Impact of Photoselective Nets on Phenolic Composition and Antioxidant Capacity in Different Apple Cultivars Under the Same Edaphoclimatic Conditions
Abstract
1. Introduction
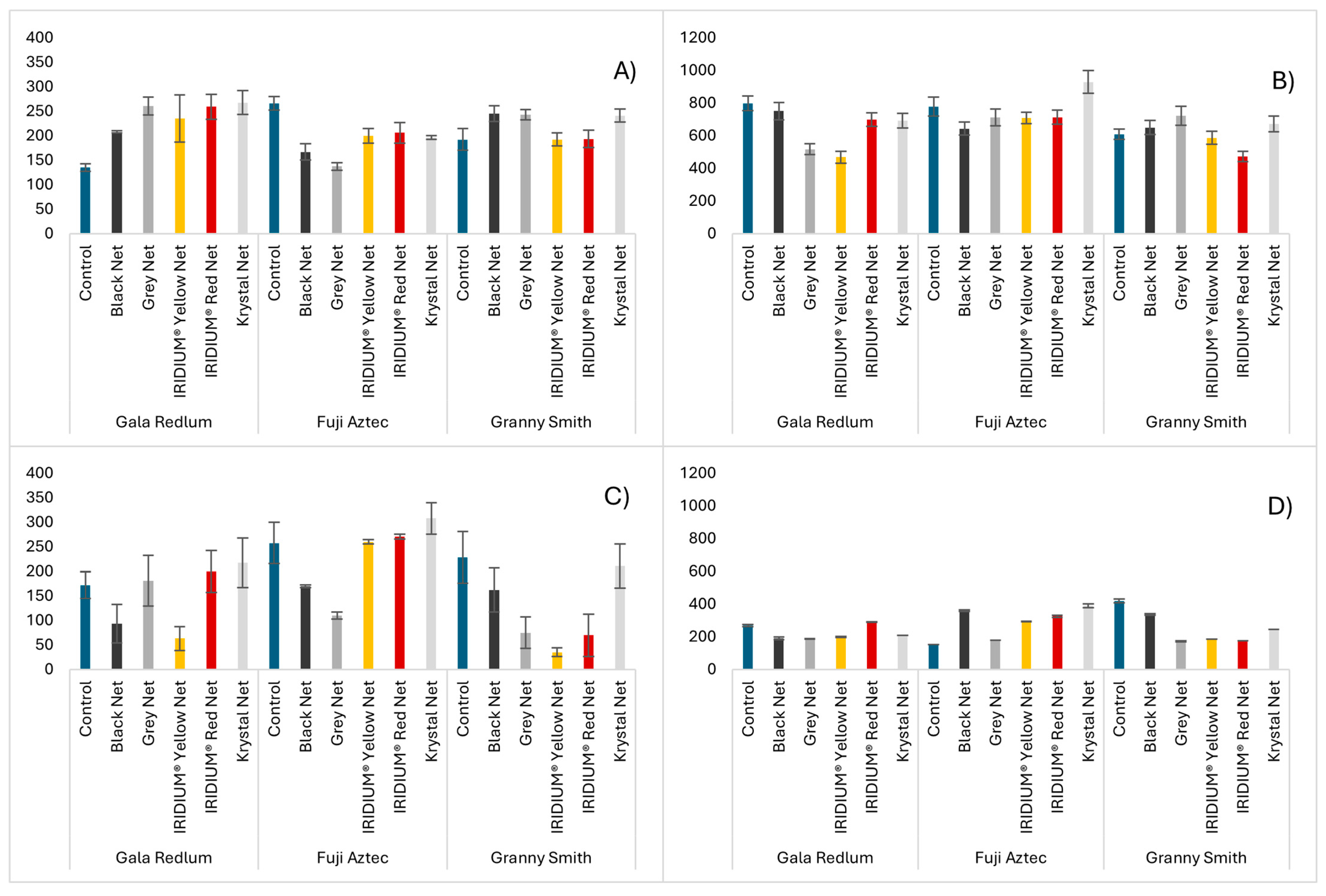
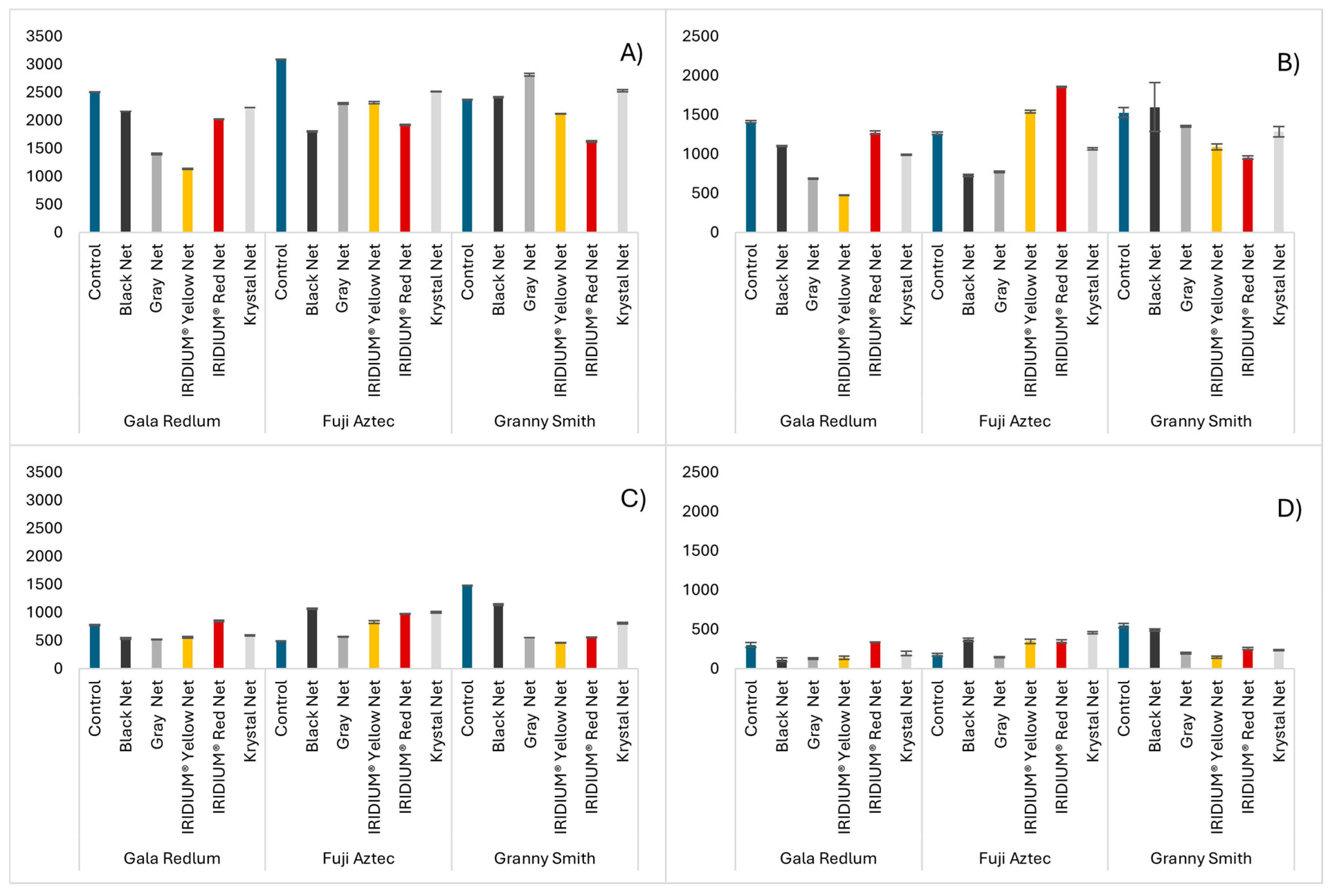
2. Results and Discussion
2.1. Liquid Chromatography
2.2. Antioxidant Capacity Assays
3. Materials and Methods
3.1. Reagents
3.2. Apple Cultivars and Photoselective Nets
3.3. Preparation of the Samples
3.4. Chromatography Instrumentation
3.5. Antioxidant Capacity Assays
- β-Carotene bleaching assay
- 2.
- DPPH radical scavenging assay
- 3.
- Total phenolic content assay
- 4.
- Total flavonoid content assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mavric-Scholze, E.; Simijonović, D.; Avdović, E.; Milenković, D.; Šaćirović, S.; Ćirić, A.; Marković, Z. Comparative Analysis of Antioxidant Activity and Content of (Poly)Phenolic Compounds in Cabernet Sauvignon and Merlot Wines of Slovenian and Serbian Vineyards. Food Chem. X 2025, 25, 102108. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, T.L.C.; Da Silva, C.; Bicas, T.C.; Ayres, B.R.B.; Zanchet, E.R.; Marafon, F.; Da Silva, A.P.; Carpes, S.T.; Bagatini, M.D.; Ascari, J.; et al. Antihyperglycemic Activity and Bioguided Isolation of Phenolic Compounds with Antioxidant and Cytotoxic Properties from Syzygium Malaccense Leaves. Fitoterapia 2025, 181, 106357. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Yan, J.; Liu, S.; Chen, S.; Zou, L.; Yang, Y.; He, L.; Li, S.; Liu, A.; Zhao, K. Extraction, Isolation and Identification of Four Phenolic Compounds from Pleioblastus Amarus Shoots and Their Antioxidant and Anti-Inflammatory Properties in Vitro. Food Chem. 2022, 374, 131743. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.A.C.D.; Levit, R.; Beres, C.; Bedani, R.; De Moreno De LeBlanc, A.; Saad, S.M.I.; LeBlanc, J.G. Tropical Fruit By-Products Water Extracts as Sources of Soluble Fibres and Phenolic Compounds with Potential Antioxidant, Anti-Inflammatory, and Functional Properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Almarfadi, O.M.; Siddiqui, N.A.; Shahat, A.A.; Fantoukh, O.I.; El Gamal, A.A.; Raish, M.; Bari, A.; Iqbal, M.; Alqahtani, A.S. Isolation of a Novel Isoprenylated Phenolic Compound and Neuroprotective Evaluation of Dodonaea Viscosa Extract against Cerebral Ischaemia–Reperfusion Injury in Rats. Saudi Pharm. J. 2024, 32, 101898. [Google Scholar] [CrossRef]
- Collins, A.; Santhakumar, A.B.; Francis, N.; Blanchard, C.; Chinkwo, K. Impact of Sorghum (Sorghum bicolor L. Moench) Phenolic Compounds on Cancer Development Pathways. Food Biosci. 2024, 59, 104177. [Google Scholar] [CrossRef]
- Millán-Laleona, A.; Cebollada, P.; Caprioli, G.; Piatti, D.; Maggi, F.; Pina, A.; Gómez-Rincón, C.; López, V. Valorization of Local Regional Apple (Malus domestica Borkh.) Cultivars versus Commercial Samples from Spain: Phenolic Compounds by HPLC-MS/MS, Cytotoxicity and Biological Potential on Nitric Oxide Radicals and Lipoxygenase Inhibition. J. Funct. Foods 2025, 124, 106631. [Google Scholar] [CrossRef]
- Rebolledo-Leiva, R.; Estévez, S.; Hernández, D.; Feijoo, G.; Moreira, M.T.; González-García, S. Environmental Insights of Bioethanol Production and Phenolic Compounds Extraction from Apple Pomace-Based Biorefinery. Clean. Circ. Bioecon. 2024, 9, 100125. [Google Scholar] [CrossRef]
- Zhang, J.; Pérez-Álvarez, E.P.; Liu, P.; Murillo-Peña, R.; Bordiga, M.; Sun, X.; Locatelli, M.; Coïsson, J.D.; Martínez-Vidaurre, J.M.; Corke, H.; et al. Identification and Influence of Soil Type and Urea Foliar Application on Phenolic Compounds in the Red Musts and Wines of Tempranillo Variety. Food Humanit. 2025, 4, 100506. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 29 January 2025).
- Elzebroek, A.T.G. Guide to Cultivated Plants; CABI: Wallingford, UK, 2008; ISBN 978-1-84593-356-2. [Google Scholar]
- Ma, Y.; Ban, Q.; Shi, J.; Dong, T.; Jiang, C.-Z.; Wang, Q. 1-Methylcyclopropene (1-MCP), Storage Time, and Shelf Life and Temperature Affect Phenolic Compounds and Antioxidant Activity of ‘Jonagold’ Apple. Postharvest Biol. Technol. 2019, 150, 71–79. [Google Scholar] [CrossRef]
- Fang, Z.; Lin-Wang, K.; Lin, Y.; Espley, R.V. Metabolomic and Transcriptomic Analyses Provide Insights into Temperature and Light Regulated Anthocyanin Accumulation in Flesh of ‘Furongli’ Plum (Prunus salicina Lindl.). Postharvest Biol. Technol. 2025, 221, 113326. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Zhu, W.; Weng, Z.; Li, F.; Zhang, Y.; Wu, H.; Zhou, K.; Strid, Å.; Qian, M. Metabolomic and Transcriptomic Analyses Reveal the Regulation Mechanism of Postharvest Light-Induced Phenolics Accumulation in Mango Peel. Lebensm. Wiss. Technol. 2024, 213, 117050. [Google Scholar] [CrossRef]
- Smrke, T.; Grohar, M.C.; Indihar, E.; Veberic, R.; Jakopic, J. Does Photoselective Netting Influence Ripening, Maturity Parameters and Chemical Composition of Highbush Blueberry (Vaccinium corymbosum L.) Fruit? Sci. Hortic. 2024, 337, 113555. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Chen, K.; Zhang, J.; Xue, T.; Cheng, Z.; Zhang, B.; Zhang, K.; Fang, Y. The Roles of Different Photoselective Nets in the Targeted Regulation of Metabolite Accumulation, Wine Aroma and Sensory Profiles in Warm Viticulture Regions. Food Chem. 2022, 396, 133629. [Google Scholar] [CrossRef]
- Boini, A.; Casadio, N.; Bresilla, K.; Perulli, G.D.; Manfrini, L.; Grappadelli, L.C.; Morandi, B. Early Apple Fruit Development under Photoselective Nets. Sci. Hortic. 2022, 292, 110619. [Google Scholar] [CrossRef]
- Boini, A.; Bresilla, K.; Perulli, G.D.; Manfrini, L.; Corelli Grappadelli, L.; Morandi, B. Photoselective Nets Impact Apple Sap Flow and Fruit Growth. Agric. Water Manag. 2019, 226, 105738. [Google Scholar] [CrossRef]
- Teixeira, J.D.; Soares Mateus, A.R.; Sanchez, C.; Parpot, P.; Almeida, C.; Sanches Silva, A. Antioxidant Capacity and Phenolics Profile of Portuguese Traditional Cultivars of Apples and Pears and Their By-Products: On the Way to Newer Applications. Foods 2023, 12, 1537. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-Lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Purves, R.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Enhancement of Phenolic Antioxidants in Industrial Apple Waste by Fermentation with Aspergillus Spp. Biocatal. Agric. Biotechnol. 2020, 25, 101562. [Google Scholar] [CrossRef]
- Yang, J.; Chen, R.; Wang, C.; Li, C.; Ye, W.; Zhang, Z.; Wang, S. A Widely Targeted Metabolite Modificomics Strategy for Modified Metabolites Identification in Tomato. J. Integr. Plant Biol. 2024, 66, 810–823. [Google Scholar] [CrossRef]
- Bastías, R.M.; Ruíz, K.; Manfrini, L.; Pierpaoli, E.; Zibordi, M.; Morandi, B.; Losciale, P.; Torrigiani, P.; Corelli-Grappadelli, L. Effects of Photoselective Nets on Phenolic Composition in Apple Fruits. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 939, Lisbon, Portugal, 22 August 2010; pp. 77–83. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Häggman, H.; Jaakola, L. Light-Controlled Flavonoid Biosynthesis in Fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Phenylpropanoid Metabolites and Expression of Key Genes Involved in Anthocyanin Biosynthesis in the Shaded Peel of Apple Fruit in Response to Sun Exposure. Plant Physiol. Biochem. 2013, 69, 54–61. [Google Scholar] [CrossRef]
- Ravaglia, D.; Espley, R.V.; Henry-Kirk, R.A.; Andreotti, C.; Ziosi, V.; Hellens, R.P.; Costa, G.; Allan, A.C. Transcriptional Regulation of Flavonoid Biosynthesis in Nectarine (Prunus persica) by a Set of R2R3 MYB Transcription Factors. BMC Plant Biol 2013, 13, 68. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, M.; Wu, R.; Niu, Q.; Teng, Y.; Zhang, D. Postharvest Pigmentation in Red Chinese Sand Pears (Pyrus pyrifolia Nakai) in Response to Optimum Light and Temperature. Postharvest Biol. Technol. 2014, 91, 64–71. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Ran, J.; Zhang, T.; Sun, H.; Dong, M.; Zhang, Z.; Zheng, H. Variation in Phenolic Compounds and Antioxidant Activity in Apple Seeds of Seven Cultivars. Saudi J. Biol. Sci. 2016, 23, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Malik, W.; Maqsood, M.; Atique, I.; Qamar, M.T. Study of Anti-Diabetic, Beta-Carotene-Bleaching Inhibiting and Iron Chelating Properties of Carissa Opaca Root Extracts. Braz. J. Pharm. Sci. 2022, 58, e18628. [Google Scholar] [CrossRef]
- Wani, S.G.; Shafi, F.; Jabeen, A.; Malik, M.A. Physicochemical, Antioxidant and Antimicrobial Properties of Peel, Pulp and Seeds of Different Pear Cultivars. Food Humanit. 2025, 4, 100521. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Ahmed, T.; Mithun, M.U.K.; Rashid, M.; Rana, M.R. Optimising Ultrasound-Assisted Extraction Conditions for Maximising Phenolic, Flavonoid Content and Antioxidant Activity in Hog Plum Peel and Seed: A Response Surface Methodology Approach. J. Agric. Food Res. 2024, 18, 101312. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Abbasnia Zare, S.K.; Sedaghathoor, S.; Padasht Dahkaei, M.-N.; Hashemabadi, D. The Effect of Light Variations by Photoselective Shade Nets on Pigments, Antioxidant Capacity, and Growth of Two Ornamental Plant Species: Marigold (Calendula officinalis L.) and Violet (Viola tricolor). Cogent Food Agric. 2019, 5, 1650415. [Google Scholar] [CrossRef]
- Zare, S.K.A.; Sedaghathoor, S.; Dahkaei, M.N.P. The Effect of Different Colored Netting on Quantitative and Qualitative Traits of Two Foliage Plant Species (Codiaeum variegatum and Aglaonema commutatum). Adv. Hortic. Sci. 2020, 34, 25–34. [Google Scholar]
- Miller, H.E. A Simplified Method for the Evaluation of Antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Sanches Silva, A.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active Polylactic Acid Film Incorporated with Green Tea Extract: Development, Characterization and Effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant Activities of Rosemary (Rosmarinus officinalis L.) Extract, Blackseed (Nigella sativa L.) Essential Oil, Carnosic Acid, Rosmarinic Acid and Sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.H.; Andrade, M.A.; Séndon, R.; Silva, A.S.; Ramos, F.; Vilarinho, F.; Khwaldia, K.; Barbosa-Pereira, L. Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods 2021, 10, 272. [Google Scholar] [CrossRef]

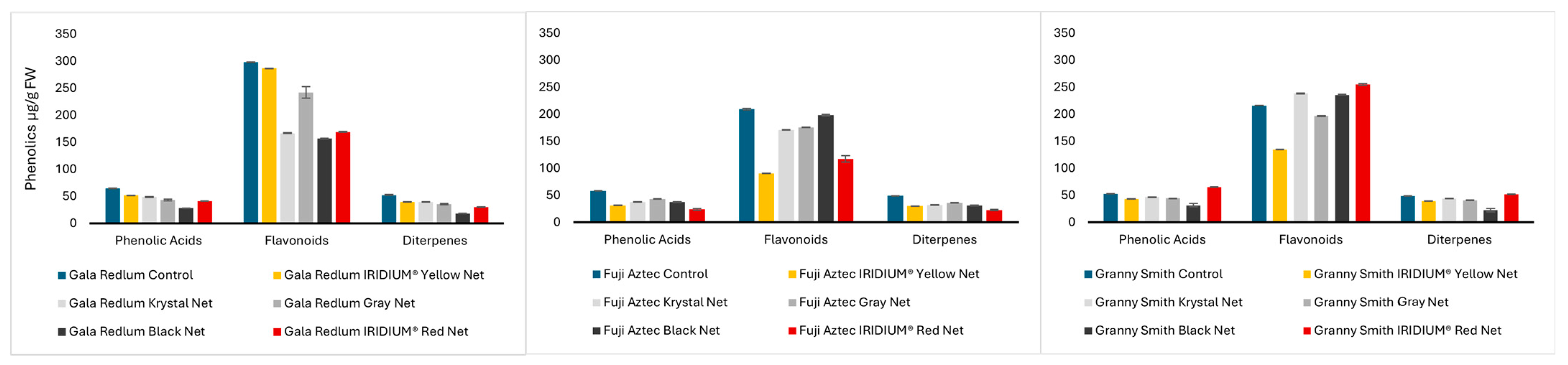
| Phenolic Compound | Gala redlum | Fuji aztec | Granny smith | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Yellow | Krystal | Gray | Black | Red | Control | Yellow | Krystal | Gray | Black | Red | Control | Yellow | Krystal | Gray | Black | Red | |
| 4-Hydroxybenzoic Acid | 4.957 a + 0.005 | 3.717 bc + 0.008 | 3.205 c + 0.023 | 3.538 bc + 0.014 | 2.072 d + 0.006 | 3.844 b + 0.060 | 1.350 a + 0.029 | n.d. | n.d. | <LOQ | n.d. | n.d. | 1.481 a + 0.002 | 1.770 a + 0.026 | 0.869 b + 0.030 | 1.572 a + 0.001 | 0.922 b + 0.016 | 1.656 a + 0.044 |
| 4-O-Caffeoylquinic Acid | 42.57 a + 0.051 | 21.32 c + 0.032 | 24.01 b + 0.044 | 24.24 b + 0.051 | 9.914 d + 0.055 | 24.93 b + 0.141 | 15.46 b + 0.448 | 7.528 c + 0.009 | 10.87 c + 0.009 | 20.13 a + 0.041 | 10.01 c + 0.020 | 9.979 c + 0.033 | 1.548 b + 0.012 | 2.575 ab + 0.000 | 1.492 b + 0.052 | 2.036 b + 0.001 | <LOQ | 3.979 a + 0.160 |
| Carnosic Acid | n.d. | n.d. | n.d. | <LOQ | <LOQ | <LOQ | n.d. | <LOQ | <LOQ | n.d. | <LOQ | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Catechin | 6.086 a + 0.024 | 4.716 bc + 0.006 | 3.874 d + 0.014 | 4.255 cd + 0.019 | 1.870 e + 0.004 | 5.123 b + 0.048 | 1.581 ab + 0.042 | <LOQ | 1.243 bc + 0.001 | 1.737 a + 0.016 | 1.363 ab + 0.001 | 0.934 c + 0.002 | 3.692 ab + 0.031 | 4.634 a + 0.003 | 2.369 b + 0.065 | 4.295 a + 0.013 | 2.357 b + 0.007 | 4.688 a + 0.221 |
| Chlorogenic Acid | 42.66 a + 0.051 | 21.41 c + 0.030 | 23.84 b + 0.043 | 24.08 b + 0.051 | 9.987 d + 0.055 | 25.01 b + 0.144 | 15.55 b + 0.444 | 7.546 c + 0.003 | 10.93 c + 0.009 | 20.20 a + 0.042 | 10.07 c + 0.020 | 10.03 c + 0.032 | 1.577 b + 0.010 | 2.590 ab + 0.002 | 1.537 b + 0.053 | 2.066 b + 0.003 | <LOQ | 3.966 a + 0.156 |
| Cyanidin-3-Glucoside | 69.59 a + 0.459 | 55.86 b + 0.057 | 44.70 c + 0.339 | 30.29 e + 0.057 | 27.65 e + 0.337 | 38.98 d + 0.195 | 44.95 b + 0.205 | 26.71 d + 0.106 | 55.03 a + 0.416 | 25.21 d + 0.186 | 36.37 c + 0.439 | 19.60 e + 0.107 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Epicatechin | 49.72 a + 0.042 | 37.43 bc + 0.321 | 33.47 d + 0.214 | 34.87 cd + 0.041 | 20.84 e + 0.036 | 39.52 b + 0.211 | 31.26 a + 0.456 | 14.50 cd + 0.106 | 18.11 bc + 0.096 | 18.70 b + 0.033 | 19.29 b + 0.011 | 13.82 d + 0.180 | 22.12 bc + 0.283 | 22.93 b + 0.097 | 16.32 d + 0.117 | 19.46 c + 0.080 | 13.82 d + 0.018 | 30.75 a + 0.173 |
| Gallic Acid | 3.858 d + 0.006 | 4.249 ab + 0.011 | 4.018 cd + 0.003 | 4.148 bc + 0.010 | 4.352 a + 0.002 | 3.900 d + 0.011 | 4.356 a + 0.062 | 1.645 b + 0.003 | 1.340 b + 0.003 | 1.482 b + 0.028 | 1.596 b + 0.026 | 1.887 b + 0.004 | 2.808 c + 0.021 | 3.034 c + 0.004 | 2.682 c + 0.014 | 2.814 c + 0.065 | 4.190 b + 0.031 | 6.160 a + 0.002 |
| Isorhamnetin-3-O-Glucoside | 0.884 a + 0.001 | <LOQ | <LOQ | 0.791 b + 0.004 | <LOQ | <LOQ | <LOQ | n.d. | <LOQ | n.d. | n.d. | n.d. | 1.149 a + 0.005 | 1.009 ab + 0.012 | 0.804 c + 0.003 | 0.913 bc + 0.011 | 0.775 c + 0.014 | 1.145 a + 0.017 |
| Kaempferol-3-O-β-Rutinoside | 22.23 a + 0.147 | 6.353 e + 0.022 | 8.213 d + 0.038 | 18.43 b + 0.027 | 12.89 c + 0.005 | 9.298 d + 0.055 | 17.02 b + 0.049 | 4.941 d + 0.011 | 13.53 bc + 0.748 | 51.47 a + 0.278 | 7.150 d + 0.042 | 10.14 cd + 0.050 | 27.53 bc + 0.155 | 34.57 b + 1.286 | 86.97 a + 1.402 | 8.657 d + 0.104 | 14.50 cd + 0.267 | 13.65 cd + 0.590 |
| o-Coumaric Acid | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| p-Coumaric Acid | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Phloridzin | 21.62 a + 0.125 | 12.65 d + 0.057 | 15.60 c + 0.020 | 16.15 c + 0.039 | 6.760 e + 0.005 | 19.44 b + 0.026 | 25.21 ab + 0.130 | 19.76 c + 0.030 | 24.32 b + 0.037 | 26.91 a + 0.001 | 21.55 c + 0.155 | 17.94 d + 0.090 | 6.242 cd + 0.044 | 6.037 cd + 0.073 | 8.724 a + 0.002 | 6.790 bc + 0.038 | 5.406 d + 0.046 | 7.296 b + 0.057 |
| Quercetin | 43.44 d + 0.261 | 88.83 c + 1.200 | 118.7 b + 0.263 | 153.2 a + 1.586 | 109.5 b + 0.285 | 105.8 b + 1.128 | 142.9 a + 0.162 | 92.28 b + 1.736 | 129.4 a + 1.800 | 81.41 b + 0.213 | 93.75 b + 0.122 | 84.15 b + 0.317 | 90.70 c + 0.388 | 125.2 a + 0.728 | 121.0 ab + 0.000 | 82.12 cd + 1.120 | 74.85 d + 0.140 | 109.0 b + 0.689 |
| Quercetin-3-β-D-Glucoside | 141.5 a + 0.684 | 63.11 d + 0.101 | 88.10 b + 0.080 | 147.4 a + 0.402 | 70.26 c + 0.044 | 72.34 c + 0.143 | 140.3 a + 0.438 | 69.72 c + 0.063 | 104.9 b + 1.949 | 86.51 c + 0.804 | 78.22 c + 0.147 | 73.61 c + 0.454 | 80.95 cd + 0.678 | 107.1 b + 1.205 | 144.6 a + 1.520 | 63.82 de + 0.508 | 60.95 e + 0.197 | 87.23 c + 1.099 |
| Quercitrin | 68.30 a + 0.995 | 55.08 b + 0.006 | 43.34 c + 0.043 | 29.09 d + 0.062 | 23.32 d + 0.037 | 37.79 c + 0.147 | 45.13 b + 0.223 | 25.77 d + 0.086 | 51.28 a + 0.147 | 24.22 d + 0.361 | 33.60 c + 0.245 | 18.14 e + 0.034 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rutin | 13.72 a + 0.063 | 2.234 d + 0.006 | 3.586 c + 0.012 | 11.90 b + 0.056 | 3.077 c + 0.007 | 3.518 c + 0.006 | 18.96 a + 0.028 | 4.784 c + 0.005 | 20.63 a + 0.089 | 22.37 a + 0.123 | 8.264 bc + 0.008 | 10.30 b + 0.169 | 18.84 c + 0.103 | 24.88 b + 0.275 | 52.00 a + 0.304 | 8.103 e + 0.080 | 12.03 d + 0.042 | 12.69 d + 0.074 |
| Taxifolin | 1.455 b + 0.006 | <LOQ | 1.134 c + 0.014 | 1.746 a + 0.001 | 1.372 b + 0.006 | 1.106 c + 0.000 | 1.915 a + 0.017 | <LOQ | 1.647 b + 0.003 | <LOQ | 1.500 b + 0.002 | 1.454 b + 0.015 | 1.338 c + 0.000 | <LOQ | 1.935 a + 0.005 | <LOQ | <LOQ | 1.629 b + 0.002 |
| Vanilic Acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Phenolic Compound | Gala redlum | Fuji aztec | Granny smith | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Yellow | Krystal | Gray | Black | Red | Control | Yellow | Krystal | Gray | Black | Red | Control | Yellow | Krystal | Gray | Black | Red | |
| 4-Hydroxybenzoic Acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | n.d. | n.d. | n.d. | <LOQ | n.d. | n.d. | 1.390 b + 0.009 | 1.170 c + 0.003 | <LOQ | 1.020 d + 0.007 | 0.921 d + 0.007 | 2.080 a + 0.011 |
| 4-O-Caffeoylquinic Acid | 52.44 a + 0.557 | 39.76 b + 0.181 | 39.72 b + 0.446 | 35.42 b + 1.279 | 18.44 c + 0.062 | 30.35 b + 0.266 | 49.35 a + 0.079 | 30.29 bc + 0.080 | 32.09 bc + 0.217 | 36.36 b + 0.020 | 31.16 bc + 0.730 | 22.51 c + 1.075 | 48.66 a + 0.128 | 39.39 ab + 0.010 | 44.08 ab + 0.036 | 41.14 ab + 0.079 | 22.48 b + 2.932 | 52.29 a + 0.205 |
| Carnosic Acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Catechin | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.845 c + 0.001 | 0.868 c + 0.008 | 1.040 b + 0.005 | 1.270 a + 0.004 | <LOQ | <LOQ | 3.900 b + 0.027 | 3.570 c + 0.008 | 1.640 e + 0.003 | 3.080 d + 0.013 | 1.500 e + 0.011 | 4.640 a + 0.010 |
| Chlorogenic Acid | 52.52 a + 0.559 | 39.84 b + 0.181 | 39.73 b + 0.458 | 35.52 b + 1.281 | 18.51 c + 0.063 | 30.45 b + 0.267 | 49.46 a + 0.081 | 30.35 bc + 0.118 | 32.17 bc + 0.218 | 36.43 b + 0.020 | 31.23 bc + 0.732 | 22.57 c + 1.079 | 48.61 a + 0.156 | 39.46 ab + 0.011 | 43.87 ab + 0.036 | 41.23 ab + 0.081 | 22.54 b + 2.936 | 52.03 a + 0.206 |
| Cyanidin-3-Glucoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | n.d. | 4.589 a + 0.002 | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Epicatechin | 6.153 a + 0.101 | 4.578 bc + 0.011 | 5.311 ab + 0.020 | 2.583 d + 0.109 | 1.775 d + 0.014 | 3.974 c + 0.034 | 7.917 a + 0.082 | 5.319 b + 0.022 | 6.293 ab + 0.007 | 7.593 ab + 0.011 | 5.683 ab + 0.043 | 3.953 b + 0.298 | 15.57 b + 0.065 | 15.47 b + 0.019 | 9.839 d + 0.002 | 12.02 c + 0.052 | 10.19 d + 0.001 | 19.50 a + 0.040 |
| Gallic Acid | 3.651 ab + 0.003 | 3.820 ab + 0.000 | 4.106 ab + 0.000 | 3.064 b + 0.088 | 3.862 ab + 0.001 | 4.854 a + 0.170 | 2.402 a + 0.106 | 1.150 a + 0.008 | 1.214 a + 0.000 | <LOQ | 1.178 a + 0.026 | 1.454 a + 0.124 | 2.540 c + 0.033 | 2.908 c + 0.023 | 2.915 c + 0.031 | 2.241 c + 0.017 | 6.897 a + 0.098 | 5.524 b + 0.083 |
| Isorhamnetin-3-O-Glucoside | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | <LOQ | <LOQ | 0.787 a + 0.005 | 0.774 a + 0.006 | 0.852 a + 0.005 | 0.753 a + 0.007 |
| Kaempferol-3-O-β-Rutinoside | 8.084 bc + 0.009 | 8.428 bc + 0.109 | 5.120 c + 0.006 | 16.87 ab + 1.410 | 6.594 bc + 0.092 | 23.24 a + 0.162 | 20.63 a + 0.170 | 11.21 b + 0.130 | 7.473 bc + 0.035 | 10.23 b + 0.025 | 22.01 a + 0.073 | 5.536 c + 0.487 | 14.44 c + 0.101 | 26.38 b + 0.009 | 9.881 e + 0.089 | 33.17 a + 0.133 | 12.15 d + 0.050 | 15.94 c + 0.202 |
| o-Coumaric Acid | 6.839 ab + 0.061 | 8.174 a + 0.031 | 3.072 c + 0.009 | 3.429 c + 0.161 | 5.912 b + 0.033 | 5.638 b + 0.026 | 6.275 ab + 0.164 | <LOQ | 4.367 c + 0.005 | 6.806 a + 7.504 | 4.955 bc + 0.004 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 3.841 a + 0.074 |
| p-Coumaric Acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Phloridzin | 259.9 a + 0.468 | 255.6 a + 0.123 | 142.2 bc + 0.782 | 181.0 b + 5.928 | 127.3 c + 0.315 | 106.3 c + 0.384 | 146.8 a + 0.831 | 60.43 c + 0.314 | 100.6 b + 0.014 | 135.6 a + 0.062 | 131.0 a + 1.245 | 80.71 bc + 3.326 | 140.1 d + 0.076 | 61.65 f + 0.038 | 184.7 a + 0.502 | 105.3 e + 0.428 | 175.3 b + 0.313 | 164.8 c + 0.484 |
| Quercetin | 14.45 a + 0.116 | 12.86 a + 0.088 | 10.89 a + 0.009 | 20.86 a + 1.427 | 12.46 a + 0.062 | 19.73 a + 0.093 | 17.78 b + 0.147 | 8.001 c + 0.043 | 27.50 a + 0.009 | 14.94 bc + 0.015 | 23.42 ab + 0.099 | 13.60 bc + 1.087 | 15.45 d + 0.062 | 17.51 cd + 0.017 | 21.56 bc + 0.141 | 22.55 b + 0.081 | 22.31 b + 0.239 | 32.94 a + 0.432 |
| Quercetin-3-β-D-Glucoside | 9.173 ab + 0.051 | 5.284 b + 0.010 | 3.028 b + 0.010 | 19.08 a + 1.584 | 8.594 ab + 0.044 | 14.97 ab + 0.074 | 14.42 ab + 0.115 | 4.885 c + 0.011 | 18.24 a + 0.054 | 6.348 c + 0.007 | 15.08 ab + 0.105 | 10.80 bc + 0.898 | 21.51 a + 0.018 | 10.27 e + 0.005 | 9.532 e + 0.002 | 18.35 b + 0.051 | 12.68 d + 0.073 | 15.03 c + 0.171 |
| Quercitrin | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | n.d. | 4.039 a + 0.008 | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rutin | <LOQ | <LOQ | n.d. | 1.316 a + 0.083 | <LOQ | 0.871 b + 0.005 | 0.961 a + 0.003 | <LOQ | 1.177 a + 0.000 | <LOQ | 1.028 a + 0.005 | 0.974 a + 0.055 | 4.650 a + 0.012 | <LOQ | 0.766 c + 0.001 | 1.327 b + 0.003 | 0.901 c + 0.016 | 1.279 b + 0.015 |
| Taxifolin | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | <LOQ | n.d. | <LOQ |
| Vanilic Acid | 1.400 a + 0.014 | <LOQ | 1.731 a + 0.004 | 1.424 a + 0.064 | <LOQ | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | <LOQ | <LOQ | <LOQ | 1.432 a + 0.008 | 1.557 a + 0.009 |
| Cultivar | Average Size | Average Weight | Brix (°Bx) | Notes |
|---|---|---|---|---|
| Gala Redlum | 65–70 mm in diameter | 140–180 g | 12–15 °Bx | Appreciated for its strong red coloration and typically sweet flavor. It maintains the crisp texture of standard Gala. |
| Granny Smith | 70–80 mm in diameter | 170–220 g | 11–13 °Bx | Known for its tartness and firm texture. It generally has lower sugar than red cultivars, and is often used in cooking or for its contrast in fresh eating. |
| Fuji Aztec | 70–80 mm in diameter | 170–220 g | 14–18 °Bx | Known for its dense, sweet flesh and can exceed typical Fuji Brix levels under good growing conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, J.D.; Leão de Sousa, M.; Barros, S.C.; Parpot, P.; Almeida, C.; Sanches Silva, A. Impact of Photoselective Nets on Phenolic Composition and Antioxidant Capacity in Different Apple Cultivars Under the Same Edaphoclimatic Conditions. Molecules 2025, 30, 1995. https://doi.org/10.3390/molecules30091995
Teixeira JD, Leão de Sousa M, Barros SC, Parpot P, Almeida C, Sanches Silva A. Impact of Photoselective Nets on Phenolic Composition and Antioxidant Capacity in Different Apple Cultivars Under the Same Edaphoclimatic Conditions. Molecules. 2025; 30(9):1995. https://doi.org/10.3390/molecules30091995
Chicago/Turabian StyleTeixeira, João David, Miguel Leão de Sousa, Sílvia Cruz Barros, Pier Parpot, Carina Almeida, and Ana Sanches Silva. 2025. "Impact of Photoselective Nets on Phenolic Composition and Antioxidant Capacity in Different Apple Cultivars Under the Same Edaphoclimatic Conditions" Molecules 30, no. 9: 1995. https://doi.org/10.3390/molecules30091995
APA StyleTeixeira, J. D., Leão de Sousa, M., Barros, S. C., Parpot, P., Almeida, C., & Sanches Silva, A. (2025). Impact of Photoselective Nets on Phenolic Composition and Antioxidant Capacity in Different Apple Cultivars Under the Same Edaphoclimatic Conditions. Molecules, 30(9), 1995. https://doi.org/10.3390/molecules30091995











