Abstract
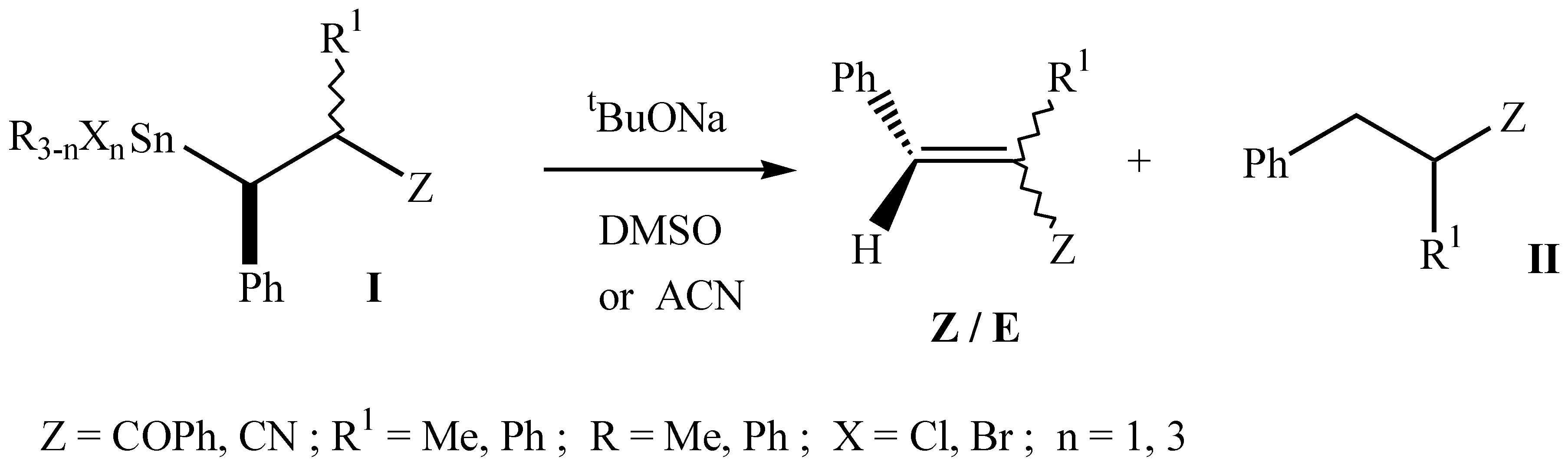
In the present work we report the results obtained in the reaction of β-stannylketones (I) with t-BuONa in dimethylsulfoxide (DMSO) and acetonitrile (ACN) as solvents. The reaction mechanisms probably involved are proposed.
Introduction
In the reaction of β-functionalized organotin compounds (I) with t-BuONa in t-BuOH there is a competition between an elimination reaction [(E1cB)R], leading to olefins with high diastereoselectivity, and a nucleofilic substitution reaction yielding the reduction product (II) [1]. Now we report on the reactions of β-stannylketones (I) with t-BuONa in DMSO and ACN in order to compare the reactivity in these solvents.
Experimental
Anhydrous solvents and sublimated t-BuONa were used. The β-stannylketones were synthesized in our laboratory [2,3]. The reaction mixtures were analysed by CGL. The reaction conditions are detailed in the Table.
Results and Discussion
The results summarised in the Table show that these reactions lead to higher yields in shorter reaction times, depending on the substrates. Thus, while β-stannylketones carrying electron-withdrawing groups attached to tin lead to the elimination product in high yield, β-trialkylstannylketones give mainly the reduction product.

Table.
Reactions of R3-nXnSn-CH(Ph)-CH(R1)-COPh with tBuONaa.
| N° | R1 | R3-nXnSn | DMSO | ACN |
| % Elim (Z/E) | % Elim (Z/E) | |||
| 1 | Me | Ph3Sn | 58 (10/90)b | 39 (1/99)c |
| 2 | Me | Ph3Sn | 86 (19/81)b | 85 (0/100)c |
| 3 | Me | Ph2BrSn | 84 (10/90) | 83 (0/100) |
| 4 | Me | Me3Sn | -d | 37 (30/70)d |
| 5 | Me | Cl3Sne | 92 (0/100) | 85 (0/100) |
| 6 | Me | Cl3Snf | 81 (100/0) | 80 (100/0) |
| 7 | Ph | Me3Sne | -d | 76 (16/84)c |
| 8 | Ph | Me3Snf | -d | 58 (17/83)c |
| 9 | Ph | Cl3Sng | 98 (89/11) | 69 (90/10) |
aSubstrate/base ratio 1/1.1 Similar results are obtained from both erythro and threo isomers; bStarting substrate is recovered like a diastereoisomeric mixture; cNo isomerization of the starting substrate was observed; dHigh yield of the reduction product; eErythro isomer; fThreo isomer; gErythro/threo 13/87.
The stereochemical results show that, in most cases, these reactions are stereoconvergent. The same ratio Z/E is obtained independently of the configuration of the starting substrate. (Table, entries 1-4,7 and 8). Taking into account the stereochemistry observed we are able to say that, probably, the elimination reaction goes through an (E1cB)R mechanism in DMSO and through an (E1cB)I in ACN.
On the other hand, β-trichlorostannylketones (Table, entries 5, 6 and 9) give high yields of olefins through a stereospecific elimination reaction. Two possible mechanisms could be proposed to explain these results: a concerted E2, or an (E1cB)I in which, because of electronic interactions between the trichlorostannyl group and the oxygen atom, the intermediate carbanions are not interconvertibles.
Acknowledgements
This work was partially supported by CIC, CONICET and UNS (Argentina). The authors thank Dr. M. González Sierra (IQUIOS, Argentina) for the NMR spectra.
References and Notes
- Murray, A.P. Thesis, 1999.
- Chopa, A.B.; Murray, A.P. Diastereoselective synthesis of β-trichlorostannyl and β-trimethylstannylketones. Main Group Metal Chem. 1998, 21, 347. [Google Scholar] [CrossRef]
- Chopa, A.B.; Murray, A.P.; Lockhart, M.T. Evidence of single electron transfer in the diastereoselective synthesis of β-stannylketones. J. Organomet. Chem. 1999, 585, 35. [Google Scholar] [CrossRef]