A Simple Method for Synthesis of Active Esters of Isonicotinic and Picolinic Acids
Abstract
:Introduction
Results and Discussion
| R (yield): |  |  |  |
 | 1a (54%) | 1b (97%) | 1c (84%) |
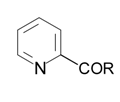 | 2a (39%) | 2b (92%) | 2c (67%) |
Conclusions
Experimental
General
Isonicotinoylchloride hydrochloride
Picolinoylchloride hydrochloride
General procedure for the preparation of active esters
Isonicotinic acid pentafluorophenyl ester
Isonicotinic acid 4-nitrophenyl ester
Isonicotinic acid N-hydroxysuccinimidyl ester
Picolinic pentafluorophenyl ester
Picolinic acid p-nitrophenyl ester
Picolinic acid N-hydroxysuccinimidyl ester
References
- Bodanzsky, M. Peptide Chemistry, 2nd ed.; Springer-Verlag: Berlin-Heidelberg-New York, 1993; p. 60. [Google Scholar]
- Folkers, K.; Ljungqvist, A. The Reaction of Pyridinecarboxylic Acids with Dicyclohexyl- carbodiimide and p-Nitrophenol. Acta Chem. Scand. 1988, B42, 408. [Google Scholar]
- Fife, T.H.; Przystas, T.J. Divalent Metal Ion Catalysis in the Hydrolysis of Esters of Picolinic Acid. Metal Ion Promoted Hydroxide Ion and Water Catalyzed Reactions. J. Am. Chem. Soc. 1985, 107, 1041. [Google Scholar] [CrossRef]
- Flouret, G.; Mahan, K.; Majewski, T. Decreased Histamine Release by Luteinizing Hormone- Releasing Hormone Antagonists Obtained upon Translocation of the Cationic Amino Acid from Position 8 to Position 7. J. Med. Chem. 1992, 35, 636. [Google Scholar] [CrossRef] [PubMed]
- Janecka, A.; Janecki, T.; Shan, S.; Bowers, C.; Folkers, K. Novel, Potent Luteinizing Hormone- Releasing Antagonistwith Improved Solubility in Water. J. Med. Chem. 1994, 37, 2238. [Google Scholar] [CrossRef] [PubMed]
- Solárzano, C.; Davis, M.A. Preparation of Arene Ruthenium(II) Complexes with Activated Ligands for Protein Labeling. Inorg. Chim. Acta 1985, 97, 135. [Google Scholar] [CrossRef]
- Bosshard, H.H.; Mory, R.; Schmid, M.; Zollinger, H. Eine Methode zur katalysierten Herstellung von Carbonsäure- und Sulfosäure-chloriden mit Thionylchlorid. Helv. Chim. Acta 1959, 42, 1653. [Google Scholar] [CrossRef]
- Sample Availability: Available from the author.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Christensen, J.B. A Simple Method for Synthesis of Active Esters of Isonicotinic and Picolinic Acids. Molecules 2001, 6, 47-51. https://doi.org/10.3390/60100047
Christensen JB. A Simple Method for Synthesis of Active Esters of Isonicotinic and Picolinic Acids. Molecules. 2001; 6(1):47-51. https://doi.org/10.3390/60100047
Chicago/Turabian StyleChristensen, Jørn B. 2001. "A Simple Method for Synthesis of Active Esters of Isonicotinic and Picolinic Acids" Molecules 6, no. 1: 47-51. https://doi.org/10.3390/60100047
APA StyleChristensen, J. B. (2001). A Simple Method for Synthesis of Active Esters of Isonicotinic and Picolinic Acids. Molecules, 6(1), 47-51. https://doi.org/10.3390/60100047




