4,5-Dimethoxy-2-nitrobenzhydrol
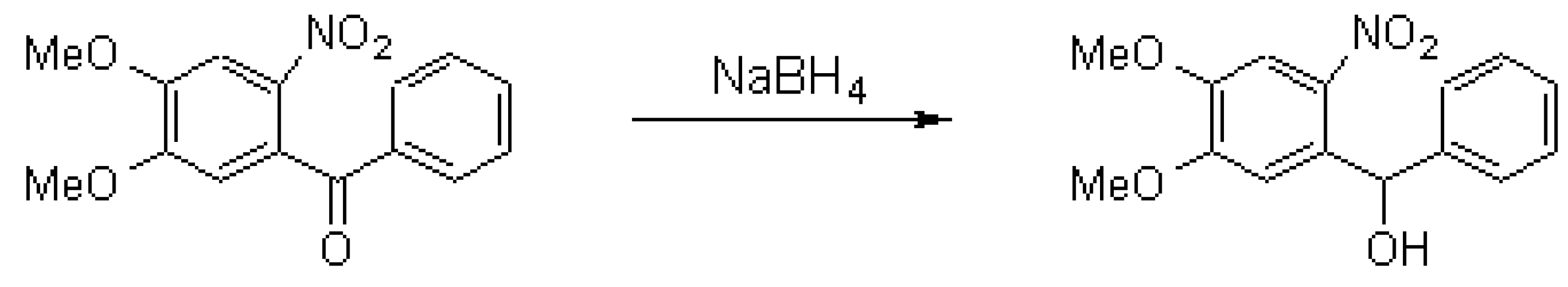
Supplementary materials
Supplementary File 1Supplementary File 2Reference
- Gutnov, A.V.; Butin, A.V.; Abaev, V.T.; Krapivin, G.D.; Zavodnik, V.E. Furyl(aryl)alkanes and Their Derivatives.19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring. Molecules 1999, 4, 204–218. [Google Scholar] [CrossRef]
- Sample availability: available from the authors and from MDPI.
© 1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/
Share and Cite
Smirnov, S.K.; Stroganova, T.A.; Butin, A.V. 4,5-Dimethoxy-2-nitrobenzhydrol. Molecules 1999, 4, M113. https://doi.org/10.3390/M113
Smirnov SK, Stroganova TA, Butin AV. 4,5-Dimethoxy-2-nitrobenzhydrol. Molecules. 1999; 4(10):M113. https://doi.org/10.3390/M113
Chicago/Turabian StyleSmirnov, Sergey K., Tat'yana A. Stroganova, and Alexander V. Butin. 1999. "4,5-Dimethoxy-2-nitrobenzhydrol" Molecules 4, no. 10: M113. https://doi.org/10.3390/M113
APA StyleSmirnov, S. K., Stroganova, T. A., & Butin, A. V. (1999). 4,5-Dimethoxy-2-nitrobenzhydrol. Molecules, 4(10), M113. https://doi.org/10.3390/M113



