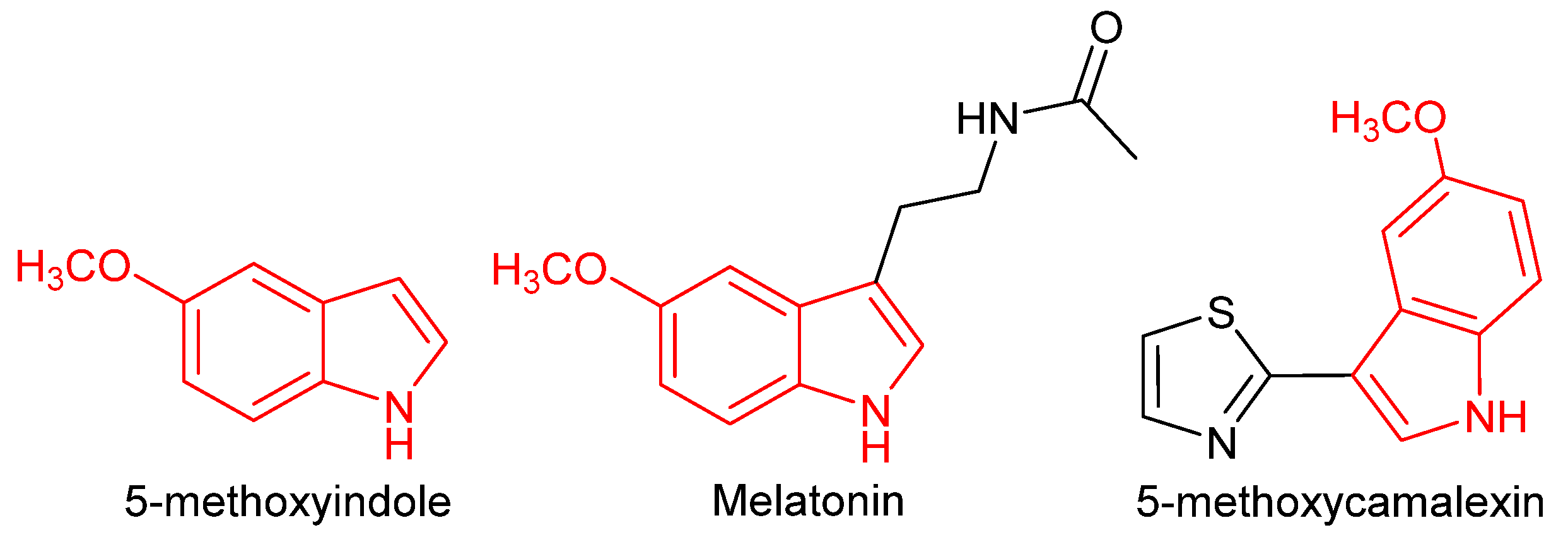
An Efficient Method for the Synthesis and In Silico Study of Novel Oxy-Camalexins
Abstract
1. Introduction
2. Results and Discussion
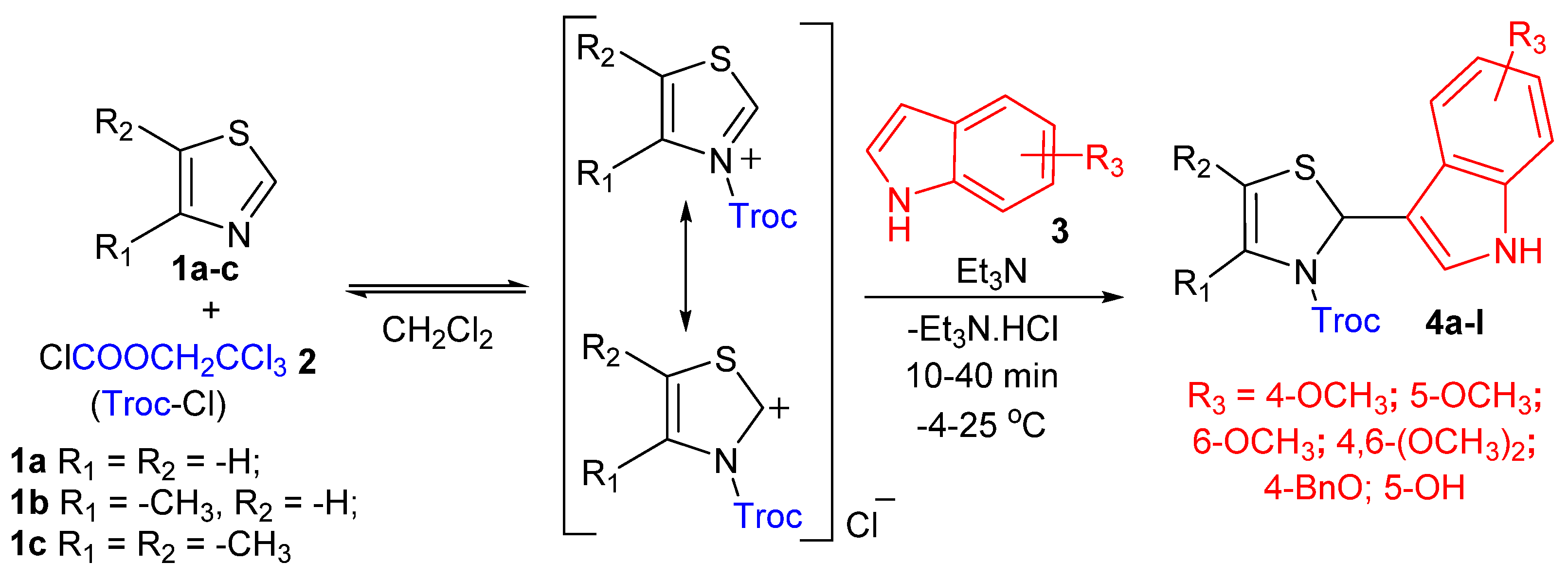
2.1. Synthesis of Novel Analogues of Oxy-Camalexins by Multicomponent Amidoalkylation of Oxy-Indoles
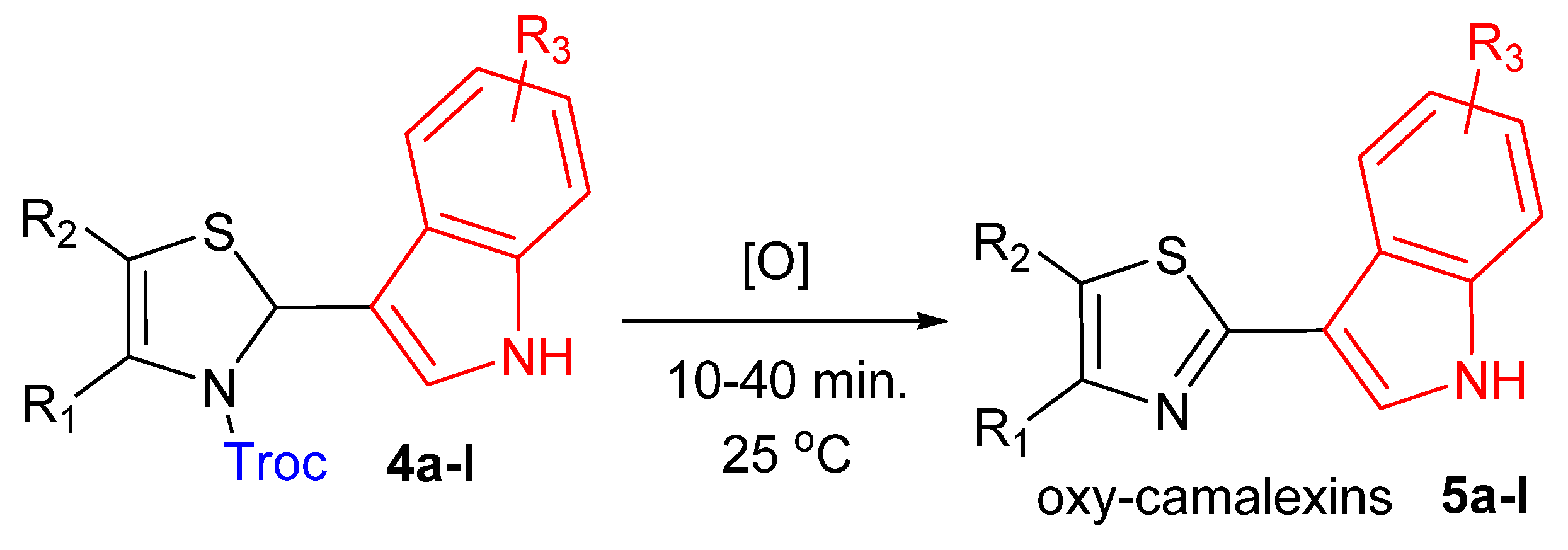
2.2. Synthesis of Oxy-Camalexins
3. In Silico QSAR Analysis
In Silico Predictions of Oxy-Camalexins Toxicity, LogP, and Various Other Parameters
4. Materials and Methods
4.1. Chemistry
4.1.1. General Information
4.1.2. General Procedure for the Synthesis of N-Acylated Oxy-Camalexin Analogues
 C)—1435, 1507, 1587; ν (C-O)—1039, 1083, 1261; ν (C-O-C)—1331; ν (C-N)—814, 852, 1389; ν (C-S-C)—730.
C)—1435, 1507, 1587; ν (C-O)—1039, 1083, 1261; ν (C-O-C)—1331; ν (C-N)—814, 852, 1389; ν (C-S-C)—730. C)—1434, 1508, 1587; ν (C-O)—1039, 1083, 1260; ν (C-O-C)—1336; ν (C-N)—837, 1399; ν (C-S-C)—732.
C)—1434, 1508, 1587; ν (C-O)—1039, 1083, 1260; ν (C-O-C)—1336; ν (C-N)—837, 1399; ν (C-S-C)—732. C)—1437, 1484, 1578; ν (C-O)—1035, 1066, 1129; ν (C-O-C)—1287; ν (C-N)—879, 1400; ν (C-S-C)—799.
C)—1437, 1484, 1578; ν (C-O)—1035, 1066, 1129; ν (C-O-C)—1287; ν (C-N)—879, 1400; ν (C-S-C)—799. C)—1454, 1485, 1539; ν (C-O)—1035, 1083, 1290; ν (C-O-C)—1305; ν (C-N)—838, 1341; ν (C-S-C)—795.
C)—1454, 1485, 1539; ν (C-O)—1035, 1083, 1290; ν (C-O-C)—1305; ν (C-N)—838, 1341; ν (C-S-C)—795. C)—1485, 1531, 1581; ν (C-O)—1026, 1063, 1280; ν (C-O-C)—1337; ν (C-N)—821, 842, 1395; ν (C-S-C)—732.
C)—1485, 1531, 1581; ν (C-O)—1026, 1063, 1280; ν (C-O-C)—1337; ν (C-N)—821, 842, 1395; ν (C-S-C)—732. C)—1451, 1539; ν (C-O)—1025, 1129, 1160; ν (C-O-C)—1236; ν (C-N)—830, 1408; ν (C-S-C)—725.
C)—1451, 1539; ν (C-O)—1025, 1129, 1160; ν (C-O-C)—1236; ν (C-N)—830, 1408; ν (C-S-C)—725. C)—1451, 1539; ν (C-O)—1025, 1102, 1158; ν (C-O-C)—1236; ν (C-N)—824, 1408; ν (C-S-C)—725.
C)—1451, 1539; ν (C-O)—1025, 1102, 1158; ν (C-O-C)—1236; ν (C-N)—824, 1408; ν (C-S-C)—725. C)—1449, 1539, 1586; ν (C-O)—1036, 1091, 1263; ν (C-O-C)—1325; ν (C-N)—806, 827, 1399; ν (C-S-C)—717.
C)—1449, 1539, 1586; ν (C-O)—1036, 1091, 1263; ν (C-O-C)—1325; ν (C-N)—806, 827, 1399; ν (C-S-C)—717. C)—1434, 1512, 1592; ν (C-O)—1036, 1090, 1270; ν (C-O-C)—1323; ν (C-N)—819, 1409; ν (C-S-C)—720.
C)—1434, 1512, 1592; ν (C-O)—1036, 1090, 1270; ν (C-O-C)—1323; ν (C-N)—819, 1409; ν (C-S-C)—720. C)—1504, 1586; ν (C-O)—1090, 1117, 1164; ν (C-O-C)—1240; ν (C-N)—879, 1400; ν (C-S-C)—731.
C)—1504, 1586; ν (C-O)—1090, 1117, 1164; ν (C-O-C)—1240; ν (C-N)—879, 1400; ν (C-S-C)—731. C)—1500, 1541; ν (C-O)—1092, 1135, 1191; ν (C-O-C)—1226; ν (C-N)—828, 1404; ν (C-S-C)—736.
C)—1500, 1541; ν (C-O)—1092, 1135, 1191; ν (C-O-C)—1226; ν (C-N)—828, 1404; ν (C-S-C)—736. C)—1455, 1582; ν (C-O)—1053, 1096, 1149; ν (C-O-C)—1186, 1340; ν (C-N)—801, 1399; ν (C-S-C)—719.
C)—1455, 1582; ν (C-O)—1053, 1096, 1149; ν (C-O-C)—1186, 1340; ν (C-N)—801, 1399; ν (C-S-C)—719.4.1.3. General Procedure for Oxidative Rearomatization of Compounds 4 to Oxy-Camalexins 5
 C)—1464, 1523, 1589; ν (C-O)—1109, 1252; ν (C-O-C)—1325; ν (C-N)—781, 1362; ν (C-S-C)—737.
C)—1464, 1523, 1589; ν (C-O)—1109, 1252; ν (C-O-C)—1325; ν (C-N)—781, 1362; ν (C-S-C)—737. C)—1460, 1532, 1589; ν (C-O)—1093, 1252; ν (C-O-C)—1326; ν (C-N)—776, 1363; ν (C-S-C)—723.
C)—1460, 1532, 1589; ν (C-O)—1093, 1252; ν (C-O-C)—1326; ν (C-N)—776, 1363; ν (C-S-C)—723. C)—1475, 1541, 1586; ν (C-O)—1076, 1213; ν (C-O-C)—1295; ν (C-N)—805, 867; ν (C-S-C)—715.
C)—1475, 1541, 1586; ν (C-O)—1076, 1213; ν (C-O-C)—1295; ν (C-N)—805, 867; ν (C-S-C)—715. C)—1472, 1525, 1592; ν (C-O)—1112, 1255; ν (C-O-C)—1315; ν (C-N)—803, 1363; ν (C-S-C)—742.
C)—1472, 1525, 1592; ν (C-O)—1112, 1255; ν (C-O-C)—1315; ν (C-N)—803, 1363; ν (C-S-C)—742. C)—1487, 1538, 1579; ν (C-O)—1079, 1211; ν (C-O-C)—1292; ν (C-N)—801, 1313; ν (C-S-C)—714.
C)—1487, 1538, 1579; ν (C-O)—1079, 1211; ν (C-O-C)—1292; ν (C-N)—801, 1313; ν (C-S-C)—714. C)—1459, 1543, 1625; ν (C-O)—1084, 1170; ν (C-O-C)—1201; ν (C-N)—801, 828; ν (C-S-C)—727.
C)—1459, 1543, 1625; ν (C-O)—1084, 1170; ν (C-O-C)—1201; ν (C-N)—801, 828; ν (C-S-C)—727. C)—1451, 1543, 1623; ν (C-O)—1084, 1164; ν (C-O-C)—1199; ν (C-N)—795, 830; ν (C-S-C)—746.
C)—1451, 1543, 1623; ν (C-O)—1084, 1164; ν (C-O-C)—1199; ν (C-N)—795, 830; ν (C-S-C)—746. C)—1454, 1527, 1587; ν (C-O)—1113, 1160, 1201; ν (C-O-C)—1330; ν (C-N)—811, 1373; ν (C-S-C)—725.
C)—1454, 1527, 1587; ν (C-O)—1113, 1160, 1201; ν (C-O-C)—1330; ν (C-N)—811, 1373; ν (C-S-C)—725. C)—1457, 1507, 1559; ν (C-O)—1151, 1202; ν (C-O-C)—1327; ν (C-N)—795, 1384; ν (C-S-C)—730.
C)—1457, 1507, 1559; ν (C-O)—1151, 1202; ν (C-O-C)—1327; ν (C-N)—795, 1384; ν (C-S-C)—730. C)—1453, 1545, 1586; ν (C-O)—1070, 1119; ν (C-O-C)—1264, 1312; ν (C-N)—756, 852, 926; ν (C-S-C)—723.
C)—1453, 1545, 1586; ν (C-O)—1070, 1119; ν (C-O-C)—1264, 1312; ν (C-N)—756, 852, 926; ν (C-S-C)—723. C)—1433, 1519, 1560; ν (C-O)—1070, 1119; ν (C-O-C)—1240, 1324; ν (C-N)—754, 781, 918; ν (C-S-C)—711.
C)—1433, 1519, 1560; ν (C-O)—1070, 1119; ν (C-O-C)—1240, 1324; ν (C-N)—754, 781, 918; ν (C-S-C)—711. C)—1424, 1541, 1625; ν (C-O)—1078, 1123; ν (C-O-C)—1211, 1326; ν (C-N)—807; ν (C-S-C)—725.
C)—1424, 1541, 1625; ν (C-O)—1078, 1123; ν (C-O-C)—1211, 1326; ν (C-N)—807; ν (C-S-C)—725.4.1.4. In Silico QSAR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic Engineering of Yeast and Plants for the Production of the Biologically Active Hydroxystilbene, Resveratrol. J. Biomed. Biotechnol. 2012, 2012, 579089. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Okada, K.; Yamane, H.; Iwai, T.; Ohashi, Y. Analysis on Blast Fungus-Responsive Characters of a Flavonoid Phytoalexin Sakuranetin; Accumulation in Infected Rice Leaves, Antifungal Activity and Detoxification by Fungus. Molecules 2014, 19, 11404–11418. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Koga, J.; Shimura, M.; Oshima, K.; Ogawa, N.; Yamauchi, T.; Ogasawara, N. Phytocassanes A, B, C and D, Novel Diterpene Phytoalexins from Rice, Oryza Sativa, L. Tetrahedron 1995, 51, 7907–7918. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; van der Graaff, E.; Roitsch, T. Phytoalexin transgenics in crop protection-Fairy tale with a happy end? Plant Sci. 2012, 195, 54–70. [Google Scholar] [CrossRef]
- Jeandet, P.; Hébrard, C.; Deville, M.-A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in Defense against Pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Jeandet, P. Structure, Chemical Analysis, Biosynthesis, Metabolism, Molecular Engineering, and Biological Functions of Phytoalexins. Molecules 2018, 23, 61. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Ahiahonu, P.W.K. Metabolism and Detoxification of Phytoalexins and Analogs by Phytopathogenic Fungi. Phytochemistry 2005, 66, 391–411. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Clément, C.; Jeandet, P.; Baillieul, F.; Aziz, A. Camalexin accumulation as a component of plant immunity during interactions with pathogens and beneficial microbes. Planta 2022, 255, 116. [Google Scholar] [CrossRef]
- Joubert, A.; Bataille-Simoneau, N.; Campion, C.; Guillemette, T.; Hudhomme, P.; Iacomi-Vasilescu, B.; Leroy, T.; Pochon, S.; Poupard, P.; Simoneau, P. Cell Wall Integrity and High Osmolarity Glycerol Pathways Are Required for Adaptation of Alternaria Brassicicola to Cell Wall Stress Caused by Brassicaceous Indolic Phytoalexins. Cell. Microbiol. 2011, 13, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.P.; Heller, J.; Daskalov, A.; Videira, A.; Glass, N.L. Regulated Forms of Cell Death in Fungi. Front. Microbiol. 2017, 8, 1837. [Google Scholar] [CrossRef] [PubMed]
- Glawischnig, E. Camalexin. Phytochemistry 2007, 68, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Yaya, E.E.; Glawischnig, E. The Phytoalexins from Cultivated and Wild Crucifers: Chemistry and Biology. Nat. Prod. Rep. 2011, 28, 1381–1405. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Baillieul, F.; Aziz, A. Priming of Camalexin Accumulation in Induced Systemic Resistance by Beneficial Bacteria against Botrytis Cinerea and Pseudomonas Syringae Pv. Tomato DC3000. J. Exp. Bot. 2022, 73, 3743–3757. [Google Scholar] [CrossRef]
- Wilson, S.K.; Pretorius, T.; Naidoo, S. Mechanisms of Systemic Resistance to Pathogen Infection in Plants and Their Potential Application in Forestry. BMC Plant Biol. 2023, 23, 404. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced Systemic Resistance (ISR) in Plants: Mechanism of Action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research Progress of Indole Compounds with Potential Antidiabetic Activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A Privileged Scaffold for the Design of Anti-Cancer Agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef]
- Umer, S.M.; Solangi, M.; Khan, K.M.; Saleem, R.S.Z. Indole-Containing Natural Products 2019–2022: Isolations, Reappraisals, Syntheses, and Biological Activities. Molecules 2022, 27, 7586. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.O.; Börger, H. Experimentelle untersuchungen über die Phytophthora resistenz der Kartoffel. Arbeit. Biol. Reichsant Land Forstwirtsch 1940, 23, 189–231. [Google Scholar]
- Jeandet, P. Phytoalexins: Current Progress and Future Prospects. Molecules 2015, 20, 2770–2774. [Google Scholar] [CrossRef]
- Jimenez, L.D.; Ayer, W.A.; Tewari, J.P. Phytoalexins Produced in the Leaves of Capsella Bursa-Pastoris (Shepherd’s Purse). Phytoprotection 1997, 78, 99–103. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Zheng, Q.A.; Sarma-Mamillapalle, V.K. The phytoalexins from Brassicaceae: Structure, biological activity, synthesis and biosynthesis. Nat. Prod. Commun. 2007, 2, 319–330. [Google Scholar] [CrossRef]
- Browne, L.M.; Conn, K.L.; Ayert, W.A.; Tewari, J.P. The Camalexins: New Phytoalexins Produced in the Leaves of Camelina Sativa (Cruciferae). Tetrahedron 1991, 47, 3909–3914. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Khan, A.Q. Biotransformation of the Phytoalexin Camalexin by the Phytopathogen Rhizoctonia Solani. Phytochemistry 2000, 53, 59–69. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Alavi, M.; Abdoli, A. Phytoalexins and Signalling Metabolites Produced in the Wild Crucifer Neslia Paniculata: Camalexins and Arabidopsides. ChemRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Liao, A.; Li, L.; Wang, T.; Lu, A.; Wang, Z.; Wang, Q. Discovery of Phytoalexin Camalexin and Its Derivatives as Novel Antiviral and Antiphytopathogenic-Fungus Agents. J. Agric. Food Chem. 2022, 70, 2554–2563. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Khan, A.Q. Unprecedented Detoxification of the Phytoalexin Camalexin by a Root Rot Pathogen. Bioorg. Med. Chem. Lett. 1997, 7, 2255–2260. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Minic, Z.; Sarma-Mamillapalle, V.K. Synthetic Inhibitors of the Fungal Detoxifying Enzyme Brassinin Oxidase Based on the Phytoalexin Camalexin Scaffold. J. Agric. Food Chem. 2009, 57, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Abdoli, A. Pathogen Inactivation of Cruciferous Phytoalexins: Detoxification Reactions, Enzymes and Inhibitors. RSC Adv. 2017, 7, 23633–23646. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Abdoli, A.; Sarma-Mamillapalle, V.K. Inhibitors of the Detoxifying Enzyme of the Phytoalexin Brassinin Based on Quinoline and Isoquinoline Scaffolds. Molecules 2017, 22, 1345. [Google Scholar] [CrossRef] [PubMed]
- Manasa, K.; Chitra, V. Evaluation of In-vitro Antioxidant Activity of Camalexin—A Novel Anti-Parkinson’s agent. Res. J. Pharm. Tech. 2020, 13, 578–582. [Google Scholar] [CrossRef]
- Egbuonu, A.C.C.; Eneogwe, J. Phytoalexins: Current and Possible Future Applications in Human Health and Diseases Control. Int. J. Mol. Biol. 2018, 3, 107–112. [Google Scholar] [CrossRef]
- Zigová, M.; Michalková, R.; Mojžiš, J. Anticancer Potential of Indole Phytoalexins and Their Analogues. Molecules 2024, 29, 2388. [Google Scholar] [CrossRef]
- Pilatova, M.; Ivanova, L.; Kutschy, P.; Varinska, L.; Saxunova, L.; Repovska, M.; Sarissky, M.; Seliga, R.; Mirossay, L.; Mojzis, J. In vitro toxicity of camalexin derivatives in human cancer and non-cancer cells. Toxicol. Vitro 2013, 27, 939–944. [Google Scholar] [CrossRef]
- Smith, B.; Randle, D.; Mezencev, R.; Thomas, L.S.; Hinton, C.; Odero-Marah, V. Camalexin-Induced Apoptosis in Prostate Cancer Cells Involves Alterations of Expression and Activity of Lysosomal Protease Cathepsin D. Molecules 2014, 19, 3988–4005. [Google Scholar] [CrossRef]
- Chripkova, M.; Drutovic, D.; Pilatova, M.; Mikes, J.; Budovska, M.; Vaskova, J.; Broggini, M.; Mirossay, L.; Mojzis, J. Brassinin and its derivatives as potential anticancer agents. Toxicol. Vitr. 2014, 28, 909–915. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Wu, W.; Yao, S.; Han, X.; He, D.; He, J.; Zheng, G.; Zhao, Y.; Cai, Z.; et al. Camalexin Induces Apoptosis via the ROS-ER Stress-Mitochondrial Apoptosis Pathway in AML Cells. Oxid. Med. Cell. Longev. 2018, 2018, 7426950. [Google Scholar] [CrossRef]
- Zigová, M.; Miškufová, V.; Budovská, M.; Michalková, R.; Mojžiš, J. Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC. Molecules 2024, 29, 1773. [Google Scholar] [CrossRef] [PubMed]
- Chripkova, M.; Zigo, F.; Mojzis, J. Antiproliferative Effect of Indole Phytoalexins. Molecules 2016, 21, 1626. [Google Scholar] [CrossRef] [PubMed]
- Mezencev, R.; Galizzi, M.; Kutschy, P.; Docampo, R. Trypanosoma cruzi: Antiproliferative effect of indole phytoalexins on intracellular amastigotes in vitro. Exp. Parasitol. 2009, 122, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.J.; Roffey, J.R.; Stephens, M.A. Stratford IJ. Synthesis and cytotoxic activity of indolyl thiazoles. Anticancer Drugs. 1997, 8, 489–499. [Google Scholar] [CrossRef]
- Smith, B.A.; Neal, C.L.; Chetram, M.; Vo, B.; Mezencev, R.; Hinton, C.; Odero-Marah, V.A. The phytoalexin camalexin mediates cytotoxicity towards aggressive prostate cancer cells via reactive oxygen species. J. Nat. Med. 2013, 67, 607–618. [Google Scholar] [CrossRef]
- Rogers, E.E.; Glazebrook, J.; Ausubel, F.M. Mode of Action of the Arabidopsis Thaliana Phytoalexin Camalexin and Its Role in Arabidopsis-Pathogen Interactions. Mol. Plant-Microbe Interact. 1996, 9, 748–757. [Google Scholar] [CrossRef]
- Ayer, W.A.; Craw, P.A.; Ma, Y.; Miao, S. Synthesis of camalexin and related phytoalexins. Tetrahedron 1992, 48, 2919–2924. [Google Scholar] [CrossRef]
- Dzurilla, M.; Kutschy, P.; Zaletova, J.; Ruzinsky, M.; Kovacik, V. Synthesis of Camalexin. Molecules 2001, 6, 716–720. [Google Scholar] [CrossRef]
- Tasch, B.O.A.; Antovic, D.; Merkul, E.; Müller, T.J.J. One-Pot Synthesis of Camalexins and 3,3′-Biindoles by the Masuda Borylation-Suzuki Arylation (MBSA) Sequence. Eur. J. Org. Chem. 2013, 2013, 4564–4569. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Abdoli, A. Methoxycamalexins and Related Compounds: Syntheses, Antifungal Activity and Inhibition of Brassinin Oxidase. Bioorganic Med. Chem. 2018, 26, 4461–4469. [Google Scholar] [CrossRef]
- Stremski, Y.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I. Synthesis of Camalexin and Related Analogues. J. Heterocycl. Chem. 2018, 55, 1589–1595. [Google Scholar] [CrossRef]
- Stremski, Y.; Ahmedova, A.; Dołega, A.; Statkova-Abeghe, S.; Kirkova, D. Oxidation Step in the Preparation of Benzocamalexin: The Crystallographic Evidence. Mendeleev Commun. 2021, 31, 824–826. [Google Scholar] [CrossRef]
- Tantak, M.P.; Wang, J.; Singh, R.P.; Kumar, A.; Shah, K.; Kumar, D. 2-(3′-Indolyl)-N-Arylthiazole-4-Carboxamides: Synthesis and Evaluation of Antibacterial and Anticancer Activities. Bioorg. Med. Chem. Lett. 2015, 25, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Liang, J.; Ali, Q.; Wen, W.; Wu, H.; Gao, X.; Gu, Q. 5-Methoxyindole, a Chemical Homolog of Melatonin, Adversely Affects the Phytopathogenic Fungus Fusarium Graminearum. Int. J. Mol. Sci. 2021, 22, 10991. [Google Scholar] [CrossRef]
- Gorunova, O.N. Modification of Heterocycles by Amidoalkylation. Ineos Open 2021, 4, 90–102. [Google Scholar] [CrossRef]
- Mazurkiewicz, R.; Październiok-Holewa, A.; Adamek, J.; Zielińska, K. α-Amidoalkylating agents: Structure, synthesis, reactivity and application. Adv. Heterocycl. Chem. 2014, 111, 43–93. [Google Scholar] [CrossRef]
- Raies, A.B.; Bajic, V.B. In Silico Toxicology: Computational Methods for the Prediction of Chemical Toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef]
- Garralaga, M.P.; Lomba, L.; Zuriaga, E.; Santander, S.; Giner, B. Key Properties for the Toxicity Classification of Chemicals: A Comparison of the REACH Regulation and Scientific Studies Trends. Appl. Sci. 2022, 12, 11710. [Google Scholar] [CrossRef]
- Thamaraikani, T.; Karnam, M.; Velapandian, C. In Silico Docking of Novel Phytoalkaloid Camalexin in the Management of Benomyl Induced Parkinson’s Disease and its In Vivo Evaluation by Zebrafish Model. CNS Neurol. Disord. Drug Targets 2022, 21, 343–353. [Google Scholar] [CrossRef]
- Mounika, K.; Sumedha, J.; Raveena, G.; Shivani, K.; Neelima, K.; Shireesha, S.M.; Anuradha Bai, S. In silico Molecular Properties Predictions of Novel Camalexin Derivatives. Int. J. All Res. Educ. Sci. Methods 2022, 10, 3230–3238. [Google Scholar]
- Martin, T.M. User’s Guide for T.E.S.T. (Toxicity Estimation Software Tool), & Todd. User’s Guide for T. E. S. T. (Toxicity Estimation Software Tool) Version 5.1 A Java Application to Estimate Toxicities and Physical Properties from Molecular Structure. 2020. Available online: https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test. (accessed on 21 February 2024).
- Guilhermino, L.; Diamantino, T.; Carolina Silva, M.; Soares, A.M.V.M. Acute Toxicity Test with Daphnia Magna: An Alternative to Mammals in the Prescreening of Chemical Toxicity? Ecotoxicol. Environ. Saf. 2000, 46, 357–362. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th ed.; Epa/821/R02/012; 2002; pp. 1–266. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/acute-freshwater-and-marine-wet-manual_2002.pdf (accessed on 21 February 2025).
- UE. ANNEX to the Commission Regulation Amending, for the Purpose of Its Adaptation to Technical Progress, the Annex to Regulation (EC) No 440/2008 Laying Down Test Methods Persuant to Regulation (EC) No 1907/2006. REACH 2023. Available online: http://data.europa.eu/eli/reg/2023/464/oj (accessed on 27 March 2023).
- OECD Guidelines for the Testing of Chemicals, Section 2. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Publishing: Paris, France, 2004; pp. 1–12. Available online: https://www.oecd-ilibrary.org/environment/test-no-202-daphnia-sp-acute-immobilisation-test_9789264069947-en (accessed on 6 November 2020).
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, M.P. Overview on the Rule of Five. Curr. Protoc. Pharmacol. 2010, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Perumal, O.P.; Panchagnula, R. Functional Role of P-Glycoprotein in Limiting Peroral Drug Absorption: Optimizing Drug Delivery. Curr. Opin. Chem. Biol. 2006, 10, 367–373. [Google Scholar] [CrossRef]
- Landrum, G. Rdkit documentation. Release 2013, 1, 1–79. [Google Scholar]
- Jana, T.; Sarkar, D.; Ganguli, D.; Mukherjee, S.K.; Mandal, R.S.; Das, S. ABDpred: Prediction of active antimicrobial compounds using supervised machine learning techniques. J. Med. Res. 2024, 159, 78–90. [Google Scholar] [CrossRef]
- US Environmental Protection Agency, Science Advisory Board Review of the Estimation Programs Interface Suite (EPI Suite™), Document EPA-SAB-07-11, US EPA, Washington, DC, USA. 2007. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 27 March 2025).
- Card, M.L.; Gomez-Alvarez, V.; Lee, W.H.; Lynch, D.G.; Orentas, N.S.; Lee, M.T.; Wong, E.M.; Boethling, R.S. History of EPI SuiteTM and Future Perspectives on Chemical Property Estimation in US Toxic Substances Control Act New Chemical Risk Assessments. Environ. Sci. Process. Impacts 2017, 19, 203–212. [Google Scholar] [CrossRef]
- Benfenati, E.; Manganaro, A.; Gini, G. VEGA-QSAR: AI inside a Platform for Predictive Toxicology. CEUR Workshop Proc. 2013, 1107, 21–28. [Google Scholar]
- Liyaqat, T.; Ahmad, T.; Saxena, C. Advancements in Molecular Property Prediction: A Survey of Single and Multimodal Approaches. arXiv 2024, arXiv:2408.09461. [Google Scholar] [CrossRef]
- Vega-Garcia, P.; Lok, C.S.C.; Marhoon, A.; Schwerd, R.; Johann, S.; Helmreich, B. Modelling the environmental fate and behavior of biocides used in façades covered with mortars and plasters and their transformation products. Build. Environ. 2022, 216, 108991. [Google Scholar] [CrossRef]
- Aires-de-Sousa, J. GUIDEMOL: A Python graphical user interface for molecular descriptors based on RDKit. Mol. Inform. 2024, 43. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Schuffenhauer, A. Estimation of Synthetic Accessibility Score of Drug-like Molecules Based on Molecular Complexity and Fragment Contributions. J. Cheminform. 2009, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Roggo, S.; Schuffenhauer, A. Natural Product-Likeness Score and Its Application for Prioritization of Compound Libraries. J. Chem. Inf. Model. 2008, 48, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Durham, E.; Dorr, B.; Woetzel, N.; Staritzbichler, R.; Meiler, J. Solvent Accessible Surface Area Approximations for Rapid and Accurate Protein Structure Prediction. J. Mol. Model. 2009, 15, 1093–1108. [Google Scholar] [CrossRef]
- Gandhi, H.A.; White, A.D. Explaining Structure—Activity Relationships Using Locally Faithful Surrogate Models. Theor. Comput. Chem. 2022, 1–17. [Google Scholar] [CrossRef]
- Ye, X.; Cui, N.; Ou, W.; Liu, D.; Bao, Y.; Ai, B.; Zhou, Y. Explainable optimized 3D-MoRSE descriptors for the power conversion efficiency prediction of molecular passivated perovskite solar cells through machine learning. J. Mater. Chem. A 2024, 12, 26224–26233. [Google Scholar] [CrossRef]
- Estrada, E.; Gutierrez, Y. The Balaban J index in the multidimensional space of generalized topological indices. Generalizations and QSPR improvements. Match Commun. Math. Comput. Chem. 2001, 44, 155–167. [Google Scholar]
- Prasanna, S.; Doerksen, R. Topological Polar Surface Area: A Useful Descriptor in 2D-QSAR. Curr. Med. Chem. 2008, 16, 21–41. [Google Scholar] [CrossRef]
- Le Fèvre, R.J.W. Molecular Refractivity and Polarizability. Adv. Phys. Org. Chem. 1965, 3, 1–90. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The Application of in Silico Drug-Likeness Predictions in Pharmaceutical Research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
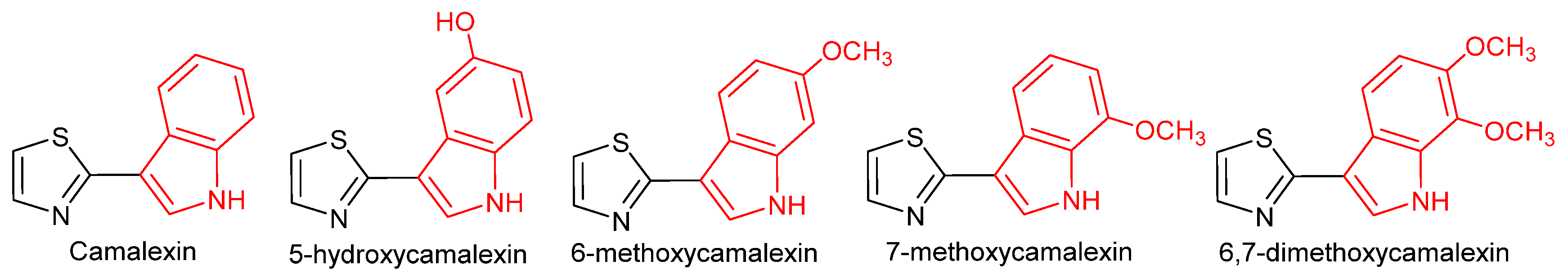
| Natural Phytoalexins | Isolated from |
|---|---|
| Camalexin | Arabidopsis thaliana and Camelina sativa (L.) [26] |
| 5-hydroxycamalexin | Metabolite in Rhizoctonia solani [30] |
| 6-methoxycamalexin | Capsella bursa-pastoris (L.) [26] |
| 7-methoxycamalexin | Neslia paniculata (L.) Desv. [28] |
| 6,7-dimethoxycamalexin | Neslia paniculata (L.) Desv. [28] |
| Product 4a–l | R1 | R2 | R3 | Reaction time, h | Yield, % | Melting Point, °C |
|---|---|---|---|---|---|---|
| 4a | CH3 | H | 4-OCH3 | 30 min | 98 | 175–177 |
| 4b | CH3 | CH3 | 4-OCH3 | 20 min | 97 | 191–193 |
| 4c | H | H | 5-OCH3 | 40 min | 93 | 162–164 [29] ** |
| 4d | CH3 | H | 5-OCH3 | 30 min | 96 | 122–124 |
| 4e | CH3 | CH3 | 5-OCH3 | 20 min | 98 * | 143–145 |
| 4f | H | H | 6-OCH3 | 30 min | 96 | Oil |
| 4g | CH3 | CH3 | 6-OCH3 | 20 min | 93 | 66–68 |
| 4h | CH3 | H | 4,6- (OCH3)2 | 10 min | 92 | 171–173 |
| 4i | CH3 | CH3 | 4,6- (OCH3)2 | 10 min | 96 | 183–185 |
| 4j | H | H | 4-BnO | 30 min | 97 | 54–56 |
| 4k | CH3 | CH3 | 4-BnO | 30 min | 93 | 141–143 |
| 4l | CH3 | CH3 | 5-OH | 10 min | 77 | Oil |
| Product 5a–l | R1 | R2 | R3 | Reaction Time, h | Yield, % | Melting Point, °C |
|---|---|---|---|---|---|---|
| 5a (p2) | CH3 | H | 4-OCH3 | 30 min | 86 | 180–181 |
| 5b (p3) | CH3 | CH3 | 4-OCH3 | 30 min | 80/93 * | 212–214 |
| 5c (p4) | H | H | 5-OCH3 | 15 min | 98 | 112–114 [29,47] ** |
| 5d (p5) | CH3 | H | 5-OCH3 | 30 min | 90 | Oil |
| 5e (p6) | CH3 | CH3 | 5-OCH3 | 30 min | 91 | 169–170 |
| 5f (p7) | H | H | 6-OCH3 | 30 min | 78 | 161–163 [47] ** |
| 5g (p9) | CH3 | CH3 | 6-OCH3 | 20 min | 91 | 163–165 |
| 5h (p11) | CH3 | H | 4,6- (OCH3)2 | 30 min | 68 | 167–169 |
| 5i (p12) | CH3 | CH3 | 4,6- (OCH3)2 | 30 min | 70/80 * | 188–190 |
| 5j (p13) | H | H | 4-BnO | 30 min | 82 | 166–168 |
| 5k (p15) | CH3 | CH3 | 4-BnO | 30 min | 90 | 168–170 |
| 5l (p18) | CH3 | CH3 | 5-OH | 40 min | 62 | 233–235 |
| Compound | MW, g/mol | Oral Rat LD50 mg/kg | T. pyriformis IGC50 (48 h) mg/L | Daphnia magna LC50 (48 h) mg/L | Water Solubility at 25 °C mg/L |
|---|---|---|---|---|---|
| p1 | 230.29 | 631.90 | 27.29 | 2.25 | 25.02 |
| p2 | 244.31 | 2133.02 | 7.60 | 1.71 | 11.34 |
| p3 | 258.34 | 3723.13 | 8.03 | 1.81 | 11.99 |
| p4 | 230.29 | 2010.54 | 27.29 | 2.25 | 22.13 |
| p5 | 244.31 | 2133.02 | 7.60 | 1.71 | 11.34 |
| p6 | 258.34 | 3723.13 | 8.03 | 11.71 | 11.99 |
| p7 | 230.29 | 2010.54 | 27.29 | 2.25 | 22.13 |
| p8 | 244.31 | 2646.44 | 7.60 | 1.71 | 61.85 |
| p9 | 258.34 | 2798.39 | 8.03 | 11.71 | 11.99 |
| p10 | 260.31 | 2819.73 | 8.09 | 0.84 | 65.90 |
| p11 | 274.34 | 3550.89 | 8.53 | 12.44 | 18.98 |
| p12 | 288.37 | 3999.40 | 8.97 | 13.07 | 19.95 |
| p13 | 306.38 | 2552.52 | 8.10 | 2.15 | 21.20 |
| p14 | 320.41 | 1519.66 | 6.53 | 2.24 | 22.17 |
| p15 | 334.44 | 524.03 | 6.81 | 15.16 | 23.14 |
| p16 | 216.26 | 1657.10 | 25.63 | 2.11 | 38.28 |
| p17 | 230.29 | 1764.60 | 27.29 | 4.47 | 58.30 |
| p18 | 244.31 | 2133.02 | 7.60 | 0.78 | 61.85 |
| Compound | Wildman & Crippen (RDkit) | Wildman & Crippen (PadelPy) | Wildman & Crippen Julia | EPI Suite | VEGA |
|---|---|---|---|---|---|
| p1 | 2.79 | 3.31 | 3.3 | 2.9 | 2.9 |
| p2 | 3.09 | 3.8 | 3.6 | 3.45 | 3.45 |
| p3 | 3.4 | 3.34 | 3.91 | 4 | 4 |
| p4 | 2.79 | 3.31 | 3.3 | 2.9 | 2.9 |
| p5 | 3.09 | 3.8 | 3.6 | 3.45 | 3.45 |
| p6 | 3.4 | 3.34 | 3.91 | 4 | 4 |
| p7 | 2.79 | 3.31 | 3.3 | 2.9 | 2.9 |
| p8 | 3.09 | 3.8 | 3.6 | 3.45 | 3.45 |
| p9 | 3.4 | 3.34 | 3.91 | 4 | 4 |
| p10 | 2.79 | 3.32 | 3.3 | 3 | 3 |
| p11 | 3.1 | 3.81 | 3.61 | 3.53 | 3.53 |
| p12 | 3.41 | 3.35 | 3.92 | 4.1 | 4.08 |
| p13 | 4.36 | 4.85 | 4.87 | 4.6 | 4.61 |
| p14 | 4.87 | 4.11 | 4.87 | 4.9 | 4.54 |
| p15 | 5.18 | 4.13 | 5.18 | 5.45 | 5.1 |
| p16 | 2.48 | 2.88 | 3 | 2.34 | 2.34 |
| p17 | 2.79 | 3.37 | 3.3 | 2.9 | 2.9 |
| p18 | 3.1 | 2.91 | 3.61 | 3.43 | 3.43 |
| Compound | Air | Water | Soil | Sediments |
|---|---|---|---|---|
| p1 | 0.05 | 37.5 | 75 | 337.5 |
| p2 | 0.05 | 37.5 | 75 | 337.5 |
| p3 | 0.05 | 37.5 | 75 | 337.5 |
| p4 | 0.05 | 37.5 | 75 | 337.5 |
| p5 | 0.05 | 37.5 | 75 | 337.5 |
| p6 | 0.05 | 37.5 | 75 | 337.5 |
| p7 | 0.05 | 37.5 | 75 | 337.5 |
| p8 | 0.05 | 37.5 | 75 | 337.5 |
| p9 | 0.05 | 37.5 | 75 | 337.5 |
| p10 | 0.05 | 37.5 | 75 | 337.5 |
| p11 | 0.05 | 37.5 | 75 | 337.5 |
| p12 | 0.05 | 37.5 | 75 | 337.5 |
| p13 | 0.05 | 37.5 | 75 | 337.5 |
| p14 | 0.3 | 37.5 | 75 | 337.5 |
| p15 | 0.33 | 60 | 120 | 541.6 |
| p16 | 0.05 | 15 | 30 | 135 |
| p17 | 0.05 | 37.5 | 75 | 337.5 |
| p18 | 0.05 | 37.5 | 75 | 337.5 |
| Compound | SA Score | NP Score | SPS | BertzCT | AvgIpc | BalabanJ |
|---|---|---|---|---|---|---|
| p1 | 3.127 | −0.6 | 12.93 | 527.17 | 2.95 | 2.16 |
| p2 | 3.047 | −0.76 | 12.94 | 581.83 | 2.93 | 2.13 |
| p3 | 3.084 | −0.67 | 12.94 | 603.98 | 2.93 | 2.13 |
| p4 | 3.126 | −0.73 | 12.93 | 527.17 | 2.95 | 2.09 |
| p5 | 3.046 | −0.89 | 12.94 | 581.83 | 2.93 | 2.07 |
| p6 | 3.083 | −0.79 | 12.94 | 603.98 | 2.92 | 2.07 |
| p7 | 3.082 | −0.59 | 12.93 | 527.17 | 2.95 | 2.04 |
| p8 | 3.004 | −0.76 | 12.94 | 581.83 | 2.95 | 2.03 |
| p9 | 3.043 | −0.67 | 12.94 | 603.98 | 2.92 | 2.03 |
| p10 | 3.157 | −0.35 | 12.66 | 582.75 | 3.04 | 2.13 |
| p11 | 3.089 | −0.5 | 12.68 | 638.05 | 3.02 | 2.12 |
| p12 | 3.126 | −0.44 | 12.7 | 660.8 | 3.01 | 2.12 |
| p13 | 2.749 | −0.74 | 12.45 | 789.31 | 3.22 | 1.64 |
| p14 | 3.079 | −0.59 | 15.6 | 833.48 | 3.19 | 1.63 |
| p15 | 3.139 | −0.56 | 15.5 | 858.15 | 3.17 | 1.65 |
| p16 | 3.368 | −0.24 | 13.2 | 512.97 | 2.82 | 2.13 |
| p17 | 3.269 | −0.43 | 13.18 | 567.47 | 2.83 | 2.1 |
| p18 | 3.296 | −0.36 | 13.7 | 589.46 | 2.82 | 2.11 |
| Compound | QED | Fraction Csp3 | Labute ASA | Mol MR | Max Estate Index | Min Estate Index | TPSA |
|---|---|---|---|---|---|---|---|
| p1 | 0.79 | 0.08 | 97.41 | 63.98 | 5.36 | 0.84 | 36.22 |
| p2 | 0.81 | 0.15 | 103.77 | 68.71 | 5.4 | 0.84 | 36.22 |
| p3 | 0.82 | 0.21 | 110.14 | 73.45 | 5.42 | 0.85 | 36.22 |
| p4 | 0.79 | 0.08 | 97.41 | 63.98 | 5.21 | 0.84 | 36.22 |
| p5 | 0.81 | 0.15 | 103.77 | 68.71 | 5.24 | 0.84 | 36.22 |
| p6 | 0.82 | 0.21 | 110.14 | 73.45 | 5.26 | 0.84 | 36.22 |
| p7 | 0.79 | 0.08 | 97.41 | 63.98 | 5.16 | 0.82 | 36.22 |
| p8 | 0.81 | 0.15 | 103.77 | 68.71 | 5.18 | 0.83 | 36.22 |
| p9 | 0.82 | 0.21 | 110.14 | 73.45 | 5.2 | 0.83 | 36.22 |
| p10 | 0.85 | 0.15 | 108.88 | 70.53 | 5.42 | 0.73 | 45.45 |
| p11 | 0.96 | 0.21 | 115.25 | 75.27 | 5.45 | 0.74 | 45.45 |
| p12 | 0.96 | 0.26 | 121.61 | 80 | 5.48 | 0.74 | 45.45 |
| p13 | 0.71 | 0.05 | 132.46 | 88.2 | 6.03 | 0.54 | 36.22 |
| p14 | 0.68 | 0.15 | 138.86 | 94.06 | 6.09 | 0.093 | 34.48 |
| p15 | 0.65 | 0.2 | 145.23 | 98.8 | 6.12 | 0.087 | 34.48 |
| p16 | 0.79 | 0 | 90.72 | 59.09 | 9.42 | 0.25 | 47.22 |
| p17 | 0.81 | 0.08 | 97.09 | 63.83 | 9.48 | 0.25 | 47.22 |
| p18 | 0.83 | 0.15 | 103.45 | 68.56 | 9.53 | 0.26 | 47.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachvarova, M.; Stremski, Y.; Ganchev, D.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I. An Efficient Method for the Synthesis and In Silico Study of Novel Oxy-Camalexins. Molecules 2025, 30, 2049. https://doi.org/10.3390/molecules30092049
Bachvarova M, Stremski Y, Ganchev D, Statkova-Abeghe S, Angelov P, Ivanov I. An Efficient Method for the Synthesis and In Silico Study of Novel Oxy-Camalexins. Molecules. 2025; 30(9):2049. https://doi.org/10.3390/molecules30092049
Chicago/Turabian StyleBachvarova, Maria, Yordan Stremski, Donyo Ganchev, Stela Statkova-Abeghe, Plamen Angelov, and Iliyan Ivanov. 2025. "An Efficient Method for the Synthesis and In Silico Study of Novel Oxy-Camalexins" Molecules 30, no. 9: 2049. https://doi.org/10.3390/molecules30092049
APA StyleBachvarova, M., Stremski, Y., Ganchev, D., Statkova-Abeghe, S., Angelov, P., & Ivanov, I. (2025). An Efficient Method for the Synthesis and In Silico Study of Novel Oxy-Camalexins. Molecules, 30(9), 2049. https://doi.org/10.3390/molecules30092049