Foaming of Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite Using Supercritical CO2: Effect of Operating Strategies on Cell Structure
Abstract
1. Introduction
2. Results and Discussion
2.1. Foaming Results from Strategies with (1T-1P), (2T-1P), and (2T-2P) Operating Conditions
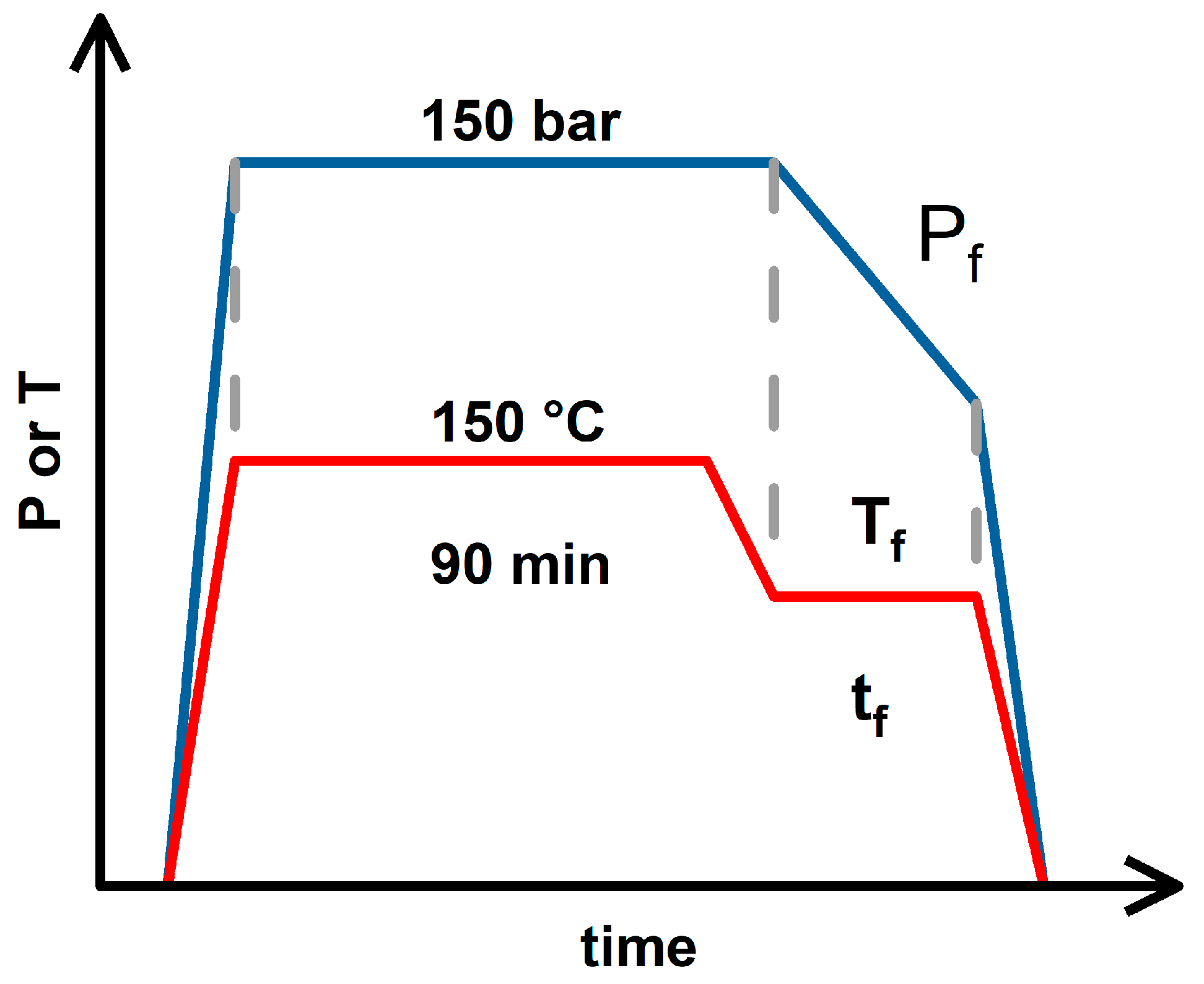
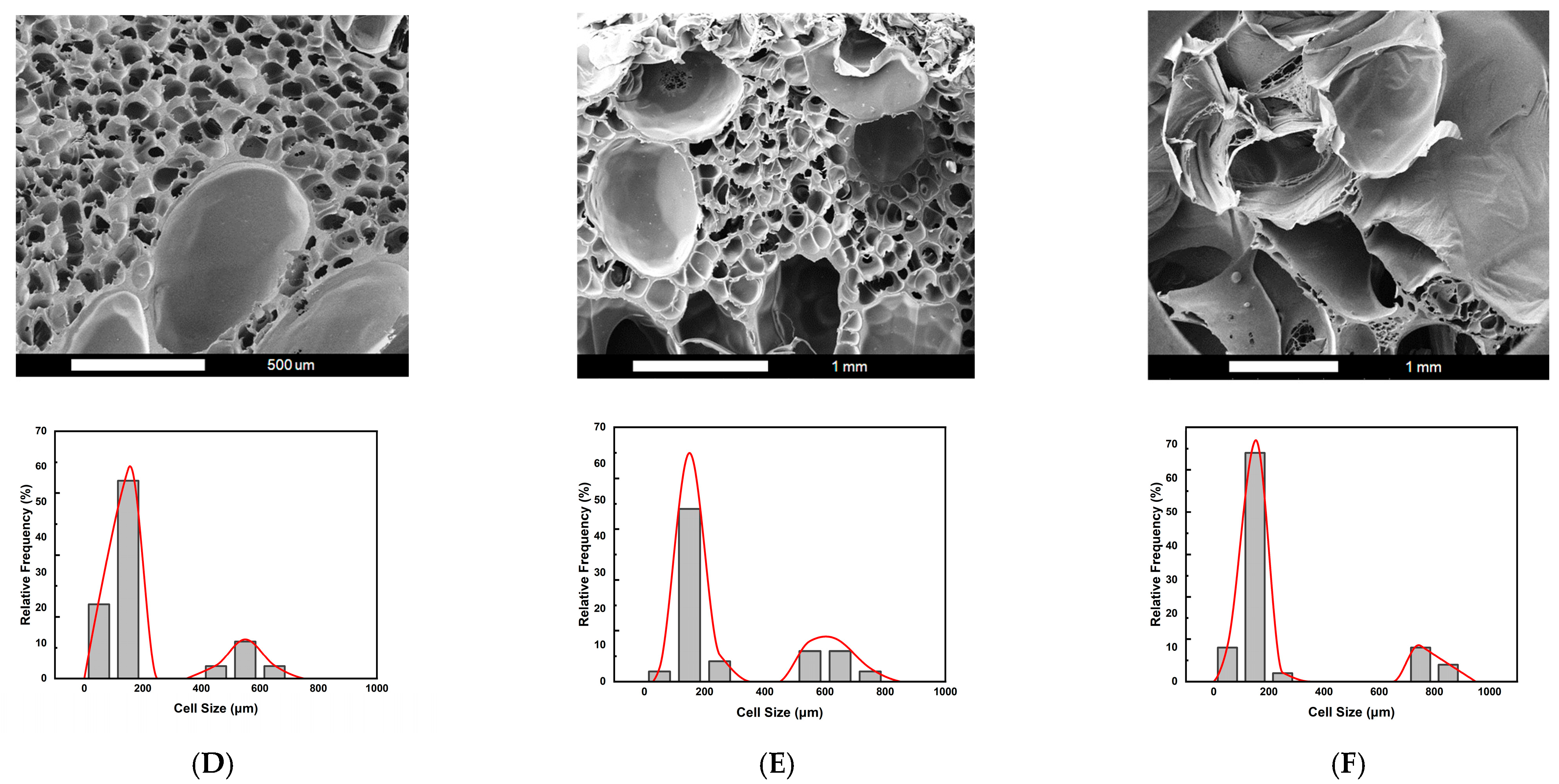
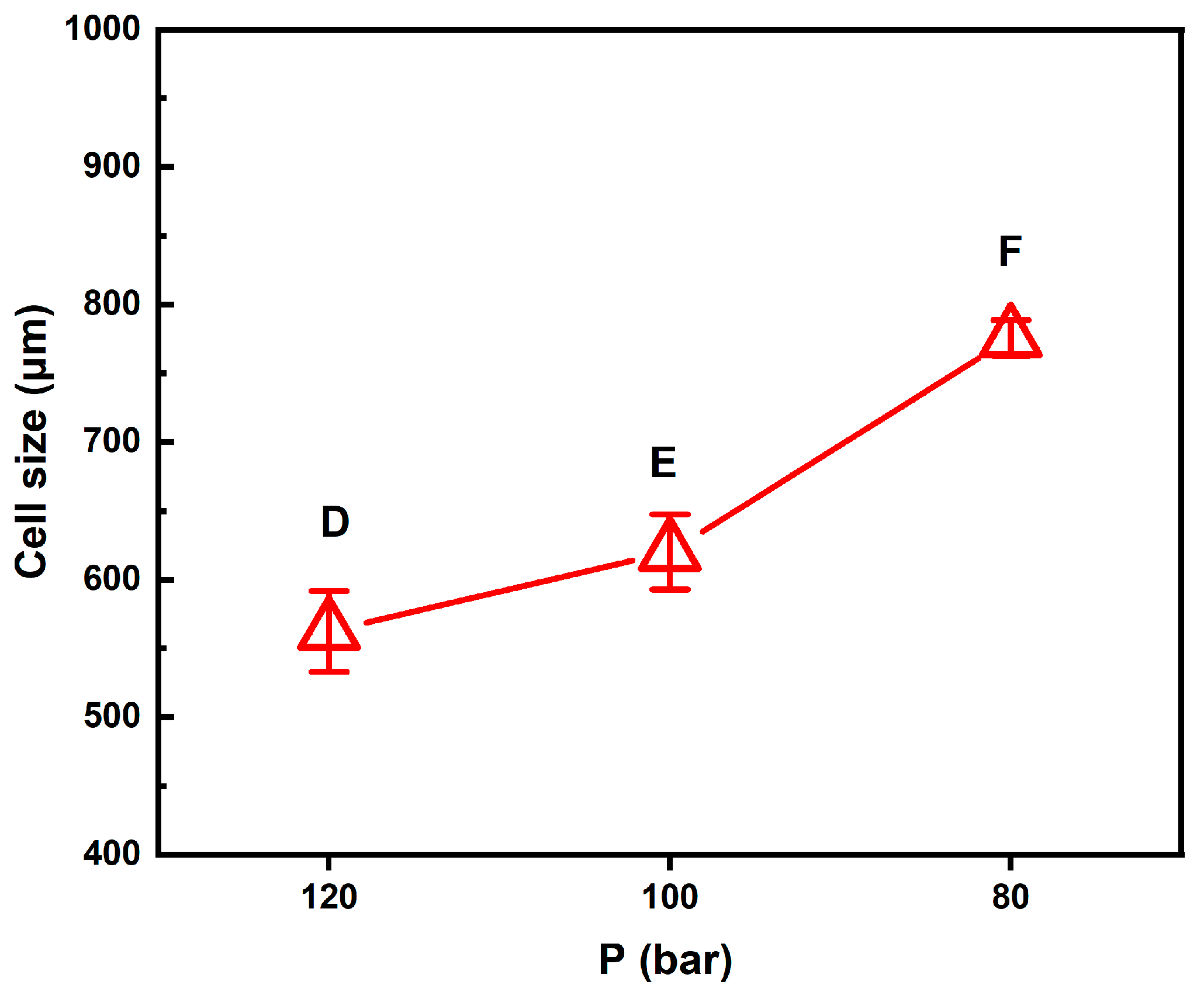
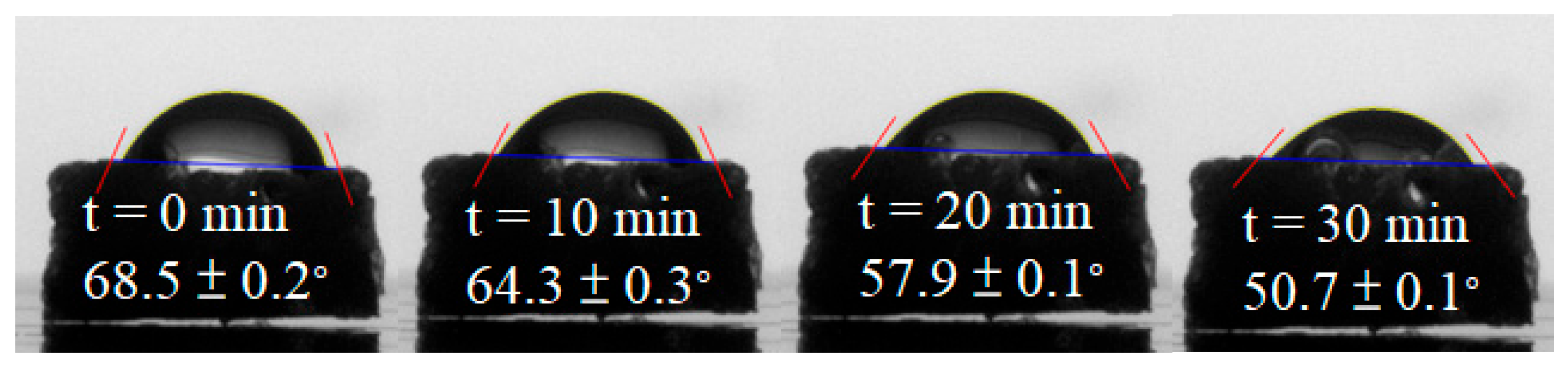
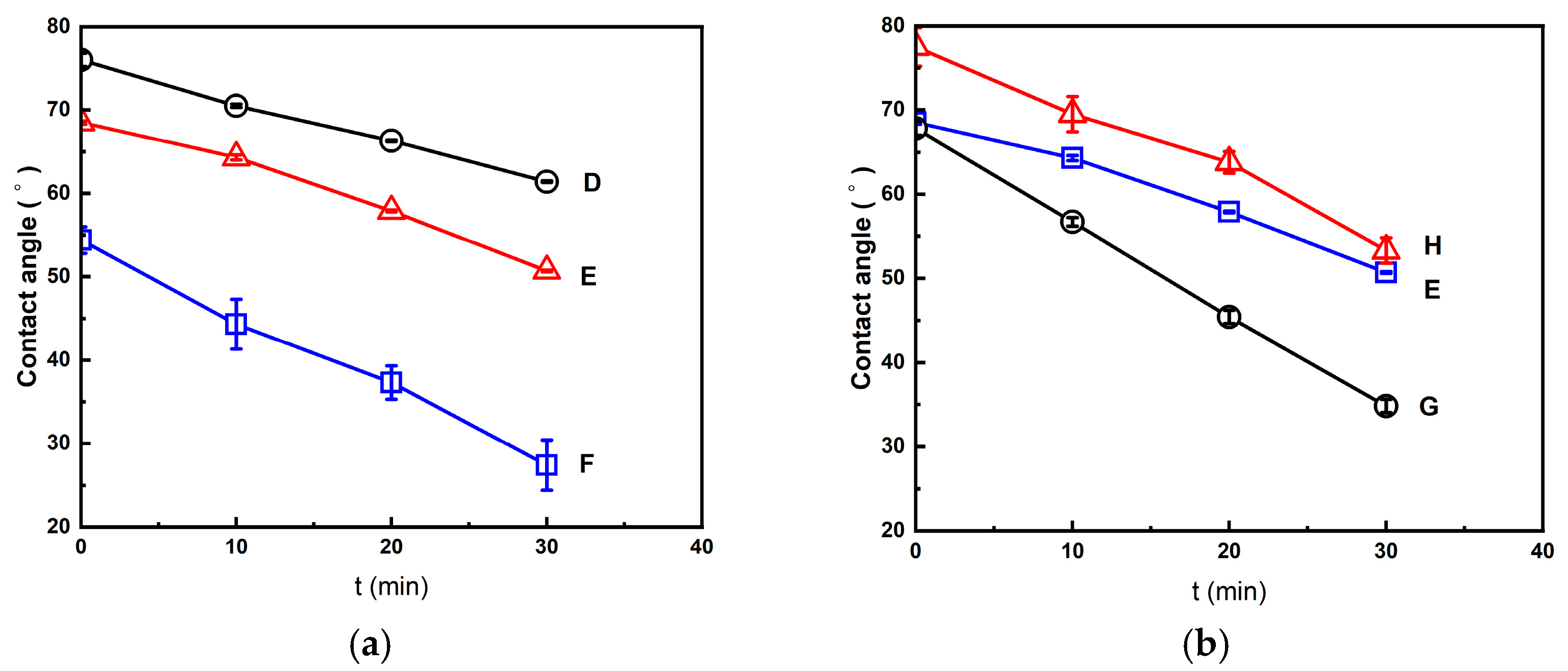
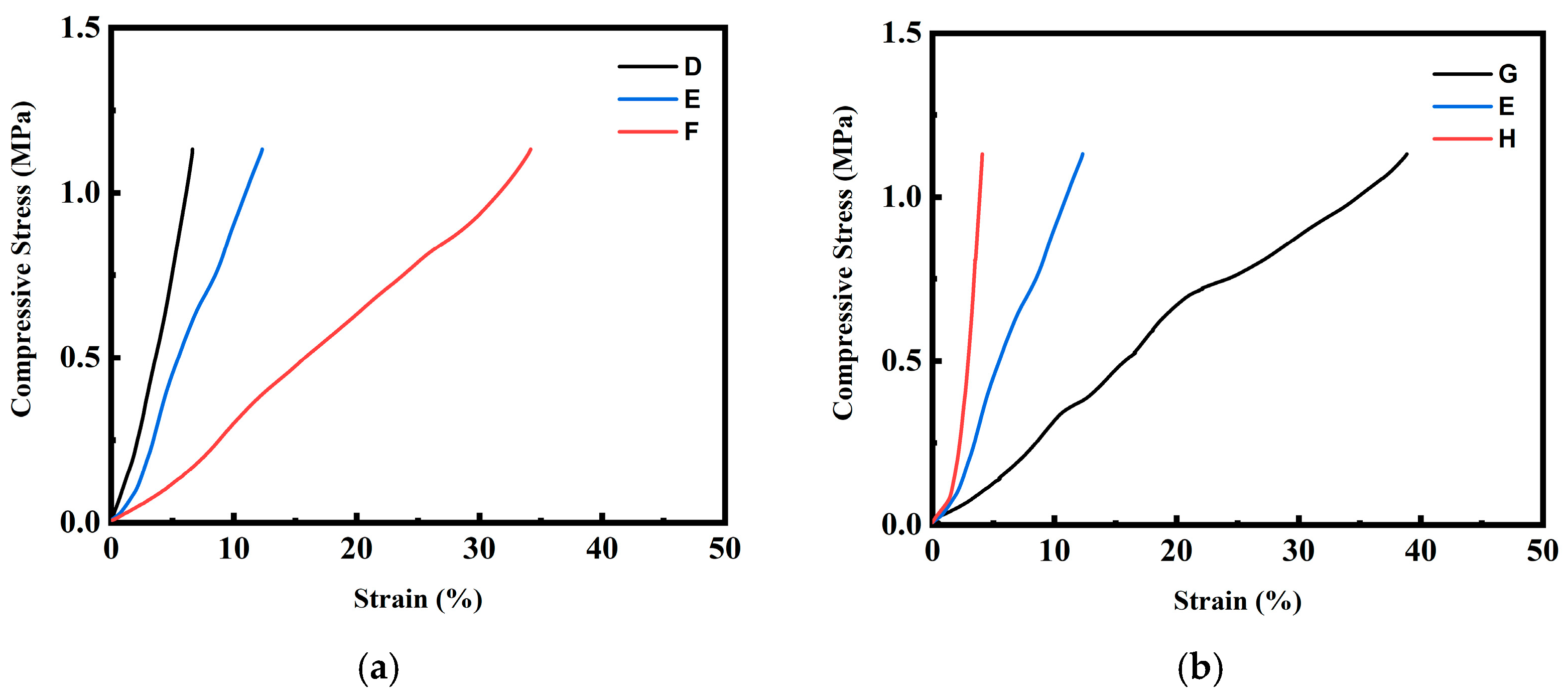
2.2. Foaming Results from Strategies with (2T-2P, Stepwise ΔP) Operating Conditions: Pressure Effect
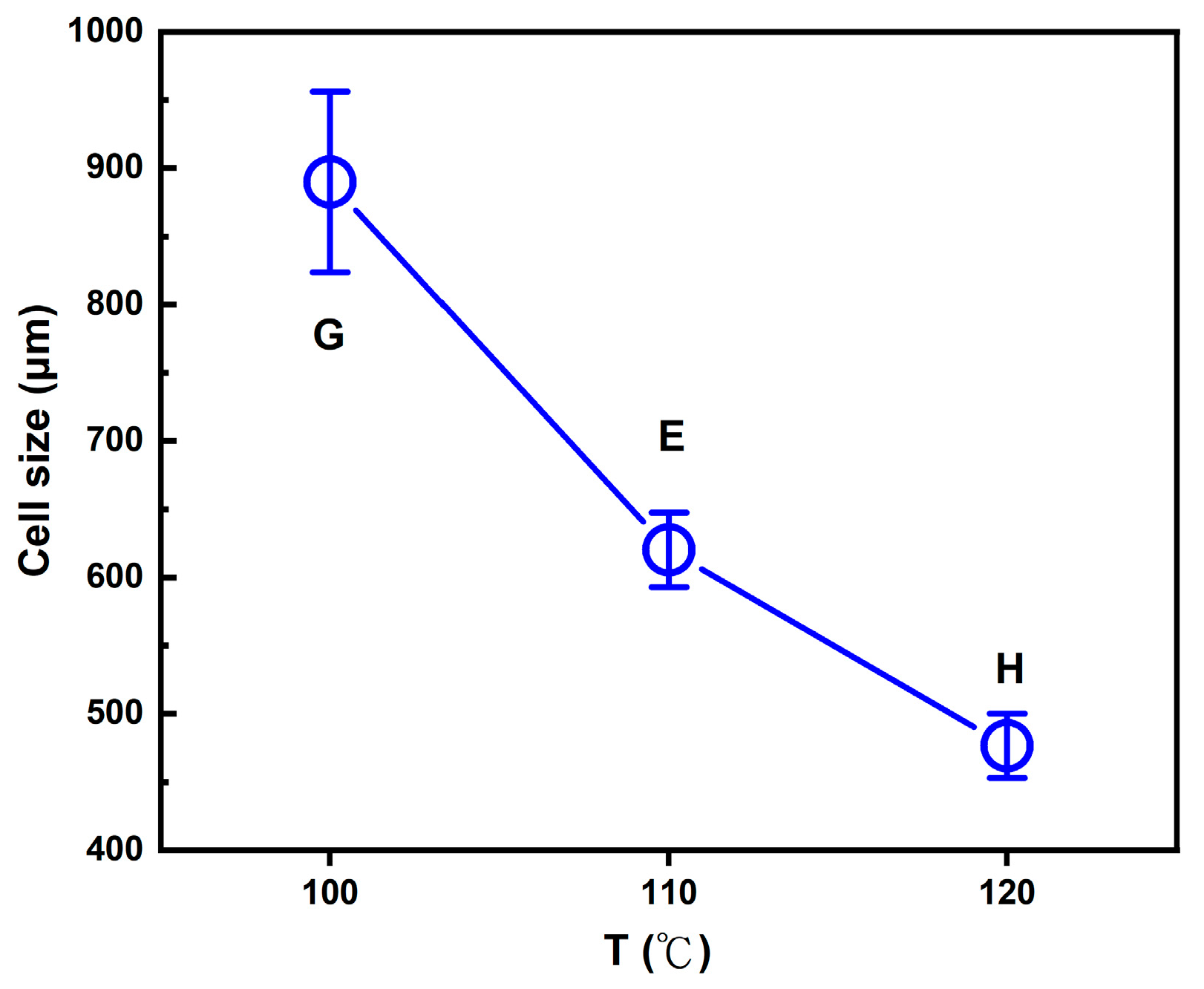
2.3. Foaming Results from Strategies with (2T-2P, Stepwise ΔP) Operating Conditions: Temperature Effect
2.4. Comparison of Foaming Results from All Operating Strategies in This Study
3. Materials and Methods
3.1. Material
3.2. Preparation of Composite Blends
3.3. Saturation and Foaming Steps
3.4. Determination of the Characteristic Structures and Properties of Foamed Polymer Blends
3.5. Water Contact Angle and Mechanical Compression Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaeili, Y.; Bidram, E.; Bigham, A.; Atari, M.; Azadani, R.N.; Tavakoli, M.; Salehi, S.; Mirhaj, M.; Basiri, A.; Mirzavandi, Z.; et al. Exploring the evolution of tissue engineering strategies over the past decade: From cell-based strategies to gene-activated matrix. Alex. Eng. J. 2023, 81, 137–169. [Google Scholar] [CrossRef]
- Al-Shalawi, F.D.; Ariff, A.H.M.; Jung, D.-W.; Ariffin, M.K.A.M.; Kim, C.L.S.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as implants in the orthopedic field for regenerative medicine: Metal versus synthetic polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Ajmal, S.; Hashmi, F.A.; Imran, I. Recent progress in development and applications of biomaterials. Mater. Today Proc. 2022, 62, 385–391. [Google Scholar] [CrossRef]
- Paternoster, J.L.; Vranckx, J.J. State of the art of clinical applications of tissue engineering in 2022. Tissue Eng. Part B Rev. 2022, 28, 592–612. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-W.; Min, K.-E.; Kim, C.; Shin, J.; Lee, J.; Yi, S. Review: Scaffold characteristics, fabrication methods, and biomaterials for the bone tissue engineering. Int. J. Precis. Eng. Manuf. 2023, 24, 511–529. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef]
- Kumeria, T. Advances on porous nanomaterials for biomedical application (drug delivery, sensing, and tissue engineering). ACS Biomater. Sci. Eng. 2022, 8, 4025–4027. [Google Scholar] [CrossRef]
- Swanson, W.B.; Ma, P.X. Textured and porous biomaterials. In Biomaterials Science, An Introduction to Materials in Medicine, 4th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 601–622. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone- tissue engineering. Nat. Rev. Mat. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Xiao, M.; Yao, J.; Shao, Z.; Chen, X. Silk-based 3D porous scaffolds for tissue Engineering. ACS Biomater. Sci. Eng. 2024, 10, 2827–2840. [Google Scholar] [CrossRef]
- Chen, H.; Song, G.; Xu, T.; Meng, C.; Zhang, Y.; Xin, T.; Yu, T.; Lin, Y.; Han, B. Biomaterial scaffolds for periodontal tissue engineering. J. Funct. Biomater. 2024, 15, 233. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Kuang, T. Oriented porous polymer scaffolds in tissue engineering: A comprehensive review of preparation strategies and applications. Macromol. Mater. Eng. 2024, 309, 2300246. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical applications of porous biomaterials: Features of porosity and tissue-specific implications for biocompatibility. Adv. Healthc. Mater. 2022, 11, e2102087. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, F.J.; de la Paz, M.F.V.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: A review. J. Mater. Chem. B 2022, 10, 8111. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A review on biomaterials for orthopaedic surgery and traumatology: From past to present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Chen, X.; Gao, Y.; Wang, W.; Zhuang, X.; Zhang, H.; Dong, X. Bone tissue engineering scaffold materials: Fundamentals, advances, and challenges. Chin. Chem. Lett. 2024, 35, 109197. [Google Scholar] [CrossRef]
- Modrák, M.; Trebunová, M.; Balogová, A.F.; Hudák, R.; Živcák, J. Biodegradable materials for tissue engineering: Development, classification and current applications. J. Funct. Biomater. 2023, 14, 159. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, H.; Tian, H. Recent advances in degradable biomedical polymers for prevention, diagnosis and treatment of diseases. Biomacromolecules 2024, 25, 7015–7057. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Shen, Y.; Ao, Q. Synthetic biodegradable polymer materials in the repair of tumor-associated bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1096525. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, J.; Fan, X.; Xie, H.; Zhang, H.; Zhang, G.; Shi, X. Influence of polylactide (PLA) stereocomplexation on the microstructure of PLA/PBS blends and the cell morphology of their microcellular foams. Polymers 2020, 12, 2362. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and its blends with poly(butylene succinate) (PBS): A brief review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Shi, J.; Ye, H.; Zhou, Q. Distinctive tensile properties of the blends of poly(l-lactic acid) (PLLA) and poly(butylene succinate) (PBS). J. Polym. Environ. 2018, 26, 1737–1744. [Google Scholar] [CrossRef]
- Ou-Yang, Q.; Guo, B.; Xu, J. Preparation and characterization of poly(butylene succinate)/polylactide blends for fused deposition modeling 3D printing. ACS Omega 2018, 3, 14309–14317. Available online: http://pubs.acs.org/journal/acsodf (accessed on 20 March 2025).
- Yu, P.; Mi, H.-Y.; Huang, A.; Geng, L.-H.; Chen, B.-Y.; Kuang, T.-R.; Mou, W.-J.; Peng, X.-F. Effect of poly(butylenes succinate) on poly(lactic acid) foaming behavior: Formation of open cell structure. Ind. Eng. Chem. Res. 2015, 54, 6199–6207. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Liu, Y.; Yang, Y. Blends of poly(butylene adipate-co-terephthalate) (PBAT) and stereocomplex polylactide with improved rheological and mechanical properties. RSC Adv. 2020, 10, 10482–10490. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Chen, P.; Shi, X.; Zhang, G.; Wang, C. Preparation of open-porous stereocomplex PLA/PBAT scaffolds and correlation between their morphology, mechanical behavior, and cell compatibility. RSC Adv. 2018, 8, 12933–12943. [Google Scholar] [CrossRef]
- Mou, Z.-L.; Zhao, L.-J.; Zhang, Q.-A.; Zhang, J.; Zhang, Z.-Q. Preparation of porous PLGA/HA/collagen scaffolds with supercritical CO2 and application in osteoblast cell culture. J. Supercrit. Fluids 2011, 58, 398–406. [Google Scholar] [CrossRef]
- Lowe, B.; Hardy, J.G.; Walsh, L.J. Optimizing nanohydroxyapatite nanocomposites for bone tissue engineering. ACS Omega 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Z.; Guo, Z.; Ma, Y.; Wang, C.; Tan, H.; Zhang, Y. Study on the properties of PLA/PBAT composite modified by nanohydroxyapatite. J. Mater. Res. Technol. 2020, 9, 11895–11904. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chen, C.-W.; Mao, H.-I.; Dai, C.-A.; Su, C.-S.; Tsai, J.-C.; Lin, F.-H. Bio-based PLA/PBS/PBAT ternary blends added with nanohydroxyapatite: A thermal, physical and mechanical study. Polymers 2023, 15, 4585. [Google Scholar] [CrossRef] [PubMed]
- Di Maioa, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Sarver, J.A.; Kiran, E. Foaming of polymers with carbon dioxide—The year-in-review-2019. J. Supercrit. Fluids 2021, 173, 105166. [Google Scholar] [CrossRef]
- Kiran, E. Supercritical fluids and polymers-The year in review—2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Y.; Peng, X. Applications and challenges of supercritical foaming technology. Polymers 2023, 15, 402. [Google Scholar] [CrossRef]
- Peng, K.; Mubarak, S.; Diao, X.; Cai, Z.; Zhang, C.; Wang, J.; Wu, L. Progress in the preparation, properties, and applications of PLA and Its composite microporous materials by supercritical CO2: A review from 2020 to 2022. Polymers 2022, 14, 4320. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J.; Shi, Z. Super high-expansion poly(lactic acid) foams with excellent oil-adsorption and thermal-insulation properties fabricated by supercritical CO2 foaming. Adv. Sustain. Syst. 2021, 5, 2000295. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhang, Q.; Xia, C.; Chen, C.; Chen, X.; Yu, P. Foaming of poly(lactic acid) with supercritical CO2: The combined effect of crystallinity and crystalline morphology on cellular structure. J. Supercrit. Fluids 2019, 145, 122–132. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Mai, Q.; Li, M.; Wu, L.; Kong, P. Foaming behavior of poly(lactic acid) with different D-isomer content based on supercritical CO2-induced crystallization. J. Cell. Plast. 2021, 57, 675–694. [Google Scholar] [CrossRef]
- Huang, J.-N.; Jing, X.; Geng, L.-H.; Chen, B.-Y.; Mi, H.-Y.; Peng, X.-F. A novel multiple soaking temperature (MST) method to prepare polylactic acid foams with bi-modal open-pore structure and their potential in tissue engineering applications. J. Supercrit. Fluids 2015, 103, 28–37. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J.; Shi, Z. Biodegradable PLA/PBS open-cell foam fabricated by supercritical CO2 foaming for selective oil-adsorption. Sep. Purif. Technol. 2021, 257, 117949. [Google Scholar] [CrossRef]
- Wang, S.; Yang, W.; Li, X.; Hu, Z.; Wang, B.; Li, M.; Dong, W. Preparation of high-expansion open-cell polylactic acid foam with superior oil-water separation performance. Int. J. Biol. Macromol. 2021, 193, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Wang, G.; Xu, Z.; Zhang, A.; Dong, G.; Zhao, G. Lightweight, low-shrinkage and high elastic poly(butylene adipate-co-terephthalate) foams achieved by microcellular foaming using N2 & CO2 as co-blowing agents. J. CO2 Util. 2022, 64, 102149. [Google Scholar] [CrossRef]
- Salerno, A.; Di Maio, E.; Iannace, S.; Netti, P.A. Solid-state supercritical CO2 foaming of PCL and PCL-HA nano-composite: Effect of composition, thermal history and foaming process on foam pore structure. J. Supercrit. Fluids 2011, 58, 158–167. [Google Scholar] [CrossRef]
- Chen, C.-X.; Peng, H.-H.; Guan, Y.-X.; Yao, S.-J. Morphological study on the pore growth profile of poly(ε-caprolactone) bi-modal porous foams using a modified supercritical CO2 foaming process. J. Supercrit. Fluids 2019, 143, 72–81. [Google Scholar] [CrossRef]
- Ju, J.; Gu, Z.; Liu, X.; Zhang, S.; Peng, X.; Kuang, T. Fabrication of bimodal open-porous poly (butylene succinate)/cellulose nanocrystals composite scaffolds for tissue engineering application. Int. J. Biol. Macromol. 2020, 147, 1164–1173. [Google Scholar] [CrossRef]
- Tammaro, D.; Lombardi, L.; Maffettone, P.L. Bimodal cell distributions in foaming of olefin block copolymers using high-throughput experimentation. Chem. Eng. Sci. 2025, 304, 121067. [Google Scholar]
- Xu, L.Q.; Huang, H.-X. Formation mechanism and tuning for bi-modal cell structure in polystyrene foams by synergistic effect of temperature rising and depressurization with supercritical CO2. J. Supercrit. Fluids 2016, 109, 177–185. [Google Scholar] [CrossRef]
- Liao, X.; Nawaby, A.V.; Whitfield, P.S. Carbon dioxide-induced crystallization in poly(L-lactic acid) and its effect on foam morphology. Polm. Int. 2010, 59, 1709–1718. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, D.; Zhu, M.; Xue, K.; Wei, X.; Park, C.B.; Wang, X.; Zhao, L. Review on poly (butylene succinate) foam: Modification, foaming behavior and applications. Sustain. Mater. Technol. 2023, 38, e00720. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Turng, L.S.; Gong, S.; Zhang, C. Measurement of gas solubility and diffusivity in polylactide. Fluid Phase Equilib. 2006, 246, 158–166. [Google Scholar] [CrossRef]
- Lin, S.; Yang, J.; Yan, J.; Zhao, Y.; Yang, B. Sorption and diffusion of supercritical carbon dioxide in a biodegradable polymer. J. Macromol. Sci. Phys. 2010, 49, 286–300. [Google Scholar] [CrossRef]
- Sato, Y.; Takikawa, T.; Sorakubo, A.; Takishima, S.; Masuoka, H.; Imaizumi, M. Solubility and diffusion coefficient of carbon dioxide in biodegradable polymers. Ind. Eng. Chem. Res. 2000, 39, 4813–4819. [Google Scholar] [CrossRef]
- Hu, D.; Xue, K.; Liu, Z.; Xu, Z.; Zhao, L. The essential role of PBS on PBAT foaming under supercritical CO2 toward green engineering. J. CO2 Utili. 2022, 60, 101965. [Google Scholar] [CrossRef]
- Yin, D.; Mi, J.; Zhou, H.; Wang, X.; Fu, H. Microcellular foaming behaviors of chain extended poly (butylene succinate)/polyhedral oligomeric silsesquioxane composite induced by isothermal crystallization. Polym. Degrad. Stab. 2019, 167, 228–240. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J. Fabrication of high-expansion PLA foams based on pre-isothermal cold crystallization and supercritical CO2 foaming. Polym. Degrad. Stab. 2018, 156, 75–88. [Google Scholar] [CrossRef]
- Zhou, H.; Song, J.; Ding, X.; Qu, Z.; Wang, X.; Mi, J.; Wang, J. Cellular morphology evolution of chain extended poly(butylene succinate)/organic montmorillonite nanocomposite foam. J. Appl. Polym. Sci. 2019, 5, 47107. [Google Scholar] [CrossRef]
- Jacobs, L.J.M.; Kemmere, M.F.; Keurentjes, J.T.F. Sustainable polymer foaming using high pressure carbon dioxide: A review on fundamentals, processes and applications. Green Chem. 2008, 10, 731–738. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, T.; Liu, B.; Zhang, C.; Zeng, X.; He, L.; Gong, W. Experimental and numerical analysis of bubble nucleation in foaming polymer. Mater. Des. 2021, 203, 109577. [Google Scholar] [CrossRef]
- Abbasi, N.; Stephen Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Huang, L.; Wang, A.; Xu, R.; Lei, C. PBS melt strength improving for supercritical CO2 Foaming molding by physical adsorption and crystallization. Polym. Bull. 2024, 81, 11629–11643. [Google Scholar] [CrossRef]
- Goncalves, L.F.F.F.; Reis, R.L.; Fernandes, E.M. Forefront research of foaming strategies on biodegradable polymers and their composites by thermal or melt-based processing technologies: Advances and perspectives. Polymers 2024, 16, 1286. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Maquet, V.; Jerome, R.; Boccaccini, A.R.; Nazhat, S.N. Mechanical properties of highly porous PDLLA/bioglass composite foams as scaffold for bone tissue engineering. Acta Biomater. 2005, 1, 643–652. [Google Scholar] [CrossRef] [PubMed]
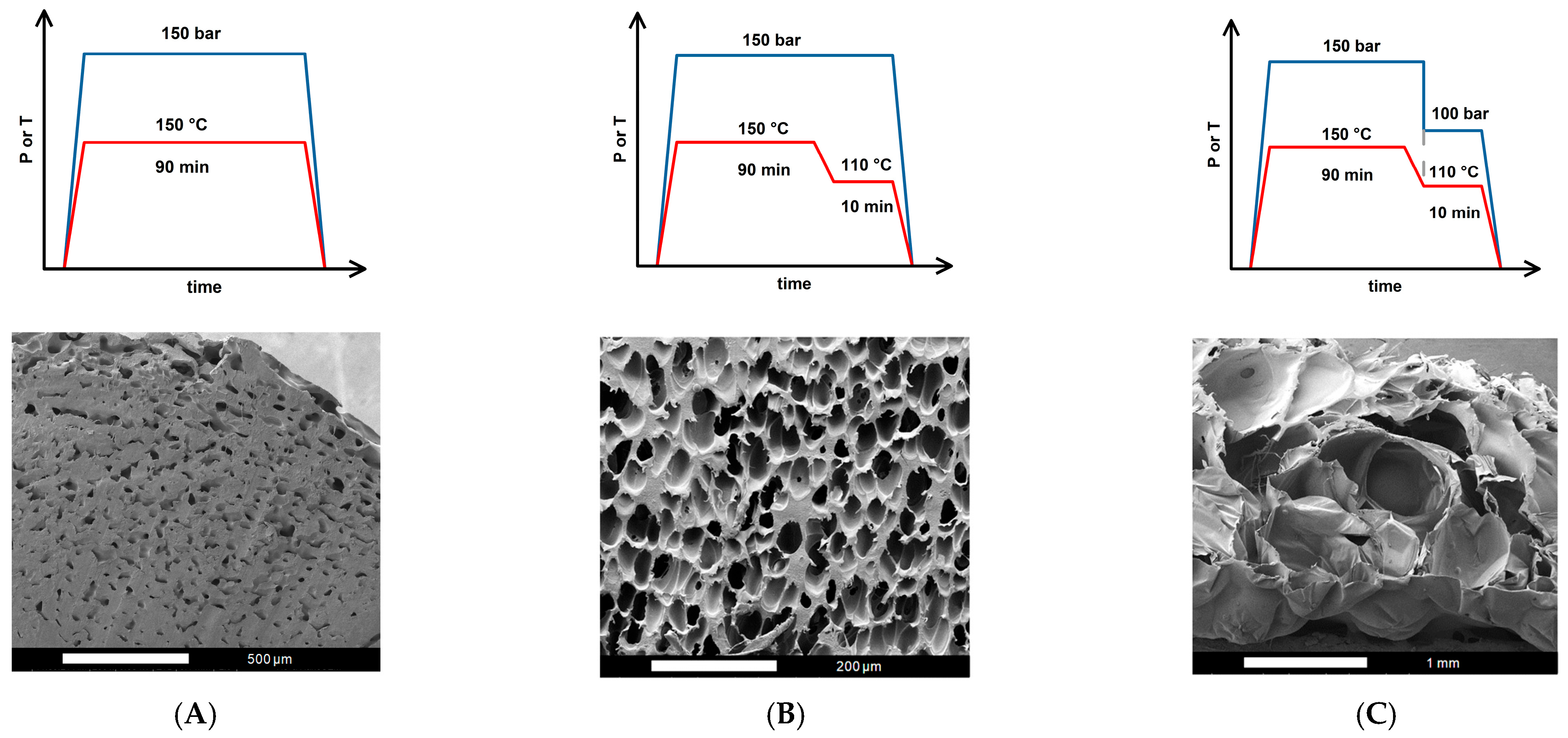




| Operating Strategy | Expansion Ratio | Porosity (%) | Cell Density (cell/cm3) | Cell Size (μm) (Small/Large) | Opening Cell Content (%) |
|---|---|---|---|---|---|
| A | 1.36 ± 0.08 | 26.5 ± 4.4 | (1.75 ± 0.17) × 107 | 27.6 ± 1.1/― | 12.7 ± 2.2 |
| B | 2.72 ± 0.21 | 65.2 ± 2.8 | (1.89 ± 0.05) × 107 | 34.2 ± 1.9/― | 45.0 ± 1.5 |
| C | 5.41 ± 0.40 | 81.5 ± 1.4 | (1.64 ± 0.02) × 104 | ―/408.7 ± 38.7 | 67.8 ± 0.5 |
| D | 4.30 ± 0.02 | 76.7 ± 0.1 | (1.05 ± 0.01) × 106 | 109.9 ± 3.2/562.0 ± 29.4 | 60.0 ± 1.2 |
| E | 7.29 ± 0.30 | 86.3 ± 0.5 | (3.24 ± 0.01) × 105 | 164.2 ± 8.4/602.1 ± 27.3 | 66.9 ± 0.7 |
| F | 14.01 ± 0.93 | 92.9 ± 0.4 | (5.86 ± 0.02) × 105 | 138.2 ± 5.4/775.6 ± 13.0 | 77.8 ± 0.6 |
| G | 17.96 ± 0.20 | 94.4 ± 0.1 | (3.27 ± 0.01) × 105 | 151.1 ± 12.1/889.8 ± 66.2 | 84.3 ± 0.4 |
| H | 4.48 ± 0.16 | 77.7 ± 0.7 | (1.06 ± 0.01) × 106 | 105.9 ± 5.1/476.4 ± 23.6 | 57.8 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Chen, C.-W.; Chan, T.-H.; Lin, H.-Y.; Tuan, K.-L.; Su, C.-S.; Tsai, J.-C.; Lin, F.-H. Foaming of Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite Using Supercritical CO2: Effect of Operating Strategies on Cell Structure. Molecules 2025, 30, 2056. https://doi.org/10.3390/molecules30092056
Chen P-H, Chen C-W, Chan T-H, Lin H-Y, Tuan K-L, Su C-S, Tsai J-C, Lin F-H. Foaming of Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite Using Supercritical CO2: Effect of Operating Strategies on Cell Structure. Molecules. 2025; 30(9):2056. https://doi.org/10.3390/molecules30092056
Chicago/Turabian StyleChen, Pei-Hua, Chin-Wen Chen, Tzu-Hsien Chan, Hsin-Ying Lin, Ke-Ling Tuan, Chie-Shaan Su, Jung-Chin Tsai, and Feng-Huei Lin. 2025. "Foaming of Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite Using Supercritical CO2: Effect of Operating Strategies on Cell Structure" Molecules 30, no. 9: 2056. https://doi.org/10.3390/molecules30092056
APA StyleChen, P.-H., Chen, C.-W., Chan, T.-H., Lin, H.-Y., Tuan, K.-L., Su, C.-S., Tsai, J.-C., & Lin, F.-H. (2025). Foaming of Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite Using Supercritical CO2: Effect of Operating Strategies on Cell Structure. Molecules, 30(9), 2056. https://doi.org/10.3390/molecules30092056








