Active Polylactic Acid (PLA) Films Incorporating Almond Peel Extracts for Food Preservation
Abstract
1. Introduction
2. Results and Discussion
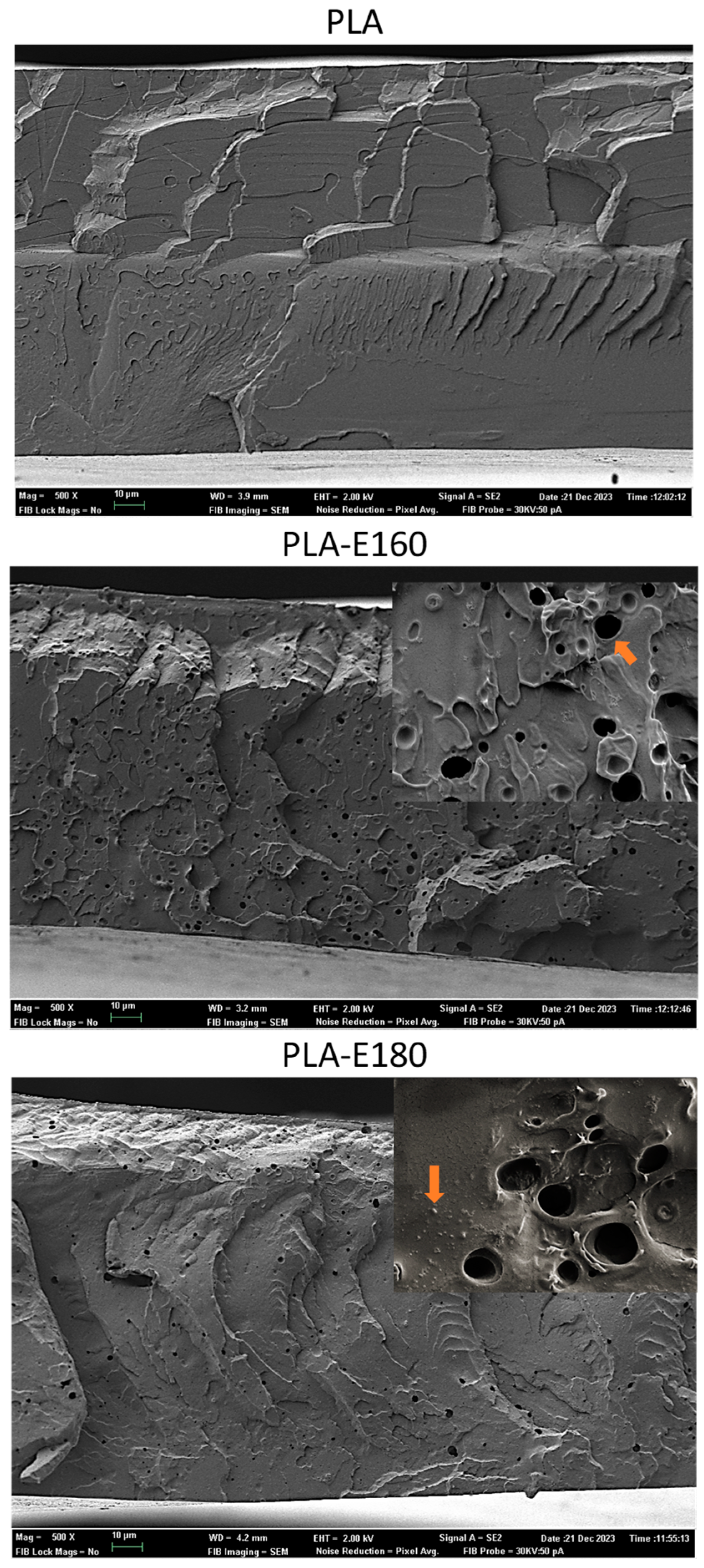
2.1. Film Microstructure
2.2. Physical Properties of the Films
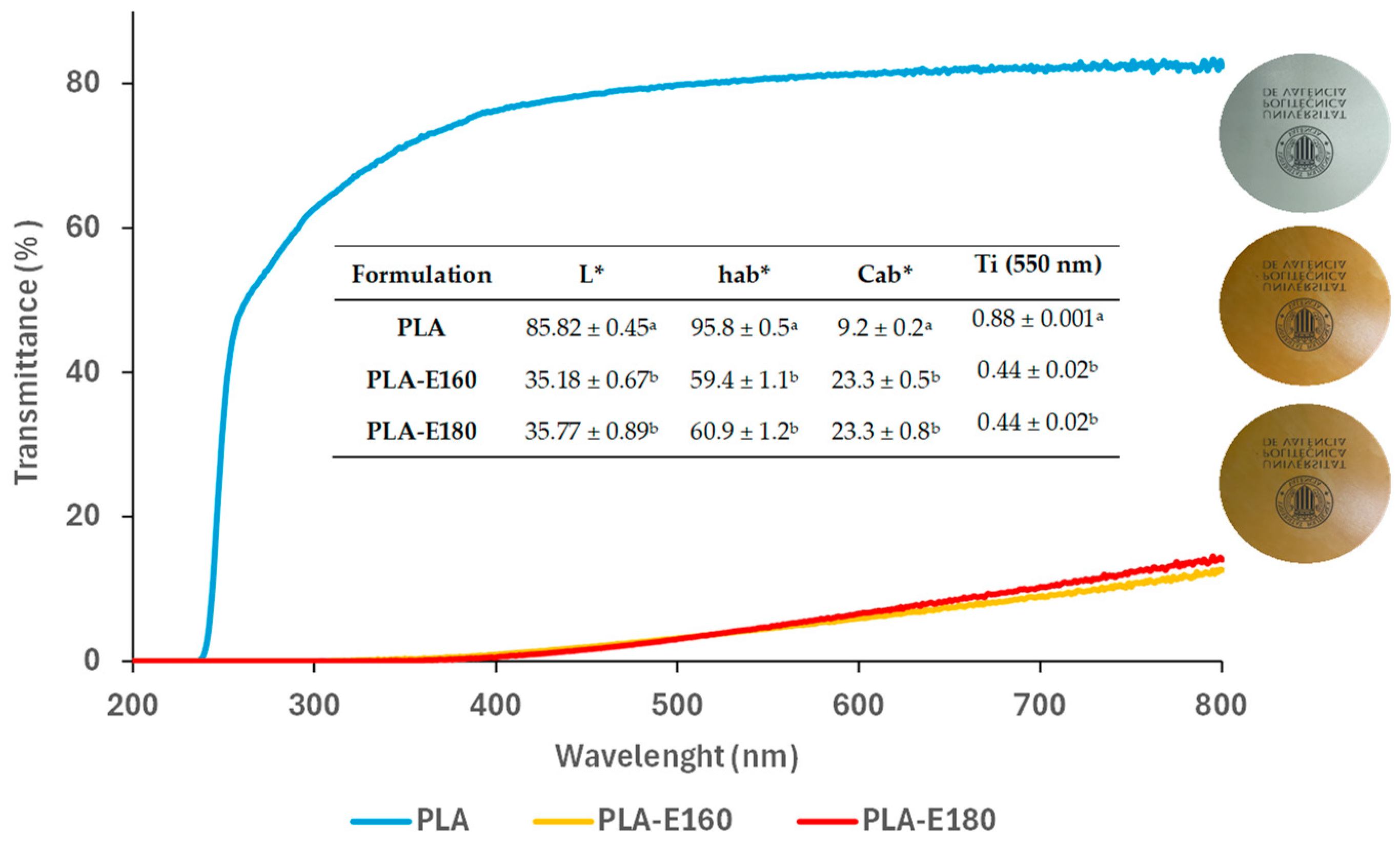
2.2.1. Film Optical Properties
2.2.2. Mechanical and Barrier Properties
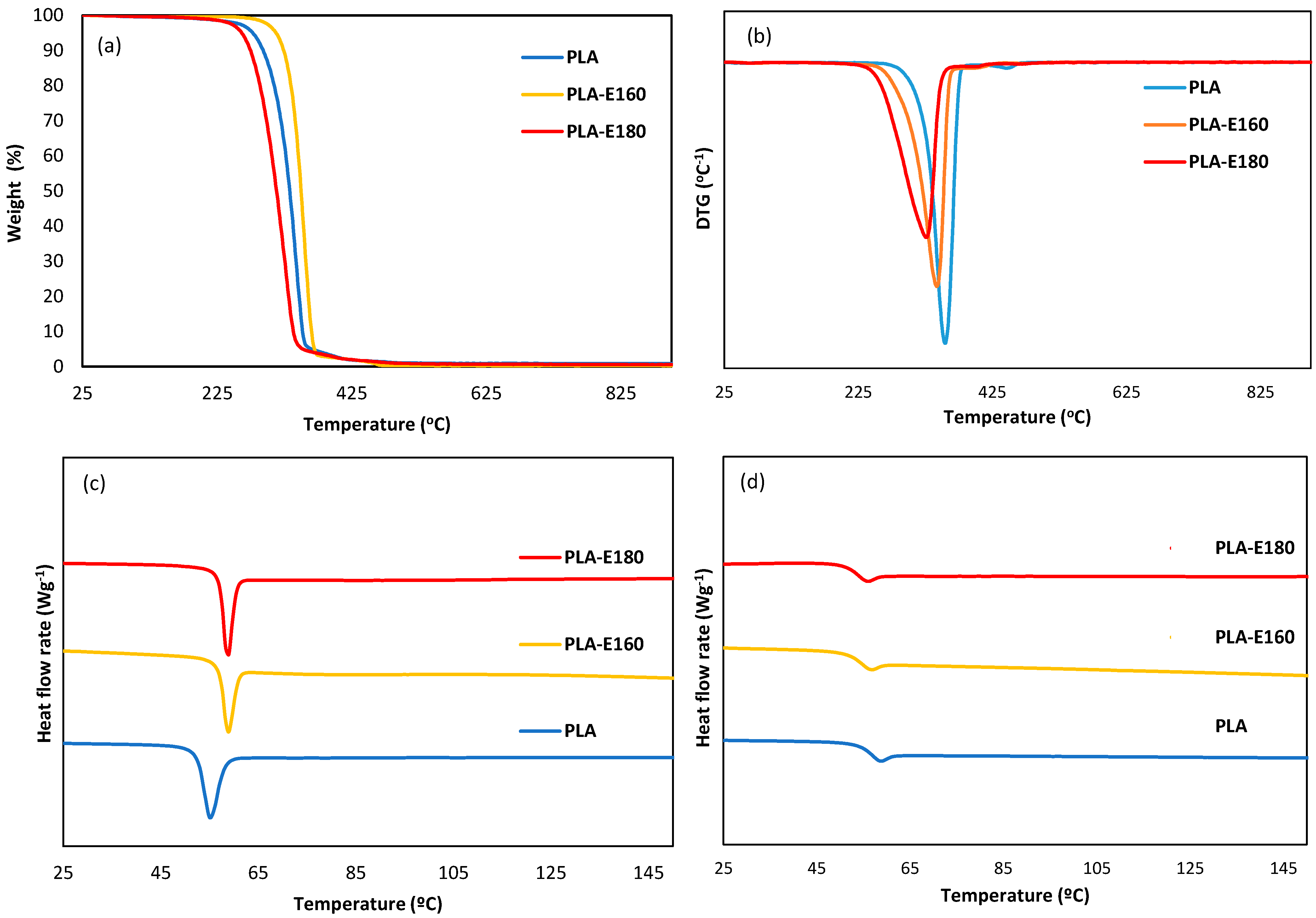
2.2.3. Thermal Behavior: Thermal Stability and Tg Values
2.3. Bioactive Potential of the Films
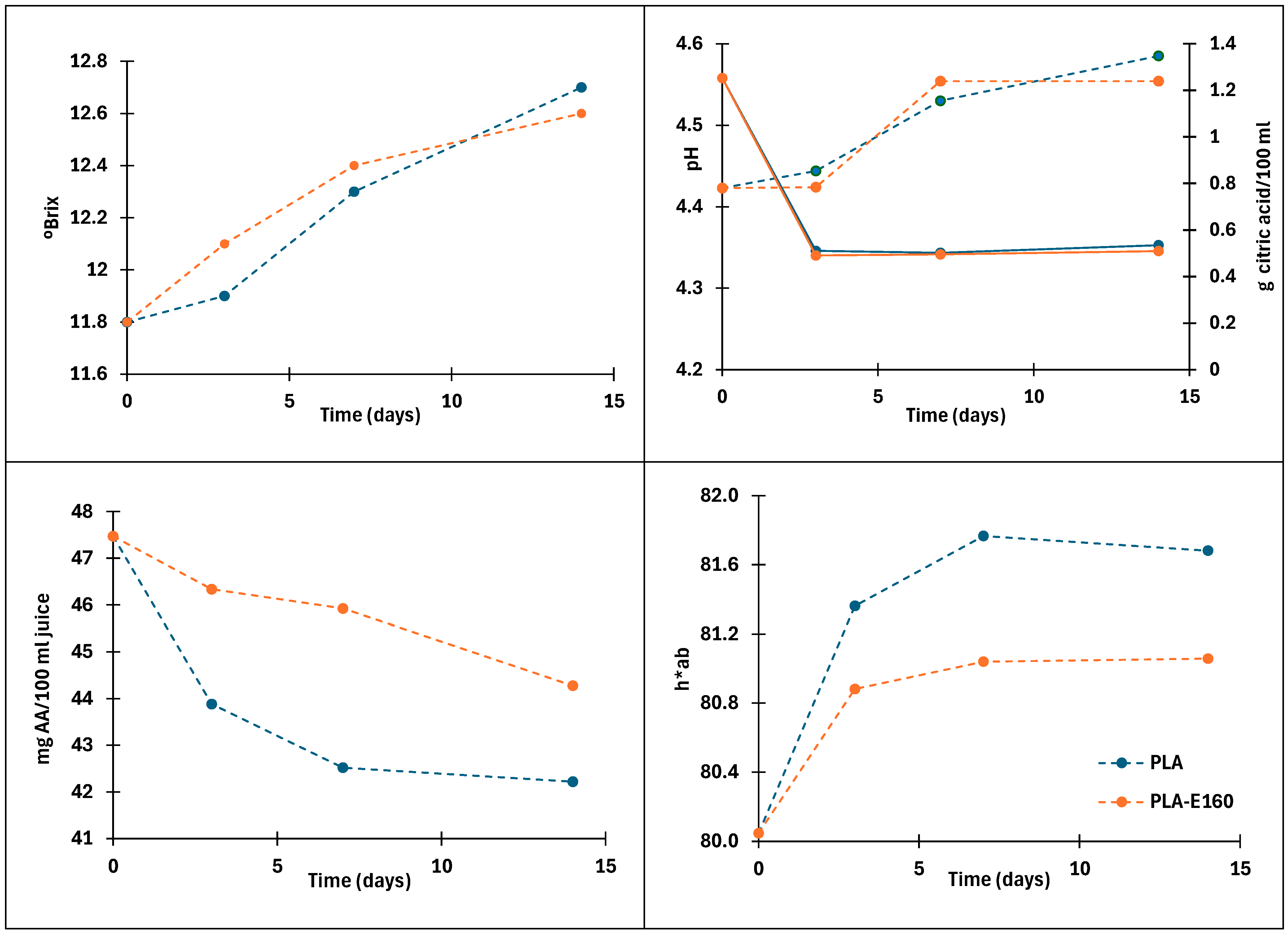
2.4. Film’s Ability to Preserve Fresh Orange Juice
2.5. Film’s Ability to Prevent Sunflower Oil Oxidation
3. Materials and Methods
3.1. Materials
3.2. Film Preparation
3.3. Film Characterization
3.3.1. Microstructure
3.3.2. Moisture Content
3.3.3. Mechanical Barrier and Optical Properties
3.3.4. Thermal Properties
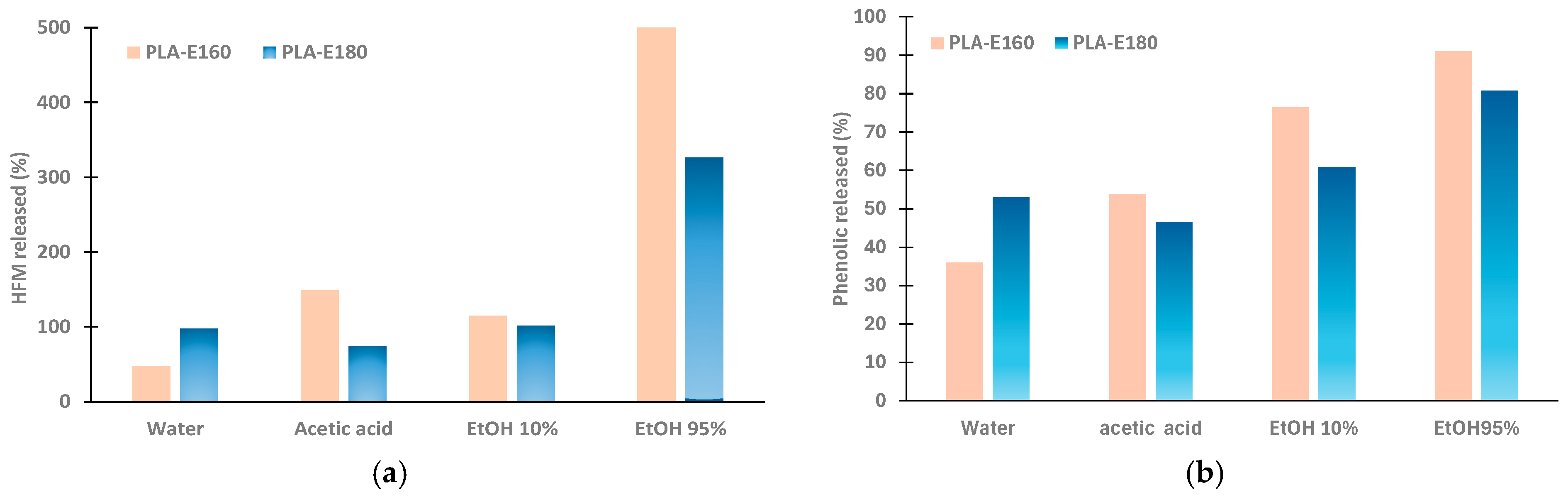
3.4. Release of 5-Hidroxymetylfurfural (HMF) and Phenolic Compounds in Different Simulants
3.5. Antibacterial Activity of the Films
3.6. Ability of Active Films to Extend Shelf Life of Food
3.6.1. Physicochemical and Microbiological Analysis of Freshly Squeezed Orange Juice
3.6.2. Sunflower Oil Oxidation Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Perez, P.; Xiao, J.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Rajoka, M.S.R.; Barros, L.; Mascoloti Sprea, R.; Amaral, J.S.; Prieto, M.A. Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review. Foods 2021, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The Importance of Almond (Prunus amygdalus L.) and Its by-Products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial Potential of Polyphenols Extracted from Almond Skins: Antimicrobials from Almond Skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic Content and Biological Properties of Avola Almond (Prunus dulcis Mill. D.A. Webb) Skin and Its Industrial Byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Gonzalez-Martinez, C.; Amparo Chiralt, A. Active Poly (Lactic acid) Films with Rice Straw Aqueous Extracts for Meat Preservation Purposes. Food Bioprocess Technol. 2023, 16, 2635–2650. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant starch- based films with encapsulated eugenol. Application to sunflower oil preservation. LWT-Food Sci. Technol. 2019, 113, 108290. [Google Scholar] [CrossRef]
- Freitas, P.A.; Martín-Pérez, L.; Gil-Guillén, I.; González-Martínez, C.; Chiralt, A. Subcritical Water Extraction for Valorisation of Almond Skin from Almond Industrial Processing. Foods 2023, 12, 3759. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical water extraction of phenolic compounds from onion skin wastes (Allium cepa cv. Horcal): Effect of temperature and solvent properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 2010, 43, 1123–1129. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Kanmaz, O.E. 5-Hydroxymethylfurfural (HMF) formation during subcritical water extraction. Food Sci. Biotechnol. 2018, 27, 981–986. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Effect of active phenolic acids on properties of PLA-PHBV blend films. Food Packag. Shelf Life 2022, 33, 100894. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Lopusiewicz, L. Recent progress on UV-light barrier food packaging films—A systematic review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103550. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Effect of ferulic and cinnamic acids on the functional and antimicrobial properties in thermo-processed PLA films. Food Packag. Shelf Life 2022, 33, 100882. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Properties of PLA films with cinnamic acid: Effect of the processing method. Food Bioprod. Process. 2022, F133, 25–33. [Google Scholar] [CrossRef]
- Rojas, A.; Velásquez, E.; Patiño Vidal, C.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C.; Active, P.L.A. Packaging Films: Effect of Processing and the Addition of Natural Antimicrobials and Antioxidants on Physical Properties, Release Kinetics, and Compostability. Antioxidants 2021, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Moll, E.; Chiralt, A. Polyhydroxybutyrate-co-hydroxyvalerate (PHBV) with Phenolic Acids for Active Food Packaging. Polymers 2023, 15, 4222. [Google Scholar] [CrossRef]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch-chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Poly(lactic) acid (PLA) and starch bilayer films, containing cinnamaldehyde obtained by compression moulding. Eur. Polym. J. 2017, 95, 56–70. [Google Scholar]
- Greco, A.; Ferrari, F. Thermal behavior of PLA plasticized by commercial and cardanol-derived plasticizers and the effect on the mechanical properties. J. Therm. Anal. Calorim. 2021, 146, 131–141. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Kus, S.; Gogus, F.; Eren, S. Hydroxymethyl furfural content of concentrated food products. Int. J. Food Prop. 2005, 8, 367–375. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active PLA–antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Desobry, S. Release of synthetic phenolic antioxidants from extruded poly lactic acid (PLA) film. Food Control 2012, 28, 445–455. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population. Food Chem. Toxicol. 2010, 48, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Ansari, F. Identification and quantification of 5-hydroxymethylfurfural in food products. Nutr. Food Sci. Res. 2015, 1, 47–53. [Google Scholar]
- Badalamenti, N.; Bruno, M.; Loizzo, M.R.; Puccio, V.; Gaglio, R.; Francesca, N. Antibacterial activity and chemical characterization of almond (Prunus dulcis L.) peel extract. Nat. Prod. Res. 2022, 10, 1680–1686. [Google Scholar] [CrossRef]
- Rodrigues, P.V.; Vieira, D.M.; Martins, P.C.; Martins, V.G.; Castro, M.C.R.; Machado, A.V. Evaluation of Active LDPE Films for Packaging of Fresh Orange Juice. Polymers 2023, 15, 50. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Polat, S. Color quality, ascorbic acid, total carotenoid, and volatile compounds of dried orange slices as influenced by packaging methods and storage conditions. J. Food Process. Preserv. 2022, 46, e15898. [Google Scholar]
- Roig, M.G.; Bello, J.F.; Rivera, Z.S.; Kennedy, J.F. Studies on the occurrence of non-enzymatic browning during storage of citrus juice. Food Res. 1999, 32, 609–619. [Google Scholar] [CrossRef]
- Ros-Chumillas, M.; Belissario, Y.; Iguaz, A.; López, A. Quality and shelf life of orange juice aseptically packaged in PET bottles. J. Food Eng. 2007, 79, 234–242. [Google Scholar]
- Wilson, R.J.; Beezer, A.E.; Mitchell, J.C. A kinetic study of the oxidation of L-ascorbic acid (vitamin C) in solution using an isothermal microcalorimeter. Thermochimica 1995, 264, 27–40. [Google Scholar]
- Zerdin, K.; Rooney, M.L.; Vermuë, J. The vitamin C content of orange juice packed in an oxygen scavenger material. Food Chem. 2003, 82, 387–395. [Google Scholar] [CrossRef]
- Solomon, O.; Svanberg, U.; Sahlström, A. Effect of oxygen and fluorescent light on the quality of orange juice during storage at 8 C. Food Chem. 1995, 53, 363–368. [Google Scholar] [CrossRef]
- Esteve, M.J.; Frígola, A.; Rodrigo, C.; Rodrigo, D. Effect of storage period under variable conditions on the chemical and physical composition and colour of Spanish refrigerated orange juices. Food Chem. Toxicol. 2005, 43, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; Ros-Chumillas, M. Packaging and the Shelf Life of Orange Juice. In Food Packaging and Shelf Life; CRC Press: Boca Raton, FL, USA, 2009; pp. 179–198. [Google Scholar]
- Abdolsattari, P.; Rezazadeh-Bari, M.; Pirsa, S. Smart Film Based on Polylactic Acid, Modified with Polyaniline/ZnO/CuO: Investigation of Physicochemical Properties and Its Use of Intelligent Packaging of Orange Juice. Food Bioprocess Technol. 2022, 15, 2803–2825. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of thermal and non-thermal treatments on the color of citrus juice: A review. Food Rev. Int. 2021, 39, 3555–3577. [Google Scholar] [CrossRef]
- Mercali, G.D.; Schwartz, S.; Marczak, L.D.F.; Tessaro, I.C.; Sastry, S. Ascorbic acid degradation and color changes in acerola pulp during ohmic heating: Effect of electric field frequency. J. Food Eng. 2014, 123, 1–7. [Google Scholar] [CrossRef]
- Meléndez-Martínez Antonio, J.; Vicario Isabel, M.; Heredia Francisco, J. Stability of carotenoid pigments in foodstuff. Arch. Latinoam. De Nutr. 2004, 54, 209–215. [Google Scholar]
- Dobarganes, M.C.; Velasco, J. Analysis of lipid hydroperoxides. Eur. J. Lipid Sci. Technol. 2002, 104, 420–428. [Google Scholar] [CrossRef]
- Mohdaly, A.A.; Sarhan, M.A.; Smetanska, I.; Mahmoud, A. Antioxidant properties of various solvent extracts of potato peel, sugar beet pulp and sesame cake. J. Sci. Food Agric. 2010, 90, 218–226. [Google Scholar] [CrossRef]
- Galarza, C.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Comparative study on the properties of films based on red rice (Oryza glaberrima) flour and starch. Food Hydrocoll. 2017, 65, 96–106. [Google Scholar]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Standard. D882. Annual Book of American Standard Testing Methods. American Society for Testing and Materials: Philadelphia, PA, USA, 2010; pp. 162–170.
- ASTM E96/E96M; Standard Test Method for Water Vapour Transmission of Materials. Standard Designations: E96-95 Annual Book of ASTM Standards. American Society for Testing and Materials: Philadelphia, PA, USA, 2005; pp. 785–792.
- ASTM D3985-05; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coloumetric Sensor. Designations (3985-05). American Society for Testing and Materials: Philadelphia, PA, USA, 2010; pp. 1–7.
- Kontogianni, V.G.; Gerothanassis, I.P. Analytical and Structural Tools of Lipid Hydroperoxides: Present State and Future Perspectives. Molecules 2022, 27, 2139. [Google Scholar] [CrossRef]

| E-160 | E-180 | |
|---|---|---|
| TPC1 (g GAE. 100 g−1 dried extract) | 10.09 ± 0.05 b | 16.1 ± 0.4 a |
| Protein (g 100 g−1 dried extract) | 2.4 ± 0.1 a | 2.2 ± 0.1 a |
| Ashes (g 100 g−1 dried extract) | 13.1 ± 0.1 a | 15.5 ± 0.7 b |
| Carbohydrates (g 100 g−1 dried extract) * | 74.4 ±0.2 a | 66.2 ± 0.2 b |
| EC50 (mg extract.mg−1 DPPH) | 1.490 ± 0.003 a | 1.063 ± 0.012 b |
| MIC L. innocua (mg.mL−1) | 90 | 34 |
| MIC E. coli (mg.mL−1) | 90 | 48 |
| MIC S. aureus (mg.mL−1) | 60 | 20 |
| Property | PLA | PLA-E160 | PLA-E180 |
|---|---|---|---|
| Thickness (μm) | 134 ± 15 ab | 141 ± 12 a | 131 ± 8 b |
| Xw (%) | 2.2 ± 0.8 a | 1.07 ± 0.03 a | 1.4 ± 0.4 a |
| EM (MPa) | 1564 ± 42 a | 1461 ± 70 a | 1244 ± 102 b |
| TS (MPa) | 48 ± 2 a | 41 ± 2 b | 8 ± 4 c |
| E% | 3.2 ± 0.2 a | 2.9 ± 0.1 b | 0.5 ± 0.2 c |
| WVP × 1011 (g/Pa.s.m) | 2.6 ± 0.6 a | 3.6 ± 0.2 a | 2.3 ± 0.1 a |
| OP × 1014 (cm3/m.s.Pa) | 124 ± 1 a | 121 ± 6 a | 111 ± 4 b |
| T onset (°C) | 323.5 ± 1.1 a | 297 ± 4 b | 263 ± 5 c |
| T peak (°C) | 354.2 ± 0.5 a | 342.17 ± 0.01 b | 327.8 ± 2 c |
| Tg1 (°C) | 58.8 ± 0.5 a | 56.2 ± 0.2 a | 53 ± 3 a |
| Tg2 (°C) | 55.06 ± 0.1 a | 53.2 ± 0.6 b | 52.3 ± 0.5 b |
| Log (UFC/mL) L. innocua | 7.62 | 7.57 | 7.54 |
| Log (UFC/mL) E. coli | 8.10 | 8.08 | 7.97 |
| Log (UFC/mL) S. aureus | 8.04 | 7.82 | 7.81 |
| HFM Released (ppm) | Water | Acetic Acid | 10% Ethanol | 95% Ethanol |
|---|---|---|---|---|
| PLA-E160 | 0.032 ± 0.008 d,2 | 0.11 ± 0.01 b,2 | 0.094 ± 0.009 b,2 | 0.35 ± 0.04 a,2 |
| PLA-E180 | 0.0147 ± 0.001 d,1 | 0.154 ± 0.009 c,1 | 0.21 ± 0.03 c,1 | 0.474 ± 0.002 a,1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Perez, L.; Contreras, C.; Chiralt, A.; Gonzalez-Martinez, C. Active Polylactic Acid (PLA) Films Incorporating Almond Peel Extracts for Food Preservation. Molecules 2025, 30, 1988. https://doi.org/10.3390/molecules30091988
Martin-Perez L, Contreras C, Chiralt A, Gonzalez-Martinez C. Active Polylactic Acid (PLA) Films Incorporating Almond Peel Extracts for Food Preservation. Molecules. 2025; 30(9):1988. https://doi.org/10.3390/molecules30091988
Chicago/Turabian StyleMartin-Perez, Laia, Carolina Contreras, Amparo Chiralt, and Chelo Gonzalez-Martinez. 2025. "Active Polylactic Acid (PLA) Films Incorporating Almond Peel Extracts for Food Preservation" Molecules 30, no. 9: 1988. https://doi.org/10.3390/molecules30091988
APA StyleMartin-Perez, L., Contreras, C., Chiralt, A., & Gonzalez-Martinez, C. (2025). Active Polylactic Acid (PLA) Films Incorporating Almond Peel Extracts for Food Preservation. Molecules, 30(9), 1988. https://doi.org/10.3390/molecules30091988







