N,N-Diethyl-1-Tosyl-3-Indoleglyoxylamide as a Dienophile in Diels-Alder Reactions. Hyperbaric vs. Thermal Conditions
Abstract
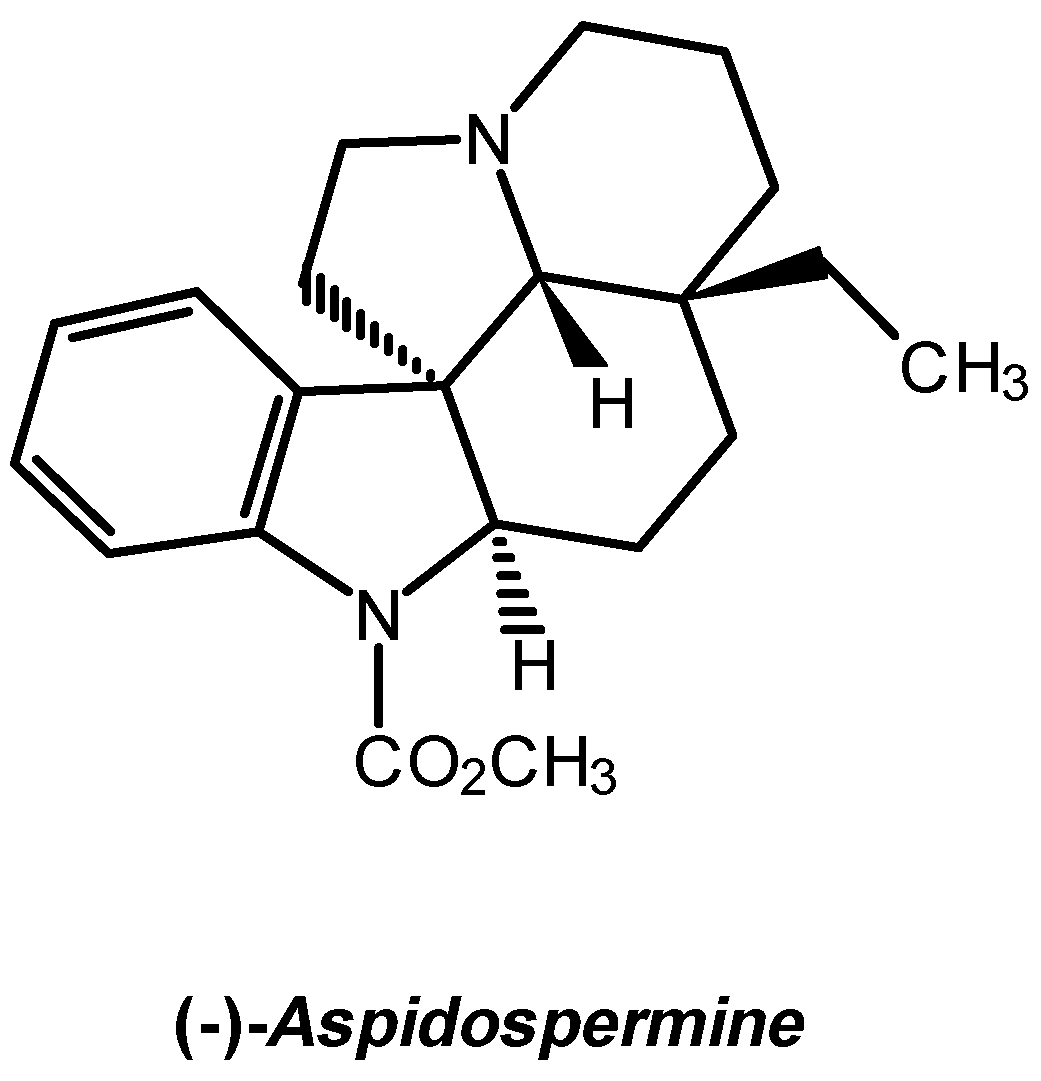
:Introduction
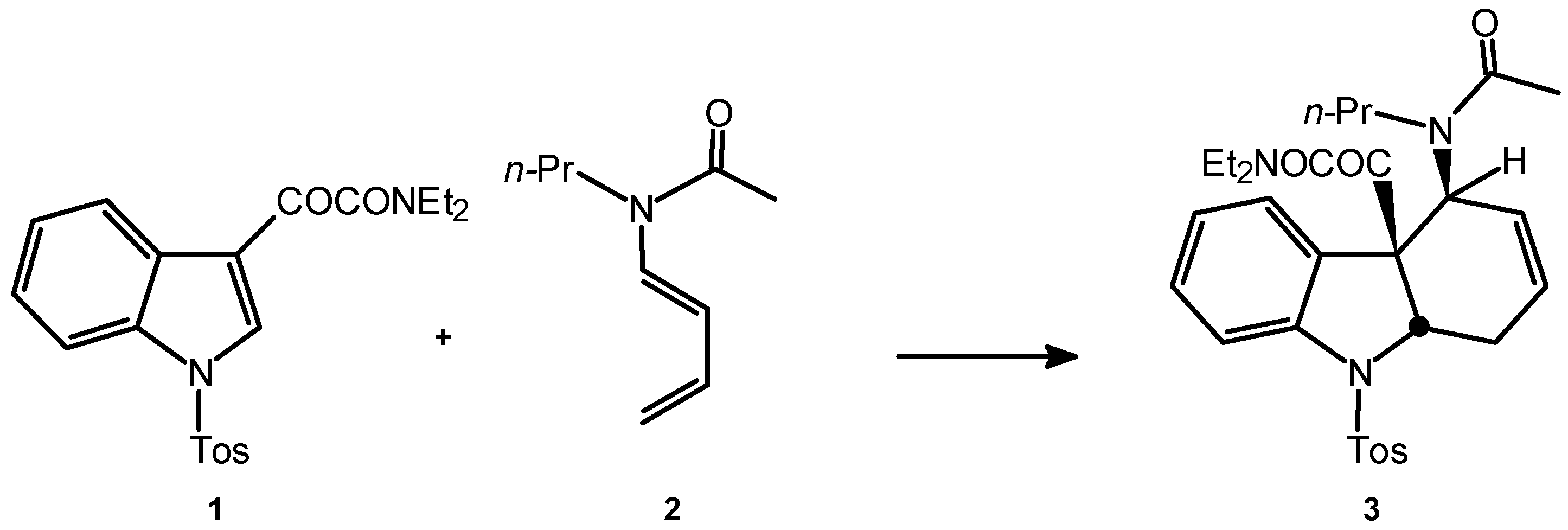
Experimental
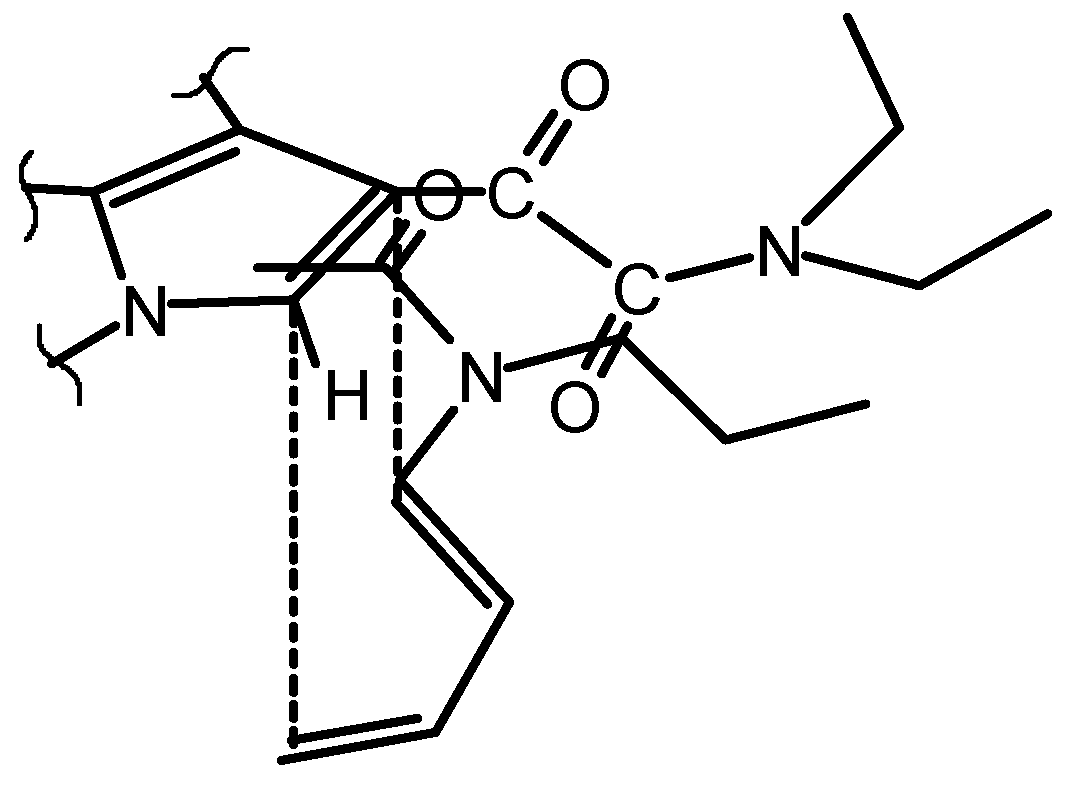
Results and Discussions
Acknowledgements
References and Notes
- Wenkert, E.; Moeller, P. D. R.; Piettre, S. J. Am. Chem. Soc. 1988, 110, 7188.
- Biolatto, B.; Kneeteman, M.; Mancini, P. Tetrahedron Lett. 1999, 40, 3343.
- Biolatto, B.; Kneeteman, M.; Gonzalez Sierra, M.; Mancini, P. M. Unpublished results.
Share and Cite
Biolatto, B.; Kneeteman, M.; Mancini, P.M. N,N-Diethyl-1-Tosyl-3-Indoleglyoxylamide as a Dienophile in Diels-Alder Reactions. Hyperbaric vs. Thermal Conditions. Molecules 2000, 5, 393-395. https://doi.org/10.3390/50300393
Biolatto B, Kneeteman M, Mancini PM. N,N-Diethyl-1-Tosyl-3-Indoleglyoxylamide as a Dienophile in Diels-Alder Reactions. Hyperbaric vs. Thermal Conditions. Molecules. 2000; 5(3):393-395. https://doi.org/10.3390/50300393
Chicago/Turabian StyleBiolatto, B., M. Kneeteman, and P. M. Mancini. 2000. "N,N-Diethyl-1-Tosyl-3-Indoleglyoxylamide as a Dienophile in Diels-Alder Reactions. Hyperbaric vs. Thermal Conditions" Molecules 5, no. 3: 393-395. https://doi.org/10.3390/50300393
APA StyleBiolatto, B., Kneeteman, M., & Mancini, P. M. (2000). N,N-Diethyl-1-Tosyl-3-Indoleglyoxylamide as a Dienophile in Diels-Alder Reactions. Hyperbaric vs. Thermal Conditions. Molecules, 5(3), 393-395. https://doi.org/10.3390/50300393



