Abstract
An empiric rule derived from the analysis of the 13C NMR spectral data, allowed us to determine 5,6-epoxide stereochemistry on decalinic systems and a discussion of the scope and limitations of this rule and its extension to other carbon squeletons, is presented.
Introduction
13C NMR and 1H NMR techniques are the most convenient methods for the elucidations of the oxirane ring stereochemistry in condensed policyclic systems, and have been widely used in the research of epoxides from natural sources [1,2,3].
Very often, however, the methods used consider mainly the effects of the oxirane ring on the γ carbons [4,5,6].
Results and Discussion
From the analysis of the 13C NMR data of a set of synthetic epoxides angularly substituted and placed on the C5-C6 position of decalin systems of known relative configurations; we could stablish an empirical correlation between the chemical shift difference of the oxirane carbons and the relative configuration of the epoxide. Therefore, using these chemical shift differences it is possible to predict the α or β orientation of the epoxide, that is, trans or cis stereochemistry of the epoxide relative to the C10 substituent (R3).
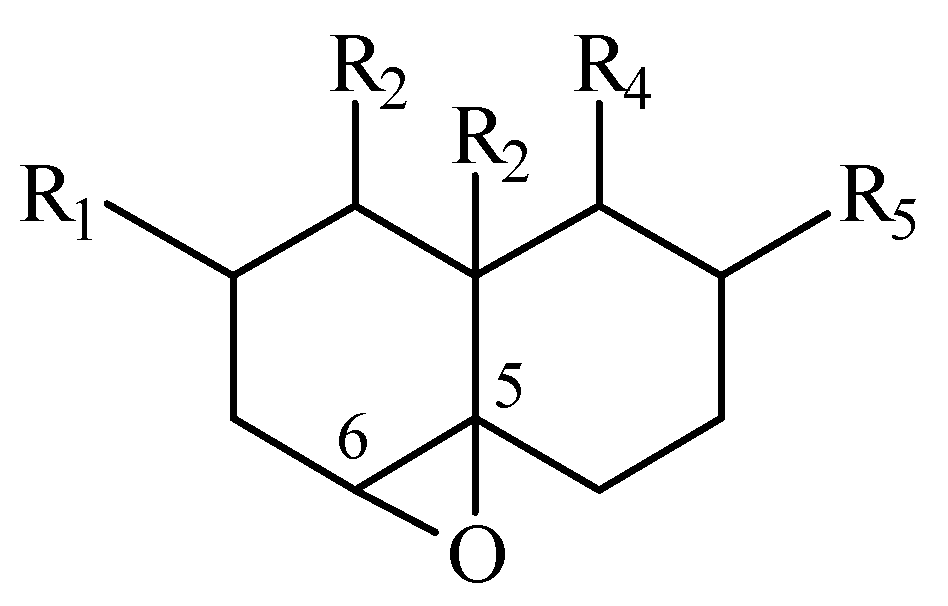
Computing for each epoxide: Δδ (epoxide)= δ C-5 - δ C-6 , the subtraction between the 13C NMR chemical shifts of both oxirane carbon signals, predicts:
Δδ α-epoxide > 5 ppm
Δδ β-epoxide < 3.8 ppm
Besides, we will present a discussion of the scope and limitations of this rule, its possible extension to other carbon skeletons and the comparative analysis with those results obtained from the existent semiempirical calculation systems [5,6].
Experimental
The epoxides were synthesized from substituted α- tetralones through a sequence involving a Birch reductive alkylation followed by the reduction and epoxidation with m-CPBA in heterogeneous phase.
The determinations of the 13C NMR spectra were realized in CDCl3 solutions using standard conditions.
Acknowledgements:
Financial supports to the authors from Universidad Nacional de Rosario, CONICET and AGENCIA are gratefully acknowledged.
References and Notes
- Cardá, M.; Sanz, J. F.; Marcos, J. A. J. Org. Chem. 1992, 57, 804–811.
- Tori, K.; Komeno, T.; Sangaré, M.; Septe, B.; Delpech, B.; Ahond, A.; Luckacs, G. Tetrahedron Lett. 1974, 14, 1157–1160.
- Sangaré, M.; Septe, B.; Berenger, G.; Luckacs, G. Tetrahedron Lett. 1977, 18, 699–702.
- Cross, A. D. J. Am. Chem. Soc. 1962, 84, 3206.
- (a) Beierbeck, H.; Saunders, J. K. Can. J. Chem. 1976, 54, 632–641. ; (b) Beierbeck, H.; Saunders, J. K. Can. J. Chem. 1976, 54, 2985–2995.
- Colombo, M.I.; Bustos, D.A.; Gonzalez Sierra, M.; Olivieri, A. C.; Ruveda, E. A. Can. J. Chem. 1986, 61, 552–555.