In Vitro Anti-Hepatitis B Virus Activity of Hydroxytyrosol from Lindernia ruellioides
Abstract
1. Introduction
2. Results
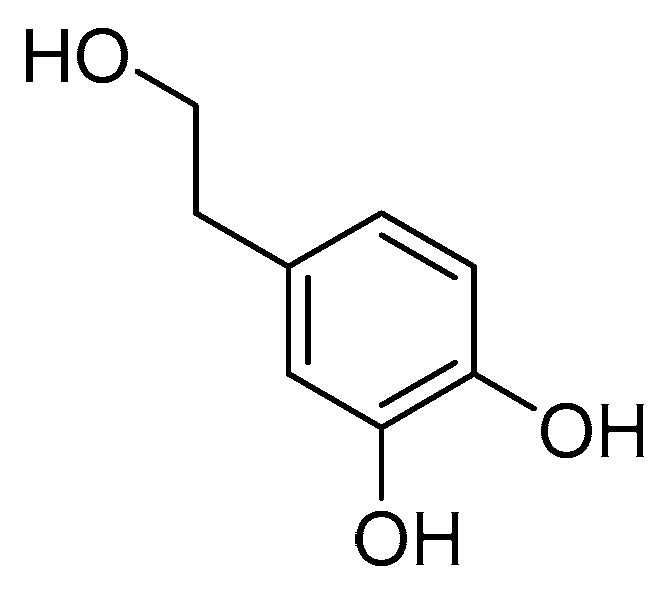
2.1. Structural Identification
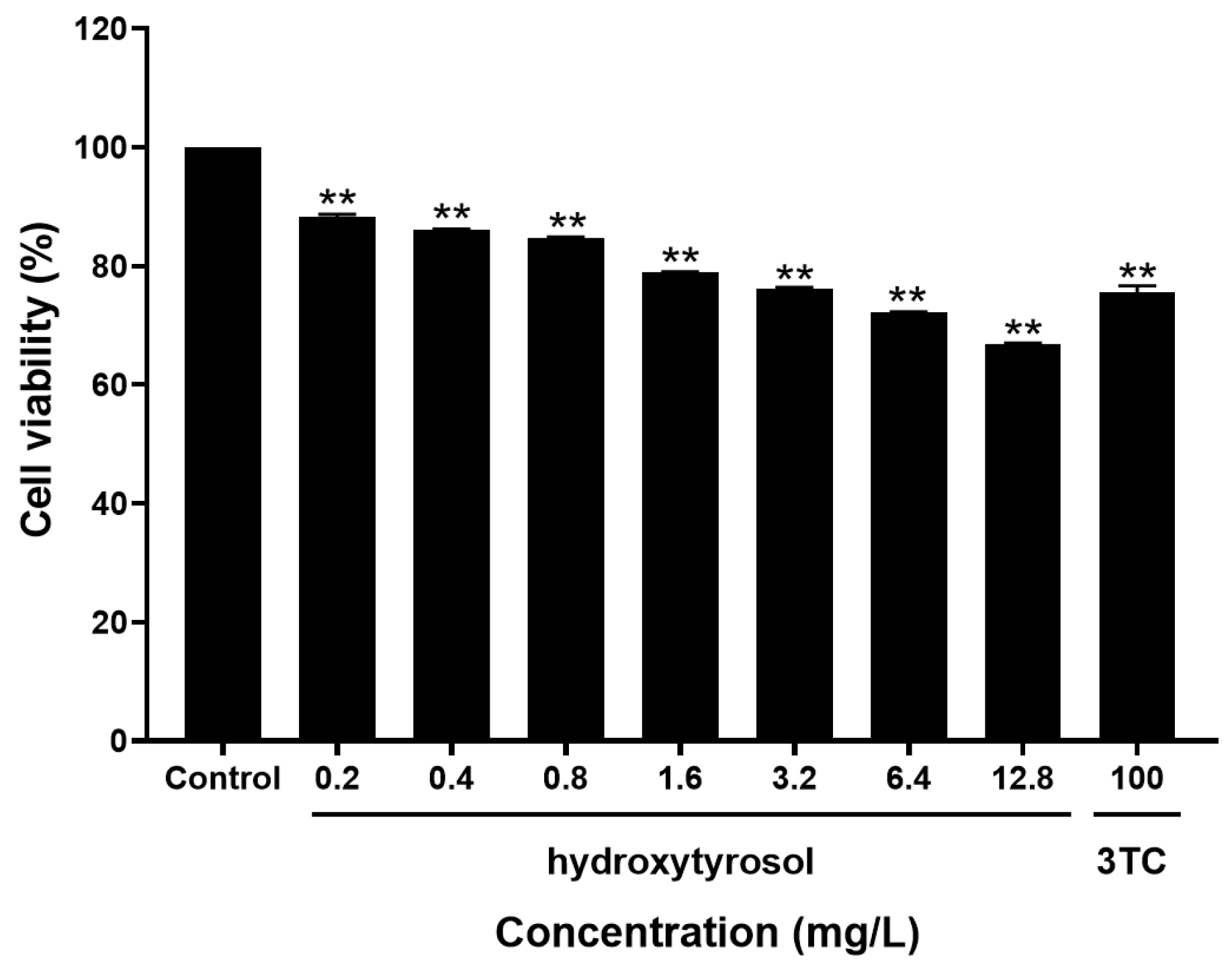
2.2. Toxicity of Hydroxytyrosol to HepG2.2.15 Cells
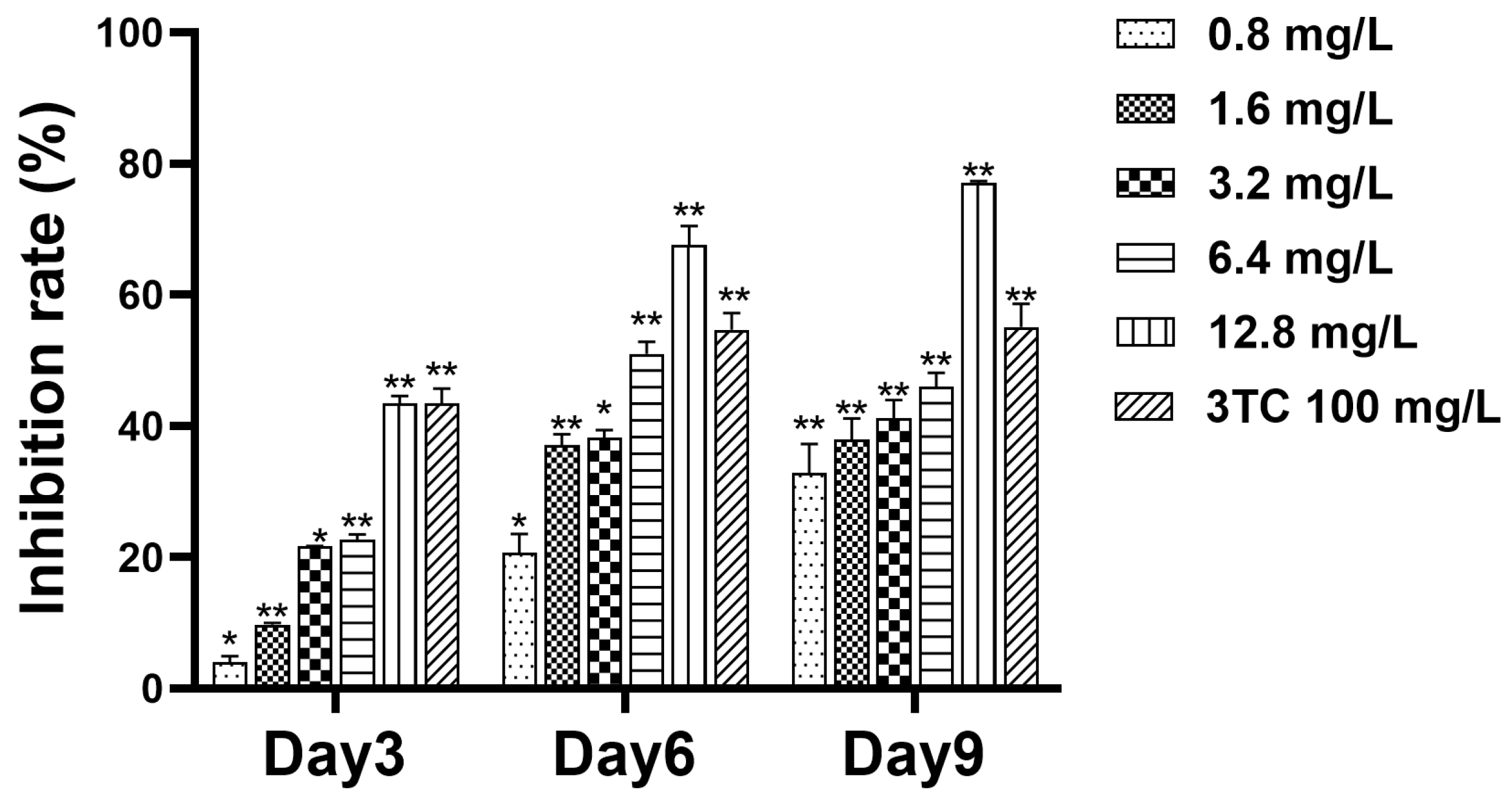
2.3. Detection of Cell Supernatant HBsAg
2.4. Detection of Cell Supernatant HBeAg
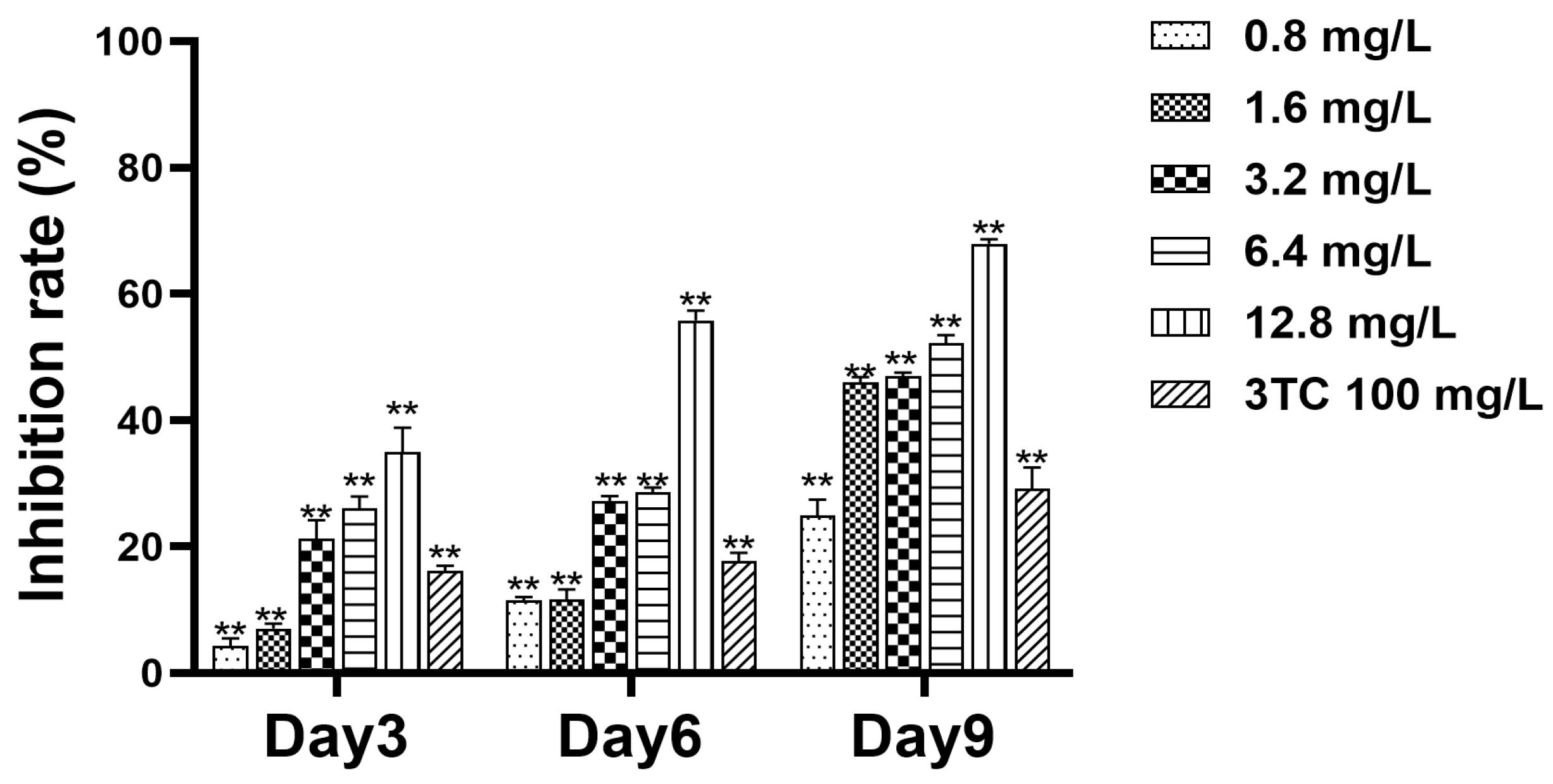
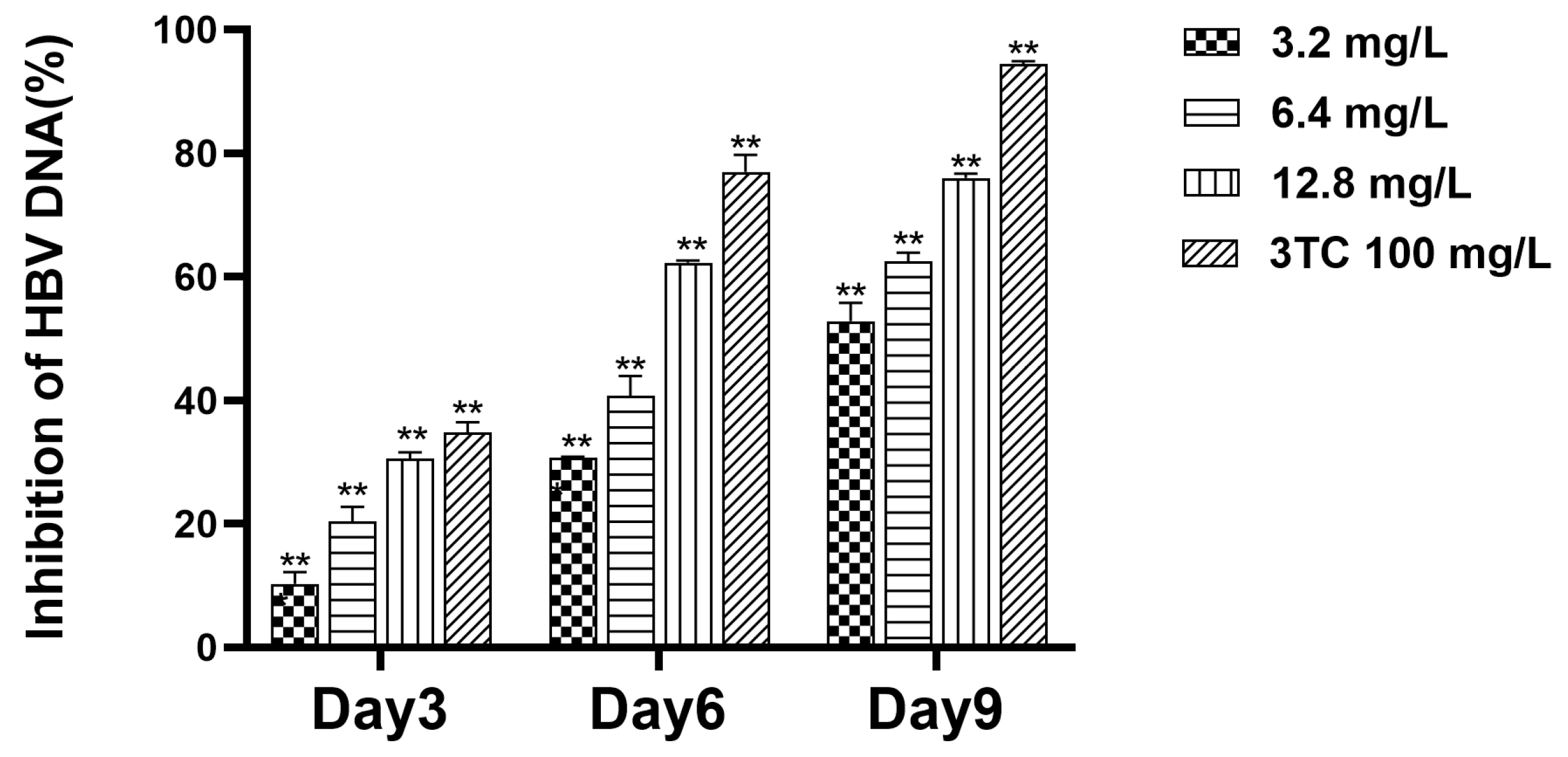
2.5. Detection of Cell Supernatant HBV DNA
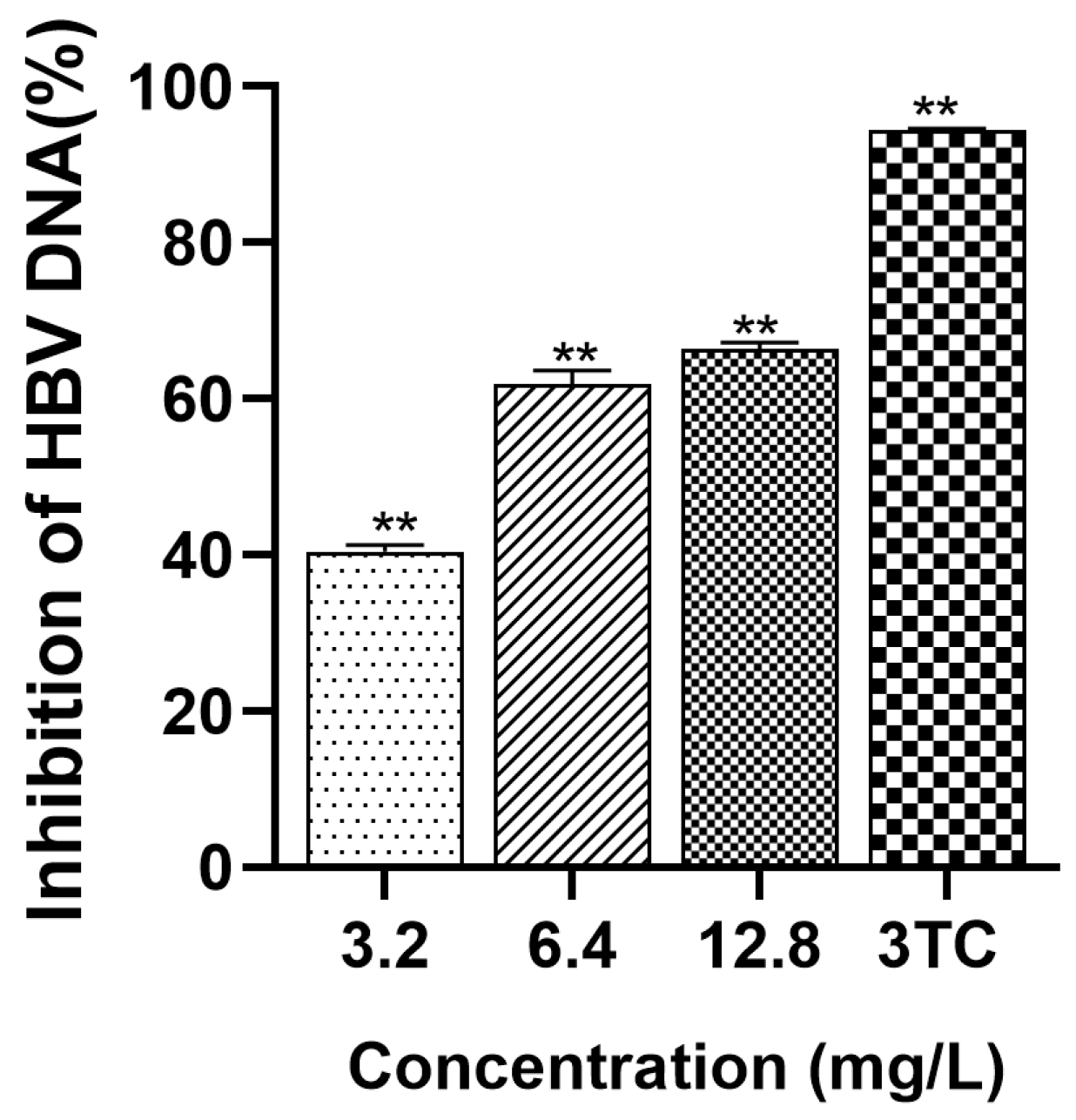
2.6. Detection of Intracellular HBV DNA
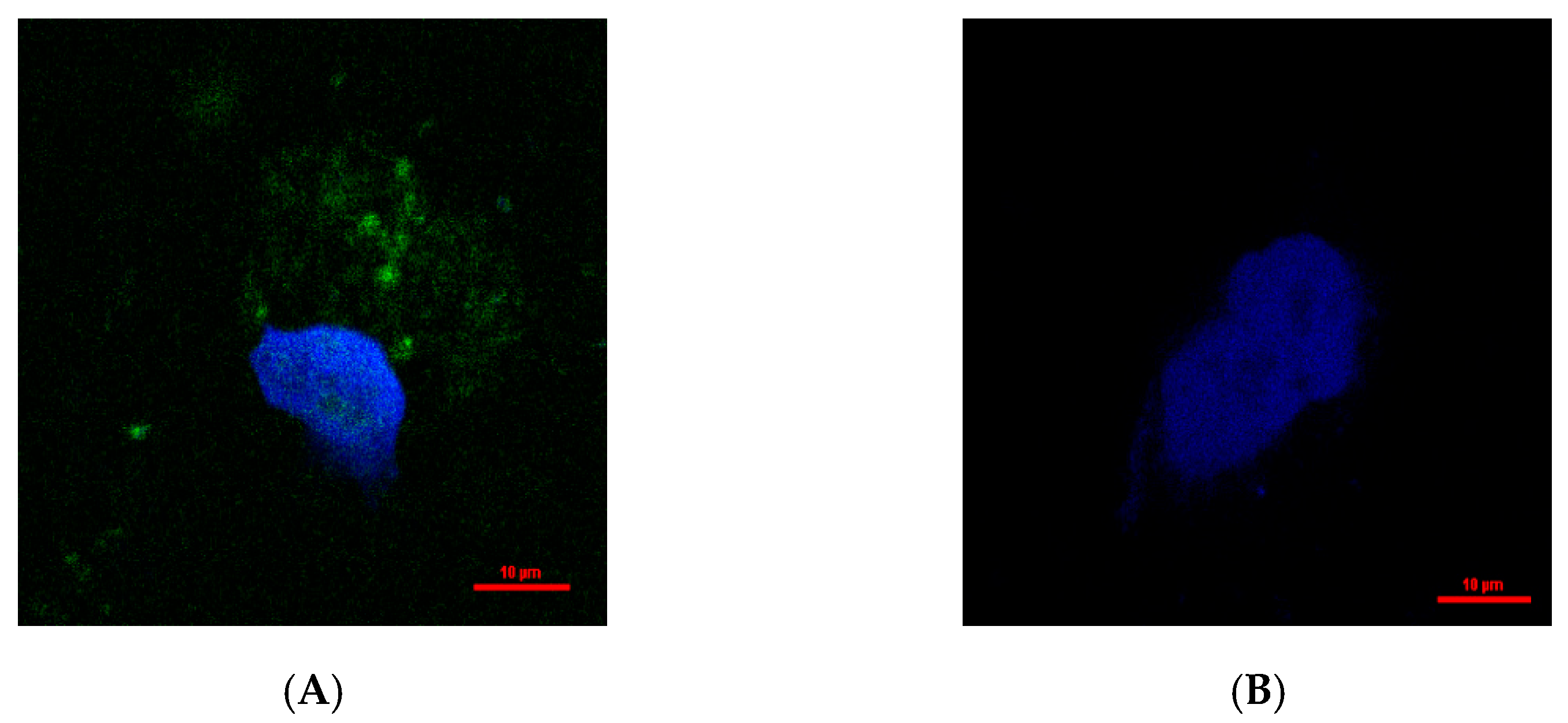
2.7. Intracellular Distribution of Hydroxytyrosol
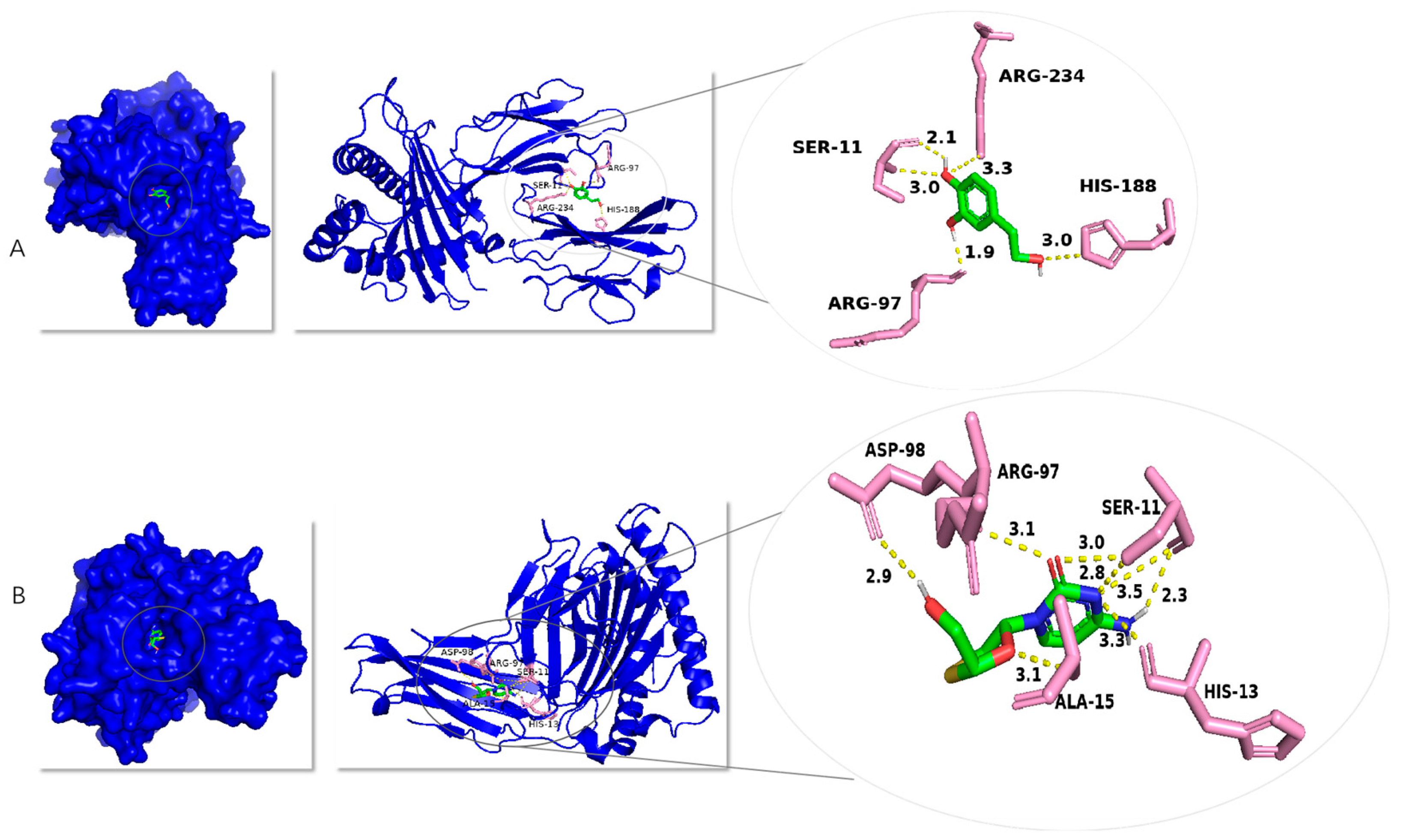
2.8. Study on Molecular Docking with Hepatitis B Virus Polymerase
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Cell Cultures
4.3. Extraction and Isolation
4.4. The Toxicity of Hydroxytyrosol to HepG2.2.15 Cells Was Determined by MTT Assay
4.5. Detection of Cell Supernatant HBsAg and HBeAg
4.6. Cell Supernatant HBV DNA Copy Number Was Detected by FQ-PCR
4.7. Intracellular HBV DNA Copy Number Was Detected by FQ-PCR
4.8. Detection of Hydroxytyrosol Distribution in HepG2.2.15 Cells
4.9. Molecular Docking Analysis of Hydroxytyrosol and Hepatitis B Virus RNA Polymerase
4.10. Statistical Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Hepatitis, B. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 4 April 2024).
- Jiang, Y.; Han, Q.; Zhao, H.; Zhang, J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.; Arbab, A.H.; ElGamal, A.A.; Parvez, M.K.; Al-Dosari, M.S. Isolation and Characterization of Two Chalcone Derivatives with Anti-Hepatitis B Virus Activity from the Endemic Socotraen Dracaena cinnabari (Dragon’s Blood Tree). Molecules 2022, 27, 952. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Salavatiha, Z.; Gogoi, U.; Mohebbi, A. An overview of anti-Hepatitis B virus flavonoids and their mechanisms of action. Front. Cell. Infect. Microbiol. 2024, 14, 1356003. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, X.; Wang, Y.; Wang, J.; Feng, S.; Zhao, Z.; Zheng, L.; Zhang, J.; Zhang, X.; Peng, Y. In vivo anti-hepatitis B activity of Artemisia argyi essential oil-loaded nanostructured lipid carriers. Study of its mechanism of action by network pharmacology and molecular docking. Phytomedicine 2023, 116, 154848. [Google Scholar] [CrossRef]
- Jose-Abrego, A.; Rivera-Iñiguez, I.; Torres-Reyes, L.A.; Roman, S. Anti-hepatitis B virus activity of food nutrients and potential mechanisms of action. Ann. Hepatol. 2023, 28, 100766. [Google Scholar] [CrossRef]
- Zhuang, A.Q.; Chen, Y.; Chen, S.M.; Liu, W.C.; Li, Y.; Zhang, W.J.; Wu, Y.H. Current Status and Challenges in Anti-Hepatitis B Virus Agents Based on Inactivation/Inhibition or Elimination of Hepatitis B Virus Covalently Closed Circular DNA. Viruses 2023, 15, 2315. [Google Scholar] [CrossRef]
- Zhu, S.; Wen, H.; Wang, W.; Chen, Y.; Han, F.; Cai, W. Anti-hepatitis B virus activity of lithospermic acid, a polyphenol from Salvia miltiorrhiza, in vitro and in vivo by autophagy regulation. J. Ethnopharmacol. 2023, 302 Pt A, 115896. [Google Scholar] [CrossRef]
- Mohebbi, A.; Azadi, F.; Hashemi, M.M.; Askari, F.S.; Razzaghi, N. Havachoobe (Onosma dichroanthum Boiss) Root Extract Decreases the Hepatitis B Virus Surface Antigen Secretion in the PLC/PRF/5 Cell Line. Intervirology 2022, 64, 22–26. [Google Scholar] [CrossRef]
- Sharma, H.N.; Catrett, J.; Nwokeocha, O.D.; Boersma, M.; Miller, M.E.; Napier, A.; Robertson, B.K.; Abugri, D.A. Anti-Toxoplasma gondii activity of Trametes versicolor (Turkey tail) mushroom extract. Sci. Rep. 2023, 13, 8667. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Sui, B.; Xu, M.; Pu, Z.; Qiu, T. Schisandrin A from Schisandra chinensis Attenuates Ferroptosis and NLRP3 Inflammasome-Mediated Pyroptosis in Diabetic Nephropathy through Mitochondrial Damage by AdipoR1 Ubiquitination. Oxidative Med. Cell. Longev. 2022, 2022, 5411462. [Google Scholar] [CrossRef]
- Jie, X.X.; Geng, C.A.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Zhang, R.P.; Chen, J.J. Five new secoiridoid glycosides and one unusual lactonic enol ketone with anti-HBV activity from Swertia cincta. Fitoterapia 2015, 102, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.A.; Yang, T.H.; Huang, X.Y.; Yang, J.; Ma, Y.B.; Li, T.Z.; Zhang, X.M.; Chen, J.J. Anti-hepatitis B virus effects of the traditional Chinese herb Artemisia capillaris and its active enynes. J. Ethnopharmacol. 2018, 224, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, Z.; He, L.; Ma, H.; Qu, W.; Yin, J.; Jia, M.; Zhao, X.; Shan, J.; Gao, Y. Hepatoprotective and inhibiting HBV effects of polysaccharides from roots of Sophora flavescens. Int. J. Biol. Macromol. 2018, 108, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, X.; Zhuo, Y.; Si, C.; Yang, L.; Meng, L.; Zhu, B. Antiviral activity of a polysaccharide from Radix Isatidis (Isatis indigotica Fortune) against hepatitis B virus (HBV) in vitro via activation of JAK/STAT signal pathway. J. Ethnopharmacol. 2020, 257, 112782. [Google Scholar] [CrossRef]
- Abookleesh, F.L.; Al-Anzi, B.S.; Ullah, A. Potential Antiviral Action of Alkaloids. Molecules 2022, 27, 903. [Google Scholar] [CrossRef]
- Editorial Board of Chinese Materia Medica, State Administration of Traditional Chinese Medicine. Chinese Materia Medica-9; Shanghai Scientific & Technical Publishers: Shanghai, China, 1999; p. 351. [Google Scholar]
- Lee, I.S.; Jung, S.H.; Lee, Y.M.; Choi, S.J.; Sun, H.; Kim, J.S. Phenolic Compounds from the Leaves and Twigs of Osteomeles schwerinae That Inhibit Rat Lens Aldose Reductase and Vessel Dilation in Zebrafish Larvae. J. Nat. Prod. 2015, 78, 2249–2254. [Google Scholar] [CrossRef]
- Arangia, A.; Marino, Y.; Impellizzeri, D.; D’Amico, R.; Cuzzocrea, S.; Di Paola, R. Hydroxytyrosol and Its Potential Uses on Intestinal and Gastrointestinal Disease. Int. J. Mol. Sci. 2023, 24, 3111. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Curieses Andrés, C.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Hydroxytyrosol and Arginine as Antioxidant, Anti-Inflammatory and Immunostimulant Dietary Supplements for COVID-19 and Long COVID. Foods 2023, 12, 1937. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, L.; Yin, H.; Shen, L.; Zheng, W.; Zhang, K.; Zeng, J.; Hu, C.; Liu, Y. Hydroxytyrosol alleviates oxidative stress and neuroinflammation and enhances hippocampal neurotrophic signaling to improve stress-induced depressive behaviors in mice. Food Funct. 2021, 12, 5478–5487. [Google Scholar] [CrossRef]
- Mou, J.F.; Lin, X.Z.; Su, H.L.; Lu, H.L.; Liu, Q.B.; Liang, B.; Chen, X.; Liang, C.Q.; Zhou, X.L. Anti-hepatitis B virus activity and hepatoprotective effect of des(rhamnosyl) verbascoside from Lindernia ruellioides in vitro. Phytother. Res. 2021, 35, 4555–4566. [Google Scholar] [CrossRef]
- Song, C.Z.; Wang, Y.H.; Hua, Y. Chemical Constituents of Clematismontana. Chin. J. Nat. Med. 2008, 6, 116–119. [Google Scholar] [CrossRef]
- Feng, J.; Xu, T.; He, M.; Li, J.; Yao, P.; Ma, C.; Yang, S.; Xu, Z.; Yan, K.; Chen, X.; et al. NSUN2-mediated m5C modification of HBV RNA positively regulates HBV replication. PLoS Pathog. 2023, 19, e1011808. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Chen, F.; Zhao, Y.; Zhou, M.; Guo, M. Anti-hepatitis B virus activities of natural products and their antiviral mechanisms. Chin. J. Nat. Med. 2023, 21, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Hu, J.; Liu, K. Complete and Incomplete Hepatitis B Virus Particles: Formation, Function, and Application. Viruses 2017, 9, 56. [Google Scholar] [CrossRef]
- Chu, C.M.; Karayiannis, P.; Fowler, M.J.; Monjardino, J.; Liaw, Y.F.; Thomas, H.C. Natural history of chronic hepatitis B virus infection in Taiwan: Studies of hepatitis B virus DNA in serum. Hepatology 1985, 5, 431–434. [Google Scholar] [CrossRef]
- Testoni, B.; Levrero, M.; Zoulim, F. Challenges to a Cure for HBV Infection. Semin. Liver Dis. 2017, 37, 231–242. [Google Scholar] [CrossRef]
- Liaw, Y.F. Clinical utility of HBV surface antigen quantification in HBV e antigen-negative chronic HBV infection. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 631–641. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, X.; Wang, Y.; Wu, X.; Zhou, P.; Miao, L.; Deng, X. Neurokinin-1 receptor antagonist aprepitant regulates autophagy and apoptosis via ROS/JNK in intrahepatic cholangiocarcinoma. Liver Int. 2024, 44, 1651–1667. [Google Scholar] [CrossRef]
- Luo, T.T.; Lu, Y.; Yan, S.K.; Xiao, X.; Rong, X.L.; Guo, J. Network pharmacology in research of Chinese medicine formula: Methodology, application and prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar] [CrossRef]
- Han, Z.; Xie, F.; He, J.; Guan, Y. Integration of molecular docking, molecular dynamics and network pharmacology to explore the multi-target pharmacology of fenugreek against diabetes. J. Cell. Mol. Med. 2023, 27, 1959–1974. [Google Scholar]
- Parvez, M.K.; Ahmed, S.; Al-Dosari, M.S.; Abdelwahid, M.A.S.; Arbab, A.H.; Al-Rehaily, A.J.; Al-Oqail, M.M. Novel Anti-Hepatitis B Virus Activity of Euphorbia schimperi and Its Quercetin and Kaempferol Derivatives. ACS Omega 2021, 6, 29100–29110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, M.; Xu, J.; Duan, A.; Yang, H.; Tao, H.; Tian, S.; Zhou, Z.; Li, W.; Tao, H.; et al. Discovery of pentacyclic triterpene conjugates as HBV polymerase/NTCP dual-targeting inhibitors with potent anti-HBV activities. Bioorg. Chem. 2025, 154, 108054. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhou, W.; Cheng, G.; Wu, J.; Guo, S.; Jia, S.; Liu, Y.; Li, B.; Zhang, X.; et al. A bioinformatics investigation into molecular mechanism of Yinzhihuang granules for treating hepatitis B by network pharmacology and molecular docking verification. Sci. Rep. 2020, 10, 11448. [Google Scholar] [CrossRef] [PubMed]
- Masters, L.; Eagon, S.; Heying, M. Evaluation of consensus scoring methods for AutoDock Vina, smina and idock. J. Mol. Graph. Model. 2020, 96, 107532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.-S.-Y.; Li, K.-Z.; Su, H.-L.; Liang, B.; Liang, C.-Q.; Gao, J.-T.; Zhou, X.-L. In Vitro Anti-Hepatitis B Virus Activity of Hydroxytyrosol from Lindernia ruellioides. Molecules 2025, 30, 2063. https://doi.org/10.3390/molecules30092063
Zhao T-S-Y, Li K-Z, Su H-L, Liang B, Liang C-Q, Gao J-T, Zhou X-L. In Vitro Anti-Hepatitis B Virus Activity of Hydroxytyrosol from Lindernia ruellioides. Molecules. 2025; 30(9):2063. https://doi.org/10.3390/molecules30092063
Chicago/Turabian StyleZhao, Tong-Shi-Yao, Kang-Zhi Li, He-Ling Su, Bin Liang, Cheng-Qin Liang, Jin-Tao Gao, and Xian-Li Zhou. 2025. "In Vitro Anti-Hepatitis B Virus Activity of Hydroxytyrosol from Lindernia ruellioides" Molecules 30, no. 9: 2063. https://doi.org/10.3390/molecules30092063
APA StyleZhao, T.-S.-Y., Li, K.-Z., Su, H.-L., Liang, B., Liang, C.-Q., Gao, J.-T., & Zhou, X.-L. (2025). In Vitro Anti-Hepatitis B Virus Activity of Hydroxytyrosol from Lindernia ruellioides. Molecules, 30(9), 2063. https://doi.org/10.3390/molecules30092063






