Catalytic Hydrogenation Reaction of Naringin-Chalcone. Study of the Electrochemical Reaction
Abstract
:Introduction
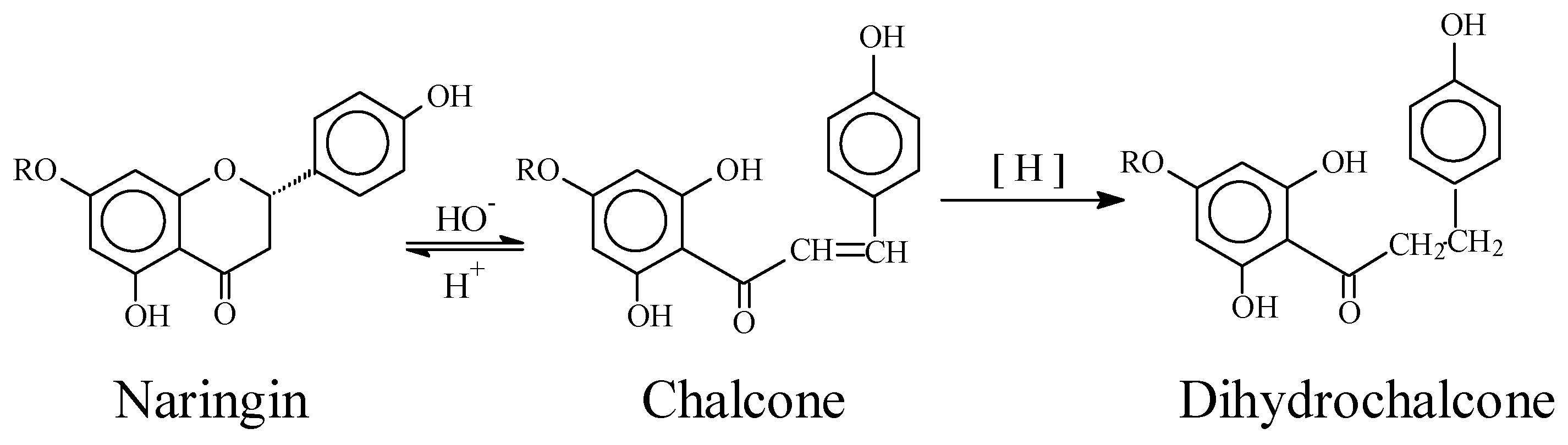
Experimental
Results and Discussion
Acknowledgements
References and Notes
- Mahdavi, B.; Marc Chapuzet, J. Lessard. J. Electrochim.Acta 1993, 38, 1377. [Google Scholar] [CrossRef]
- Pintauro, P.N.; Phan, H.; Baizer, M.M.; Nobe, K. AIChE Symposium Series 1987. n°254, 83, 34. Park, K.; Pintauro, P.N.; Baizer, M.M.; Nobe, K. J. Electrochem. Soc. 1985, 132, 1850. [PubMed]
- Yusem, G.; Pintauro, P. N.; Cheng, P. C.; An, W. J. J. Appl. Electrochem. 1996, 26, 1779.
- Horowitz, R. M.; Gentili, B. U. U. S. Patent.
Share and Cite
Nazareno, M.A.; Giannuzzo, A.N.; Mishima, H.T.; López de Mishima, B.A. Catalytic Hydrogenation Reaction of Naringin-Chalcone. Study of the Electrochemical Reaction. Molecules 2000, 5, 589-590. https://doi.org/10.3390/50300589
Nazareno MA, Giannuzzo AN, Mishima HT, López de Mishima BA. Catalytic Hydrogenation Reaction of Naringin-Chalcone. Study of the Electrochemical Reaction. Molecules. 2000; 5(3):589-590. https://doi.org/10.3390/50300589
Chicago/Turabian StyleNazareno, M. A., A. N. Giannuzzo, H. T. Mishima, and B. A. López de Mishima. 2000. "Catalytic Hydrogenation Reaction of Naringin-Chalcone. Study of the Electrochemical Reaction" Molecules 5, no. 3: 589-590. https://doi.org/10.3390/50300589
APA StyleNazareno, M. A., Giannuzzo, A. N., Mishima, H. T., & López de Mishima, B. A. (2000). Catalytic Hydrogenation Reaction of Naringin-Chalcone. Study of the Electrochemical Reaction. Molecules, 5(3), 589-590. https://doi.org/10.3390/50300589



