, reaction solvent, concentration of the solution and aggregation state of the catalyst. Concentration of the catalyst on the support and the molar ratio of WD to substrate were also varied.
Reactions using bulk catalyst
When 1,2-dichloroethane was the solvent, the bulk catalyst did not dissolve, even at 80°C. The reaction is slow under these conditions and a maximum yield of 80% is reached in 5 hours. Secondary products begin to appear at longer reaction times. On the other hand, the use of methanol as the solvent allows the catalyst to dissolve, the reaction is fast and the yields are excellent, e.g. 98% within 45 min or 100% after 1 hr. reaction at 65°C (Table 1).
Methanol is therefore a good solvent for the reaction, but it has the disadvantage that it dissolves the WD off the silica when the supported catalyst was used. Consequently, we examined the use of mixtures of solvents in which the catalyst is insoluble and small amounts (1 – 5%) of methanol. Yields are very good using a 1,2-dichloroethane-methanol mixture. Among other potential solvents, THF was also studied. Yields are low and reactions are slow when pure THF is used; however, addition of 1% methanol rises the yield, being quantitative in 40 min. In all the experiments 1% catalyst (moles/ mole of substrate) was used.
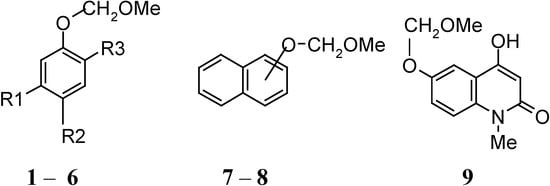
Scheme 1.
Phenol methoxymethyl ether substrates.

Table 1.
Deprotection of 1 using 1% of bulk Well-Dawson catalyst
| Run | Solvent | T (°C) | t (min) | % yield |
|---|---|---|---|---|
| 1 | Cl2(CH2)2 | 80 | 300 | 80 |
| 2 | MeOH | 65 | 45 | 98 |
| 3 | Cl2(CH2)2-MeOH 5% | 70 | 45 | 95 |
| 4 | Cl2(CH2)2-MeOH 1% | 70 | 45 | 96 |
| 5 | THF | 70 | 180 | 53 |
| 6 | THF-MeOH 1% | 65 | 40 | 98 |
Reactions using supported catalyst
In view of the results obtained when bulk catalyst and different solvents were employed (see Table 1), in all the experiments involving supported catalysts, solvents with 1% added methanol were used. Since the solvents checked showed no substantial differences, we did not study further the use of the chlorinated one. Formation of various by-products was observed when the reactions were carried out at a temperature of 75°C or higher. The use of WD(20)/SiO2 catalyst, or use of greater percentages of catalyst (in moles with respect to the substrate) affords similar yields to those obtained with the WD(60)/SiO2 catalyst [Table 2, runs 11 and 13 vs. 3]. The yields obtained were similar, regardless of whether WD, WD(20)/SiO2 or WD(60)/SiO2 were used [Table 1, run 6, vs. Table 2, run 13]. However, use of the supported ones allows for the easy separation and recovery of the catalyst for its immediate reutilization, without any lowering of its catalytic activity. Dilution turns the reaction slower [Table 2, runs 6, 7, 8 vs. 3], and raising the quantity of catalyst used have not a substantial effect [Table 2, runs 9 and 10 vs. 3]. Deprotection of compounds 2-9 was performed using standard reaction conditions to give clean reactions, with yields higher than 90% in most of the examples. Optimised results are included in Table 2.

Table 2.
Deprotection using supported Well-Dawson catalysts
| Run | Substrate | Catalyst | Catalyst (mol %) | Solvent | T (°C) | t (min) | Dilution | % yield |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | b | 1 | c | 80 | 30 | --- | 70 |
| 2 | 1 | b | 1 | c | 70 | 45 | --- | 95 |
| 3 | 1 | b | 1 | d | 70 | 45 | --- | 100 |
| 4 | 1 | b | 1 | d | 50 | 105 | --- | 97 |
| 5 | 1 | b | 1 | d | 75 | 45 | --- | 70 |
| 6 | 1 | b | 1 | d | 65 | 90 | x 2 | 100 |
| 7 | 1 | b | 1 | d | 65 | 120 | x 4 | 95 |
| 8 | 1 | b | 1 | d | 65 | 45 | x 1/2 | 90 |
| 9 | 1 | b | 5 | d | 65 | 60 | --- | 99 |
| 10 | 1 | b | 10 | d | 65 | 45 | --- | 95 |
| 11 | 1 | a | 1 | d | 65 | 50 | --- | 100 |
| 12 | 1 | a | 1 | d | 70 | 40 | --- | 90 |
| 13 | 1 | a | 5 | d | 65 | 55 | --- | 95 |
| 14 | 2 | b | 1 | d | 65 | 60 | --- | 100 |
| 15 | 2 | a | 1 | d | 65 | 55 | --- | 100 |
| 16 | 2 | a | 1 | d | 65 | 45 | x 1/2 | 90 |
| 17 | 3 | b | 1 | d | 65 | 60 | --- | 100 |
| 18 | 3 | a | 1 | d | 65 | 60 | --- | 95 |
| 19 | 4 | b | 1 | d | 65 | 60 | --- | 85 |
| 20 | 4 | a | 1 | d | 65 | 60 | --- | 80 |
| 21 | 5 | b | 1 | d | 65 | 60 | --- | 95 |
| 22 | 6 | b | 1 | d | 65 | 60 | --- | 90 |
| 23 | 7 | b | 1 | d | 65 | 60 | --- | 92 |
| 24 | 8 | b | 1 | d | 65 | 60 | --- | 95 |
| 25 | 9 | b | 1 | d | 65 | 60 | --- | 72 |
a) WD(20)/SiO2; b) WD(60)/SiO2; c) Cl2(CH2)2-MeOH 1%; d) THF-MeOH 1%;
Conclusions
The MOM-ether deprotection reactions performed using Wells-Dawson catalysts were clean, their workup was very simple and the yields ranged were good to excellent. The use of these solid catalysts allows replacement of the usual soluble inorganic acids. It also contributes to waste reduction.
Acknowledgements
Financial support from CONICET (Argentina), and Universidad Nacional de La Plata is gratefully acknowledged.
Experimental
General
Methoxymethyl ethers 1, 2, 5-8 were obtained by refluxing the appropriate phenol with ClCH2OCH3, in the presence of anhydrous K2CO3 and acetone for 3-6 hours, depending on the starting phenol. Acylation of 1 gave 3 [4] and Baker-Venkataraman rearrangement of 3 gave 4 [4]. The 2-quinolone 9 was prepared as already described [5]. Purification of the MOM-ethers was accomplished by flash column chromatography on silica gel. Structure and substitution patterns of the starting MOM-compounds is shown in Scheme 1. Reaction products were characterised by GC-MS (their spectra were in agreement with the expected ones in all cases) and by comparison of their TLC with authentic samples.
The Wells-Dawson acid (H6P2W18O62.aq.) was prepared as described elsewhere [6] from an aqueous solution of α/β K6P2W18O62·10H2O salt, which was treated with ether and concentrated (37%) HCl solution.
Silica-supported Wells-Dawson acids (WD) were prepared by wet impregnation of Grace Davison silica (Grade 59, specific area= 250 m2/g) with an aqueous solution of the synthesised WD acid. Two catalysts containing 20 and 60 wt% of WD were prepared (WD(20)/SiO2 and WD(60)/SiO2, respectively). After impregnation, samples were dried at room temperature in a vacuum-dessicator for 8 h.
Typical procedure
In a deprotection experiment, 40 – 60 mg of substrate were dissolved in 1.0 mL of solvent (see Table 2) and the catalyst was added to the solution. The mixture was then stirred at the indicated temperature, and reactions were assumed complete when the TLC analysis did not show any starting material. The reaction mixture was filtered; the filtrate was concentrated and subjected to flash column chromatography on silica. In all the experiments, the desired products were obtained with high selectivity, and the starting material could be quantitatively recovered. The results are collected in the Tables.
References
- Yardley, J.P.; Fletcher, H., III. Synthesis 1976, 244.
- Wiesner, K. Pure Appl. Chem. 1979, 51, 689.
- Fieser, L.F.; Fieser, M. Reagents for Organic Synthesis; J. Wiley and Sons, Inc.: New York, NY, 1967; Volume 1, p. 132. [Google Scholar]
- Jios, J.L.; Autino, J.C.; Pomilio, A.B. An. Asoc. Quim. Argent. 1995, 83, 183.
- Corral, R.A.; Orazi, O.O.; Autino, J.C. Tetrahedron Lett. 1983, 2359.
- Baronetti, G.T.; Briand, L.; Sedran, U.; Thomas, H. Appl. Catal. A: General 1998, 172, 265. [CrossRef]
- Sample Availability: Samples of compounds 1 and 3 are available from MDPI.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.