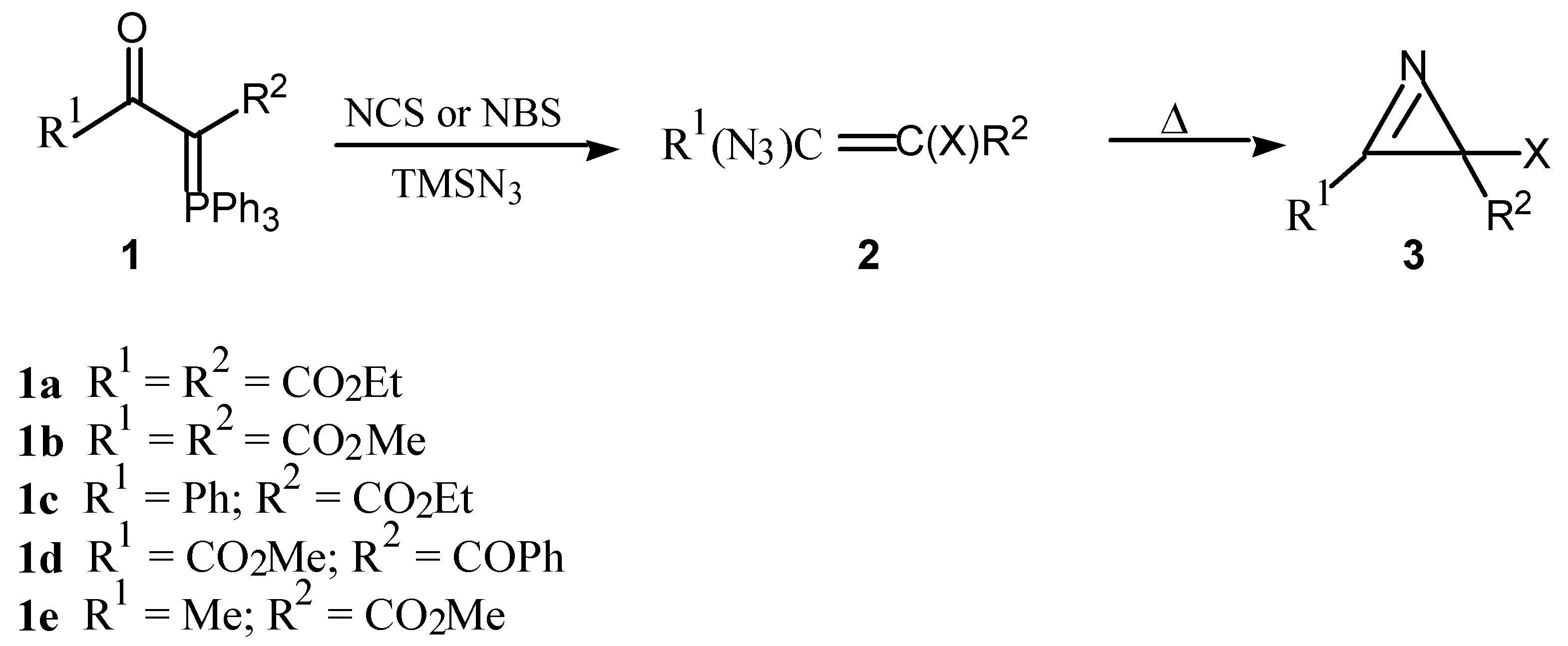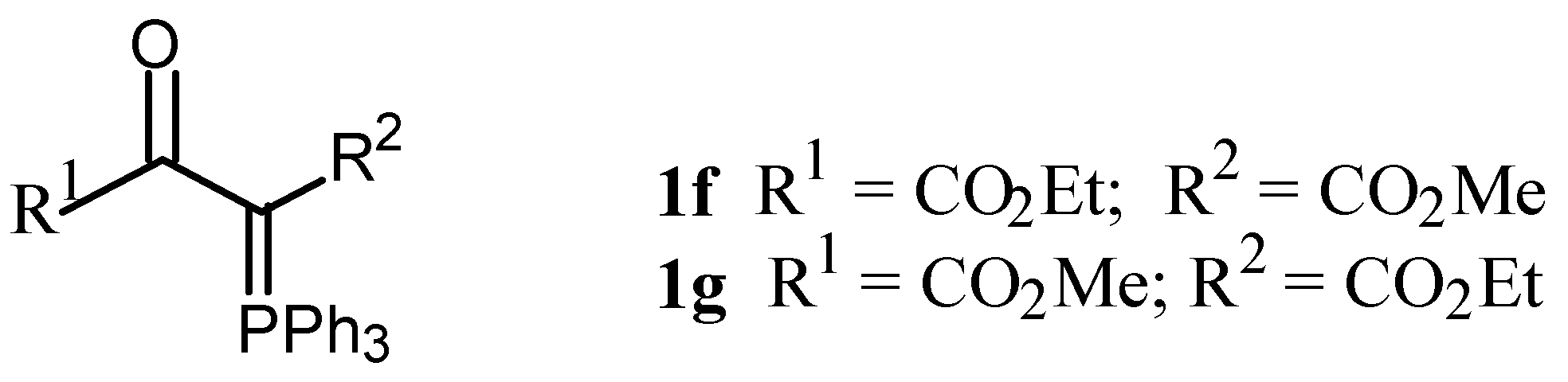Experimental
General
Unless otherwise indicated all common reagents and solvents were used as obtained from commercial suppliers without further purification. IR spectra were recorded on a Perkin Elmer 1720X FTIR spectrometer. 1H and 13C NMR spectra were recorded in deuteriochloroform on a Bruker AMX300 spectrometer. Mass spectra were recorded on a VG Micromass 7070E instrument by chemical ionisation (CI) with isobutane (except where indicated otherwise) or where indicated under electron impact. M.p.’s were recorded on a Leitz Wetzlar 799 hot stage and are uncorrected. Flash column chromatography was performed with Merck 9385 silica as the stationary phase.
1-Ethyl 4-methyl 2-oxo-3-triphenylphosphoranylidenebutanedioate (1f)
Methyl triphenylphosphoranylideneacetate [
6] (10.93 g, 32.7 mmol) was dissolved in toluene (80 mL) and the resulting solution was cooled at 5–10 °C. Ethyl oxalyl chloride (3.6 mL, 32.83 mmol) was added dropwise. The reaction mixture was stirred at 5–10 °C for 5 min then at room temperature for 30 min. Diethyl ether (80 mL) was added and an oil separated out. The solution was decanted from the oil and the solvent was removed under reduced pressure. The residue was triturated with ether to give a colourless solid which was isolated by filtration. Water was added to the oil separated by decantation and the resulting solution was extracted with chloroform. After evaporating the solvent and addition of ether, more solid was obtained, giving altogether 8.66 g (70%).
Mp 170 - 172 °C (lit. [
7], 173 - 174°C).
1H NMR δ: 1.37 (t, 3H, CH3CH2), 3.32 (s, 3H, CH3), 4.33 (q, 2H, CH3CH2), 7.27 - 7.73 (m, 15H, Ar-H).
13C NMR δ: 14.05, 50.13, 60.84, 122.95, 124.80, 128.52, 128.77, 132.41, 132.36, 133.39, 133.59, 154.39, 154.51, 167.22, 167.41, 184.59, 184.51.
IR (KBr) cm−1 2920, 1730, 1665.
4-Ethyl 1-methyl 2-oxo-3-triphenylphosphoranylidenebutanedioate (1g)
Ethyl triphenylphosphoranylideneacetate [
6] (10.39 g, 29.84 mmol) was dissolved in toluene (75 mL) and the resulting solution was cooled at 5–10 °C. Methyl oxalyl chloride (2.1 mL, 29.96 mmol) was added dropwise. The reaction mixture was stirred at 5–10 °C for 5 min then at room temperature for 30 min. Diethyl ether (70 mL) was added and an oil separated out. The solution was decanted from the oil and the solvent was removed under reduced pressure. The residue was triturated with ether to give a colourless solid which was isolated by filtration. Water was added to the oil separated by decantation and the resulting solution was extracted with chloroform. After evaporating the solvent and addition of ether, more solid was obtained, giving altogether 7.46 g (62%).
Mp 110 °C (lit. [
7], 115 - 118°C).
1H NMR δ: 0.76 (t, 3H, CH3CH2), 3.82 (q, 2H, CH3CH2), 3.85 (s, 3H, OCH3), 7.47 - 7.86 (m, 15H, Ar-H).
IR (KBr) cm−1 2980, 1724, 1674.
Ethyl 3-oxo-3-phenyl-2-triphenylphosphoranylidenepropanoate [5] (1c)
Ethyl triphenylphosphoranylideneacetate [
6] (5 g, 14.4 mmol) was dissolved in dry THF (50 mL) and NEt
3 was added (2 ml, 19.4 mmol). Benzoyl chloride (2 g, 16 mmol) was added dropwise. The reaction mixture was stirred under nitrogen at room temperature for 19 hours. The solution was filtered and the solid was washed with THF. The solvent was removed under reduced pressure and the residue was dissolved in chlroform. The organic phase was washed with water and dried over MgSO
4. The residue obtained upon removal of the solvent was purified by crystallization [ethyl acetate–hexane (2:1)] leading to
1c, as a white solid (4.5 g, 72%).
Mp 94.3 - 97 °C.
1H NMR δ: 0.58 (t, 3H, CH3CH2), 3.67 (q, 2H, CH3CH2), 7.30 - 7.36 (m, 3H, Ar-H), 7.43 - 7.56 (m, 9H, Ar-H), 7.67 - 7.70 (m, 2H, Ar-H),7.73 - 7.81 (m, 6H, Ar-H).
IR (KBr) cm−1 3051, 1671, 1530.
MS (EI) m/z (%) 452 (100), 423 (23), 379 (64), 347 (30), 77 (65).
Anal. Calc. for C29H25PO3: C, 76.96; H, 5.57. Found: C, 76.34; H, 5.46.
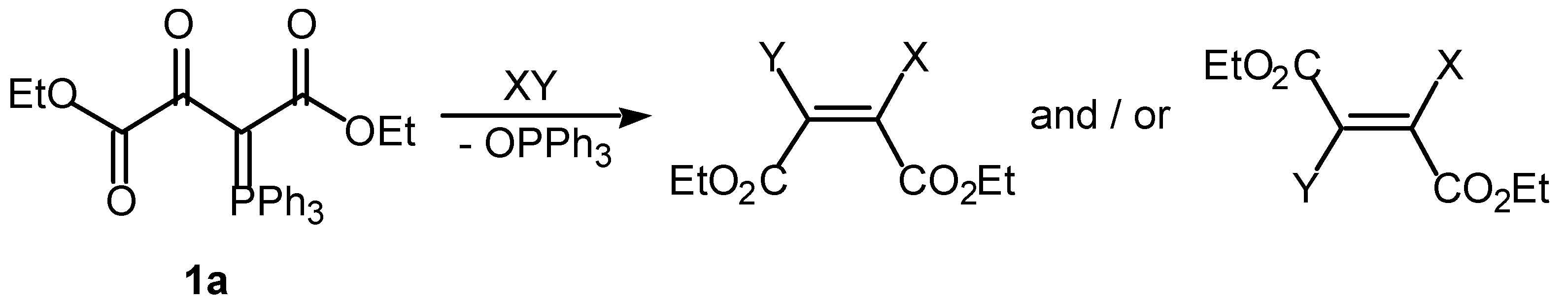
Ethyl methyl (Z)-2,3-dichlorobutenedioate (Z)-4
The ylide 1f (5.82 g, 13.35 mmol) was dissolved in chloroform (120 mL) and a solutions of chlorine (1 g, 14.1 mmol) in chloroform (120 mL) was added The mixture was stirred at room temperature for 5 min. The organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (2:1)] leading to the separation of triphenylphosphine oxide and isolation of the diester (Z)-4, an oil, that was purified by distillation at 73 °C/0.1.7 Torr (2.18 g, 72%).
1H NMR δ: 1.35 (t, 3H, CH3CH2), 3.88 (t, 3H, CH3) and 4.34 (q, 2H, CH3CH2)..
13C NMR δ: 13.81 (q), 53.45 (q), 63.25 (t), 130.06 (s), 130.81 (s), 161.12 (s),161.72 (s).
IR (film) cm−1 1745, 1600
MS (CI) m/z (%) 227 [M(35Cl) + H+] (17), 191 (25), 181 (55), 167 (12).
1-Ethyl 4-methyl (Z)-2-acetoxy-3-bromobutenedioate (Z)-5
The ylide 1f (3.87 g, 8.9 mmol) was dissolved in a mixture of acetic acid (35 mL) and chloroform (75 mL) and solutions of bromine (1.17 g, 7.3 mmol) and sodium bicarbonate (0.87 g, 10.3 mmol) in water (75 mL) were added. The reaction mixture was stirred at room temperature for 24 h and the organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (2:1)] leading to the separation of triphenylphosphine oxide and isolation of the diester (Z)-5, an oil, that was purified by distillation at 112.5 °C/0.1.7 Torr (2.04 g, 78%).
1H NMR δ: 1.32 (t, 3H, CH3CH2), 2.25 (s, 3H, CH3CO), 2.32 (s, 3H, CO2CH3), 4.29 (q, 2H, CH3CH2).
13C NMR δ: 13.71, 20.11, 53.51, 62.64, 115.12 (3-C), 141.25 (2-C), 159.07, 163.10, 165.17
IR (film) cm−1 2986, 1762, 1736
MS (CI) m/z (%) 295 [M(79Br) + H+] (82), 279 (12), 265 (22), 221 (29).
1-Ethyl 4-methyl (Z)- and (E)-2-acetoxy-3-chlorobutenedioate (Z)-6 / (E)-6
The ylide 1f (5.82 g, 13.35 mmol) was dissolved in a mixture of acetic acid (52.5 mL) and chloroform (110 mL) and a solution of chlorine (7.8 g, 10.95 mmol) and sodium bicarbonate (1.32 g, 15.45 mmol) in water (110 mL) was added. The reaction mixture was stirred at room temperature for 24 h and the organic phase was then washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (2:1)] leading to the separation of triphenylphosphine oxide and the isolation of a mixture of the alkenes (Z)-6 and (E)-6 (49:51) (1.76 g, 49%) as an oil
1H NMR (Z)-6 δ: 1.26 (t, 3H, CH3CH2), 2.24 (s, 3H, COCH3), 3.87 (s, 3H, CO2CH3), 4.27 (q, 2H, CH3CH2).
13C NMR (Z)-6 δ: 13.62, 19.94, 53.40, 62.52, 124.54 (3-C), 139.82 (2-C), 159.48, 161.41, 166.88
1H NMR (E)-6 δ: 1.34 (t, 3H, CH3CH2), 2.30 (s, 3H, COCH3), 3.89 (s, 3H, CO2CH3), 4.34 (q, 2H, CH3CH2).
13C NMR (E)-6 δ 13.75, 19.94, 53.40, 62.41, 122.28 (3-C), 141.32 (2-C), 159.94, 162.23, 167.72.
IR (film) cm−1 2995, 2980, 1785, 1740, 1640.
MS (CI) m/z (%) 251 [M(35Cl) + H+] (84), 221 (12), 177 (10).
4-Ethyl 1-methyl 2-chloro-3-methoxybutenedioate (E)-7
The ylide 1f (5.82 g, 13.35 mmol) was dissolved in chloroform (60 mL) and a solution of chlorine (2.55 g, 36 mmol) in methanol (45 mL) was added. The reaction was complete after 5 min at room temperature. The organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (2:1)] leading to the separation of triphenylphosphine oxide and the isolation of the enol ether (E)-7 (1.33 g, 44%) as an oil.
1H NMR δ: 1.39 (t, 3H, CH3CH2), 3.80 (s, 3H, CH3O), 3.89 (s, 3H, CO2CH3), 4.40 (q, 2H, CH3CH2).
13C NMR δ: 13.83, 52.80, 58.20, 62.74, 105.20 (2-C), 156.01 (3-C), 161.73, 163.22.
IR (film) cm−1 2955, 1742, 1612.
MS (CI) m/z (%) 223 [M(35Cl) + H+] (88), 177 (100), 163 (31), 149 (70).
4-Ethyl 1-methyl (Z)-2-acetoxy-3-bromobutenedioate (Z)-8 / (E)-8
The ylide 1c (3.87 g, 8.9 mmol) was dissolved in a mixture of acetic acid (35 mL) and chloroform (75 mL) and solutions of bromine (1.17 g, 7.3 mmol) and sodium bicarbonate (0.87 g, 10.3 mmol) in water (75 mL) were added. The reaction mixture was stirred at room temperature for 24 h and the organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (2:1)] leading to the separation of triphenylphosphine oxide and isolation of the mixture (Z)-8 / (E)-8 (78:22), an oil, that was purified by distillation at 112 °C/1.7 Torr (2.31 g, 88%).
1H NMR (Z)-8 δ: 1.36 (t, 3 H, CH3CH2), 2.31 (s, 3 H, CH3CO), 3.81 (s, 3 H, CO2CH3), 4.35 (q, 2 H, CH3CH2).
13C NMR (Z)-8 δ: 13.79, 20.17, 53.11, 63.04, 116.97 (3-C), 140.84 (2-C), 159.77, 162.59 and 167.68.
1H NMR (E)-8 δ: 1.34 (t, 3 H, CH3CH2), 2.23 (s, 3 H, CH3CO), 2.86 (s, 3 H, CO2CH3), 4.32 (q, 2 H, CH3CH2).
13C NMR (E)-8 δ:13.94, 20.06, 52.99, 62.95, 111.37 (3-C), 140.83 (2-C), 160.83, 161.79 and 167.33.
IR (film) cm−1 3000, 1782, 1738, 1638
MS (CI-CH4) m/z (%) 296 [M(81Br)] (100), 262 (8) and 252 (55).
Anal. Calc. for C9H11BrO6: C, 36.63; H, 3,76. Found: C, 36.69; H, 3.73.
4-Ethyl 1-methyl (Z)- and (E)-2-acetoxy-3-chlorobutenedioate (Z)-9 / (E)-9
The ylide 1g (5.82 g, 13.35 mmol) was dissolved in a mixture of acetic acid (52.5 mL) and chloroform (110 mL) and a solution of chlorine (7.8 g, 10.95 mmol) and sodium bicarbonate (1.32 g, 15.45 mmol) in water (110 mL) was added. The reaction mixture was stirred at room temperature for 24 h and the organic phase was then washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (2:1)] leading to the separation of triphenylphosphine oxide and the isolation of a mixture of the alkenes (Z)-9 and (E)-9 (1.67 g, 50%) as an oil, that was purified by distillation at 133 °C/1.7 Torr.
1H NMR (Z)-9 δ: 1.24 (t, 3H, CH3CH2), 2.26 (s, 3H, COCH3), 3.74 (s, 3H, CO2CH3), 4.13 (q, 2H, CH3CH2).
13C NMR (Z)-9 δ: 13.92, 20.08, 53.08, 62.96, 125.23 (3-C), 139.82 (2-C), 160.24, 161.05, 167.41.
1H NMR (E)-9 δ: 1.31 (t, 3H, CH3CH2), 2.20 (s, 3H, COCH3), 3.77 (s, 3H, CO2CH3), 4.34 (q, 2H, CH3CH2).
13C NMR (E)-9 δ:14.00, 20.08, 53.02, 62.81, 123.28 (3-C), 140.71 (2-C), 160.63, 161.79 and 167.04.
IR (film) cm−1 2988, 1741, 1636.
MS (EI) m/z (%) 250 [M(35Cl)]+ (53), 218 (8) and 156 (26).
4-Ethyl 1-methyl 3-chloro-2-methoxybutenedioate (E)-10
The ylide 1g (3.84 g, 8.8 mmol) was dissolved in chloroform (40 mL) and a solution of N-chlorosuccinimide (1.2 g, 8.8 mmol) in methanol (64 mL) was added. The reaction was complete after 5 min at room temperature. The organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (2:1)] leading to the separation of triphenylphosphine oxide and the isolation of the enol ether (E)-10 (1.33 g, 44%) as an oil, that was purified by distillation at 119 °C/1.7 Torr.
1H NMR δ: 1.33 (t, 3H, CH3CH2), 3.87 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 4.24 (q, 2H, CH3CH2).
13C NMR δ: 13.68, 52.83, 58.01, 61.81, 105.54 (3-C), 155.30 (2-C), 161.98, 162.34.
IR (film) cm−1 2957, 1744, 1613.
MS (EI) m/z (%) 222 [M(35Cl)]+ (30), 207 (10), 163 (28) and 59 (100).
Ethyl (Z)-2,3-dichloro-3-phenylpropenoate (12)
The ylide 1c (4.0 g, 8.82 mmol) was dissolved in chloroform (82 mL) and a solutions of chlorine (3.3 g, 47 mmol) in chloroform (80 mL) was added The mixture was stirred at room temperature for 1 h. The organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (1:1)] leading to the separation of triphenylphosphine oxide and isolation of 12, an oil, that was purified by distillation at 23 °C / 2.5 Torr (1.5 g, 69%).
1H NMR δ: 1.17 (t, 3H, CH3CH2), 4.31 (q, 2H, CH3CH2), 7.45 - 7.50 (m, 2H, Ar-H), 7.59 - 7.64 (m, 1H, Ar-H), 8.02 - 8.05 (m, 2H, Ar-H).
13C NMR δ: 13.49, 64.68, 82.01 (2-C), 128.74, 129.97, 130.85, 134.30, 163.90 (3-C), 183.09.
IR (film) cm−1 1765, 1689, 1250.
Ethyl (Z)-3-acetoxy-2-bromo-3-phenylpropenoate (13)
The ylide 1c (2.0 g, 4.45 mmol) was dissolved in a mixture of acetic acid (17.5 mL) and chloroform (37.5 mL) and solutions of bromine (0.23 ml, 4.45 mmol) and sodium bicarbonate (0.528 g, 6.59 mmol) in water (45.5 mL) were added. The reaction mixture was stirred at room temperature for 20 h and the organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (1:1)] leading to the separation of triphenylphosphine oxide and isolation of 13, an oil, that was purified by distillation at 82.5°C/1.5 Torr (0.84 g, 60%).
1H NMR δ: 1.14 (t, 3 H, CH3CH2), 2.25 (s, 3 H, AcO), 4.29 (q, 2 H, CH3CH2), 7.42 - 7.48 (m, 2 H, Ar-H), 7.57 - 7.62 (m, 1 H, Ar-H), 8.04 - 8.06 (m, 2 H, Ar-H).
13C NMR δ: 13.49, 20.79, 64.68, 105.87 (2-C), 128.21, 128.58, 130.07 (3-C), 130.59, 133.94, 155.42 and 182.95..
IR (film) cm−1 2985, 1728, 1707, 1694.
MS (CI-CH4) m/z (%) 313 [M(79Br) + H+] (28), 253 (42) and 233 (100).
Ethyl (E)-2-chloro-3-methoxy-3-phenylpropenoate (14)
The ylide 1c (0.5 g, 1.1 mmol) was dissolved in chloroform (5 mL) and a solution of N-chlorosuccinimide (0.15 g, 1.1 mmol) in methanol (8 mL) was added. The reaction was complete after 10 min at room temperature. The organic phase was washed with an aqueous solution of sodium bisulfite and dried over MgSO4. The residue obtained upon removal of the solvent was purified by column chromatography [ethyl acetate–hexane (3:1)] leading to the separation of triphenylphosphine oxide and the isolation of 14 (0.26 g, 98%) as an oil, that was purified by distillation at 91.7 °C/1.5 Torr.
1H NMR δ: 1.37 (t, 3H, CH3CH2), 3.51 (s, 3H, OMe), 4.39 (q, 2H, CH3CH2), 7.44 - 7.49 (m, 2H, Ar-H), 7.59 - 7.62 (m, 1H, Ar-H), 8.02 - 8.05 (m, 2H, Ar-H).
13C NMR δ: 13.48, 58.95, 64.61, 81.85 (2-C), 128.59, 129.99, 130.78, 134.16, 163.96 (3-C), 183.12.
IR (film) cm−1 2986, 1767, 1715, 1646.
MS (EI) m/z (%) 240 [M(35Cl)]+ (3), 195 (3), 153 (2), 77 (45).