Abstract
A convenient procedure for the synthesis of 5-(4-acetamidophenyl)-10,15,20-tris(4-substituted phenyl) porphyrins from dipyrrolomethane is reported. meso-(4-Substituted phenyl) dipyrrolomethanes were obtained in yields of 72-84%. The amide porphyrins were isolated with appreciable yields of 15-17%.
Introduction
The design of new material systems involves the synthesis of asymmetric porphyrins. Thus, porphyrin covalentely linked to carotenoids has been used in the design of artificial photosynthetic membranes, which mimic the natural process of solar energy conversion [1]. Also, electroactive porphyrins have been employed in the design of molecular electronic systems [2]. In these cases, the synthesis of porphyrins substituted in meso-position by phenyl groups, where one differs of the other three (AB3-porphyrins), results particularly interesting [3].
Experimental
All the products were characterized by 1HNMR spectroscopy, MS spectroscopy, and elemental analysis of C, H and N. The reactions were performed according to the methodology described in ref. 3.
Results and Discussion
Synthesis of dipyrrolomethanes
The meso-substituted dipyrrolomethanes were synthesized by the condensation of the corresponding benzaldehydes and excess pyrrole. The reaction is catalyzed by acids. Under these conditions, pyrrole acts as reactant and solvent, causing direct formation of dipyrrolomethane. These compounds were isolated with high purity and used in the AB3-porphyrin synthesis.
Synthesis of asymmetric tetraphenylporphyrins
The tetraphenylporphyrins (AB3-porphyrins) were synthesized by the condensation of the appropriated benzaldehydes and dipyrrolomethanes (Scheme). The reaction requires catalytic amount of BF3·O(Et)2 in chloroform. This condensation produces porphyrin in its reduced form, therefore the reaction mixture was oxidized with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). This reaction yielded a mixture of three porphyrins, which were separated with high purity by flash chromatography (yields 15-17%).
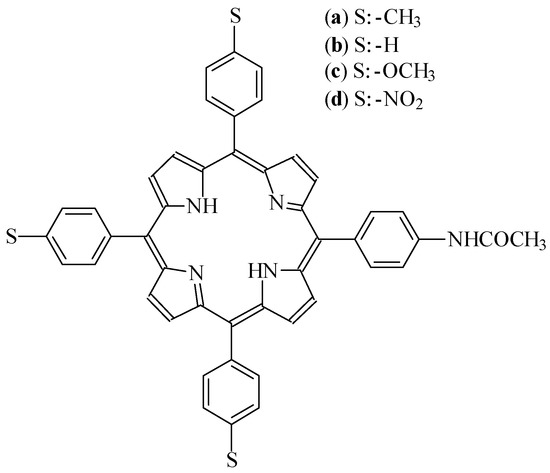
Scheme.
Acknowledgements
Authors are grateful to Fundación Antorchas and Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina, for financial support.
References and Notes
- Steinberg-Yfrach, G.; Rigaud, J.-L.; Durantini, E.N.; Moore, A.L.; Gust, D.; Moore, T.A. Nature (London) 1998, 392, 479.
- Han, W.; Durantini, E.N.; Moore, T.A.; Moore, A.L.; Gust, D.; Rez, P.; Leatherman, G.; Seely, G.; Tao, N.; Lindsay, S.M. J. Phys. Chem. B 1997, 101, 10719. [CrossRef]
- Durantini, E.N.; Silber, J.J. Synth. Commun. 1999, 29, 19.