Research Progress on the Reaction of Carbon Dioxide with Hydrazones and Their Derivatives
Abstract
1. Introduction
2. Reaction of Carbon Dioxide with Hydrazones and Their Derivatives
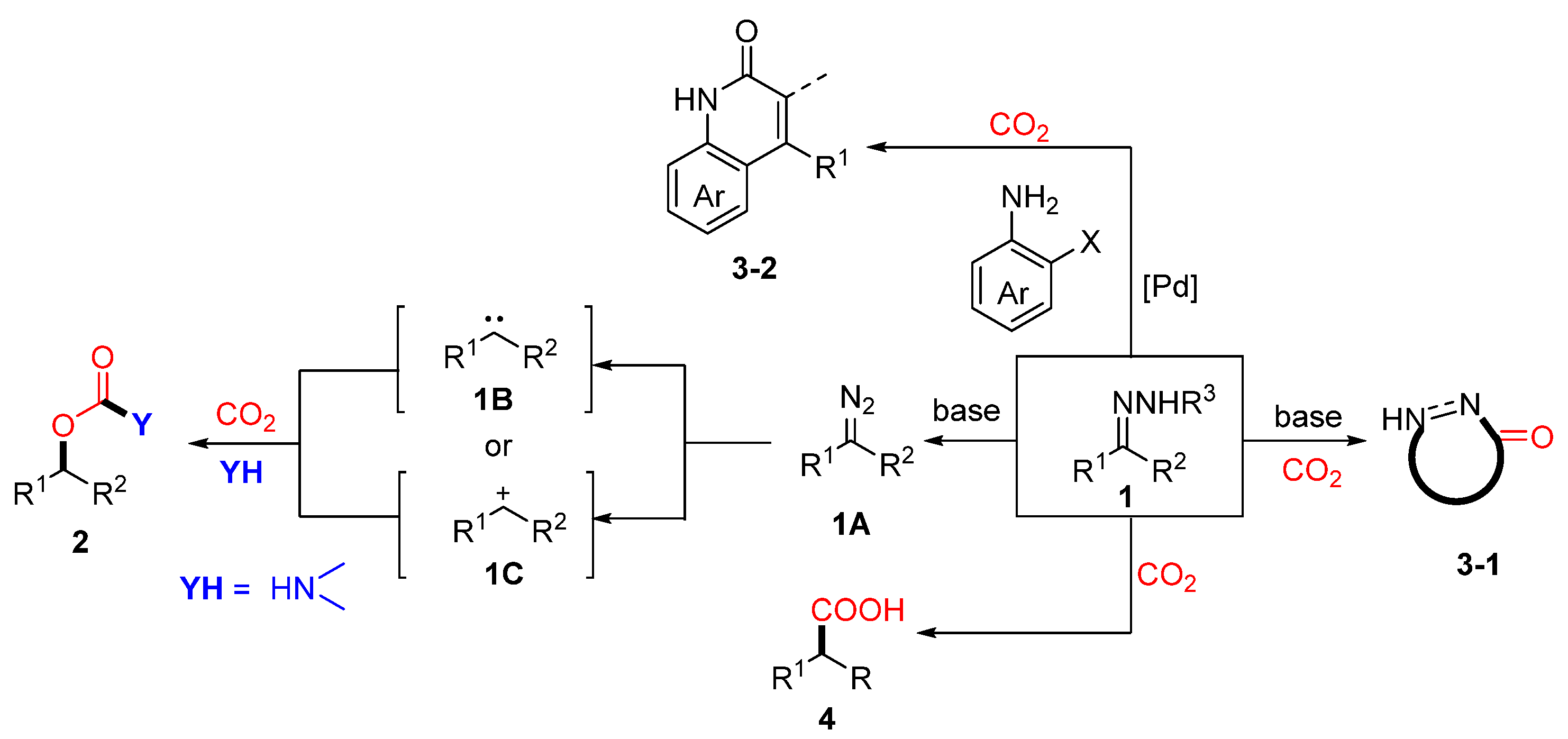
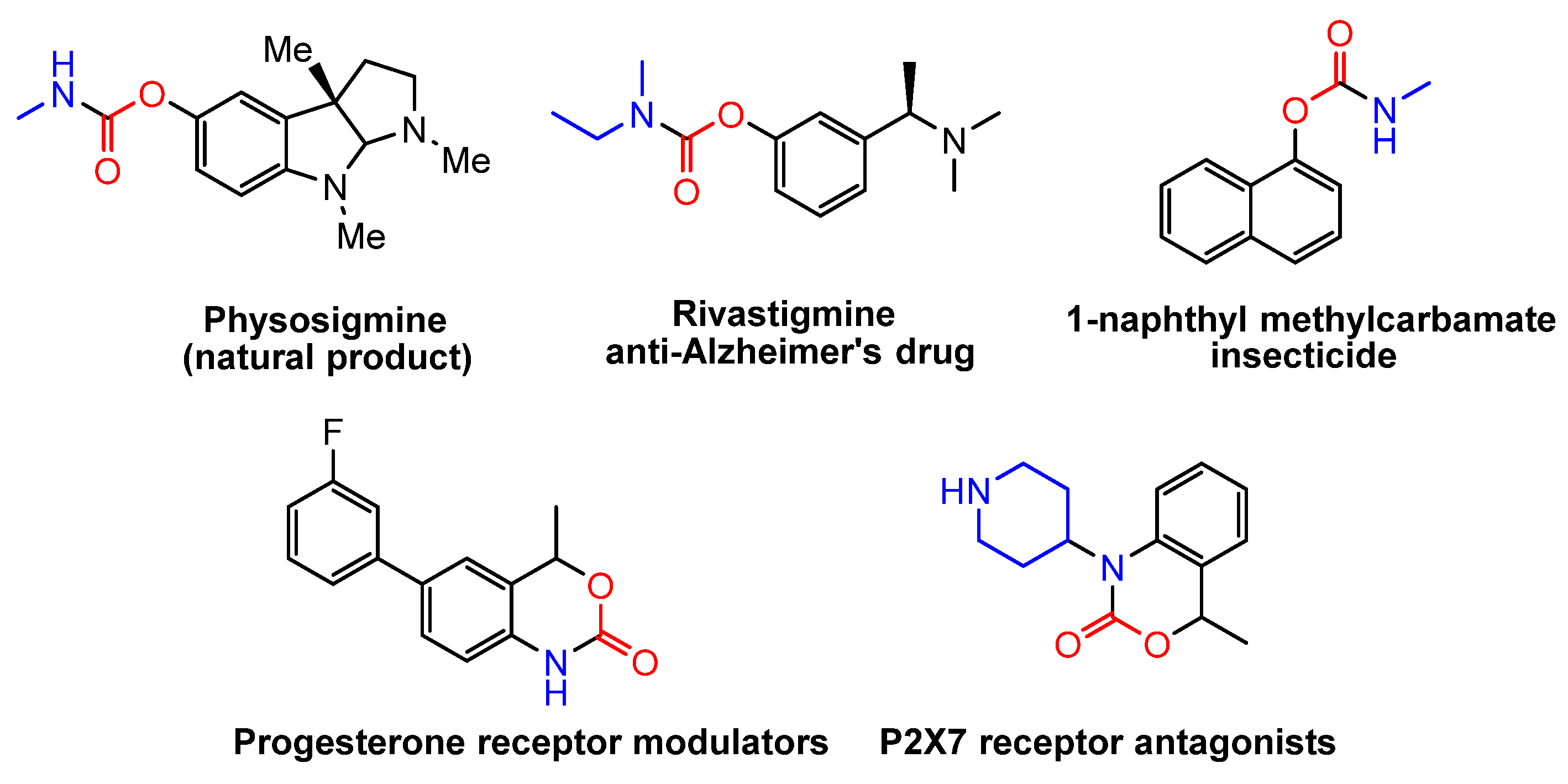
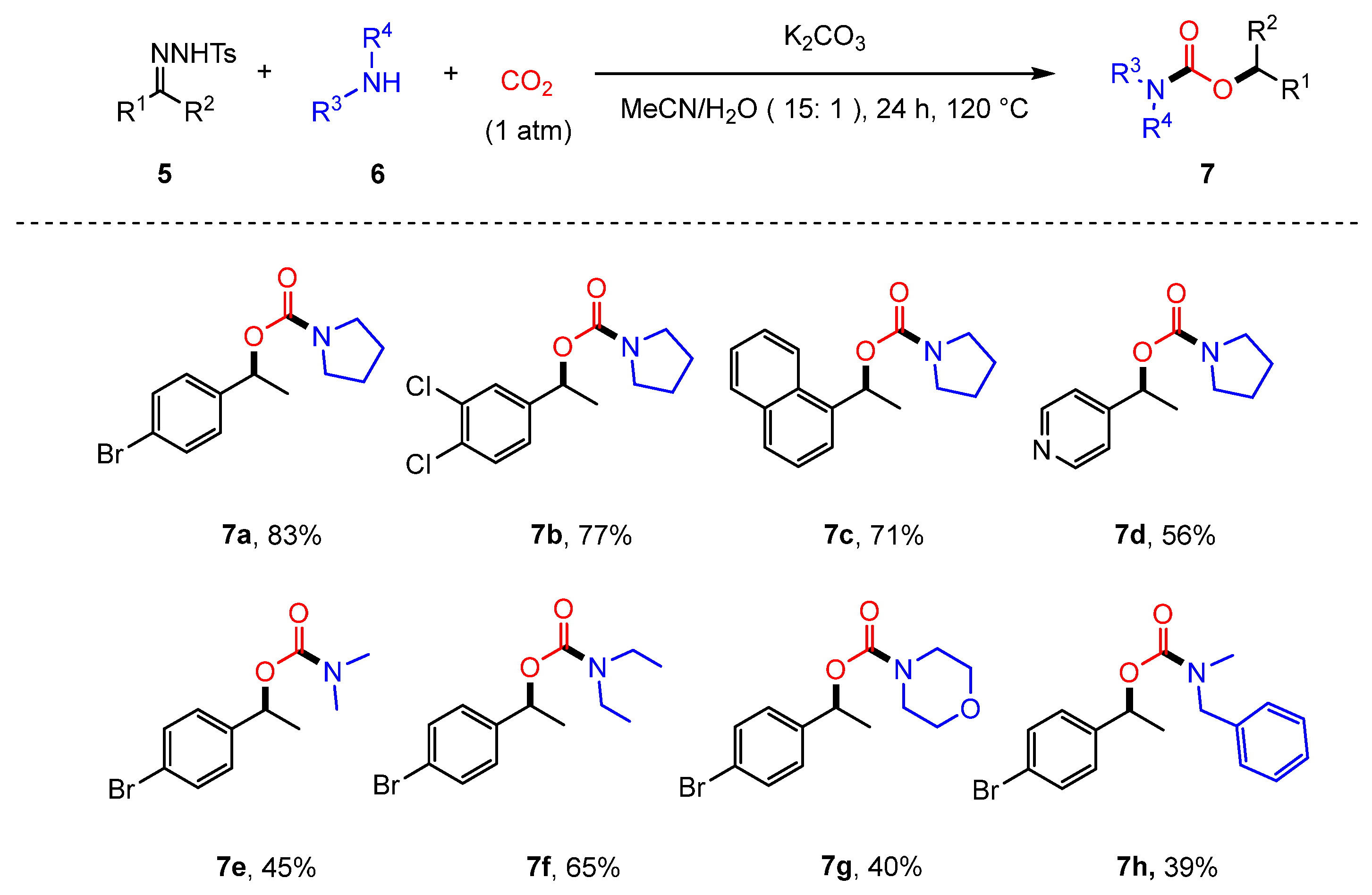
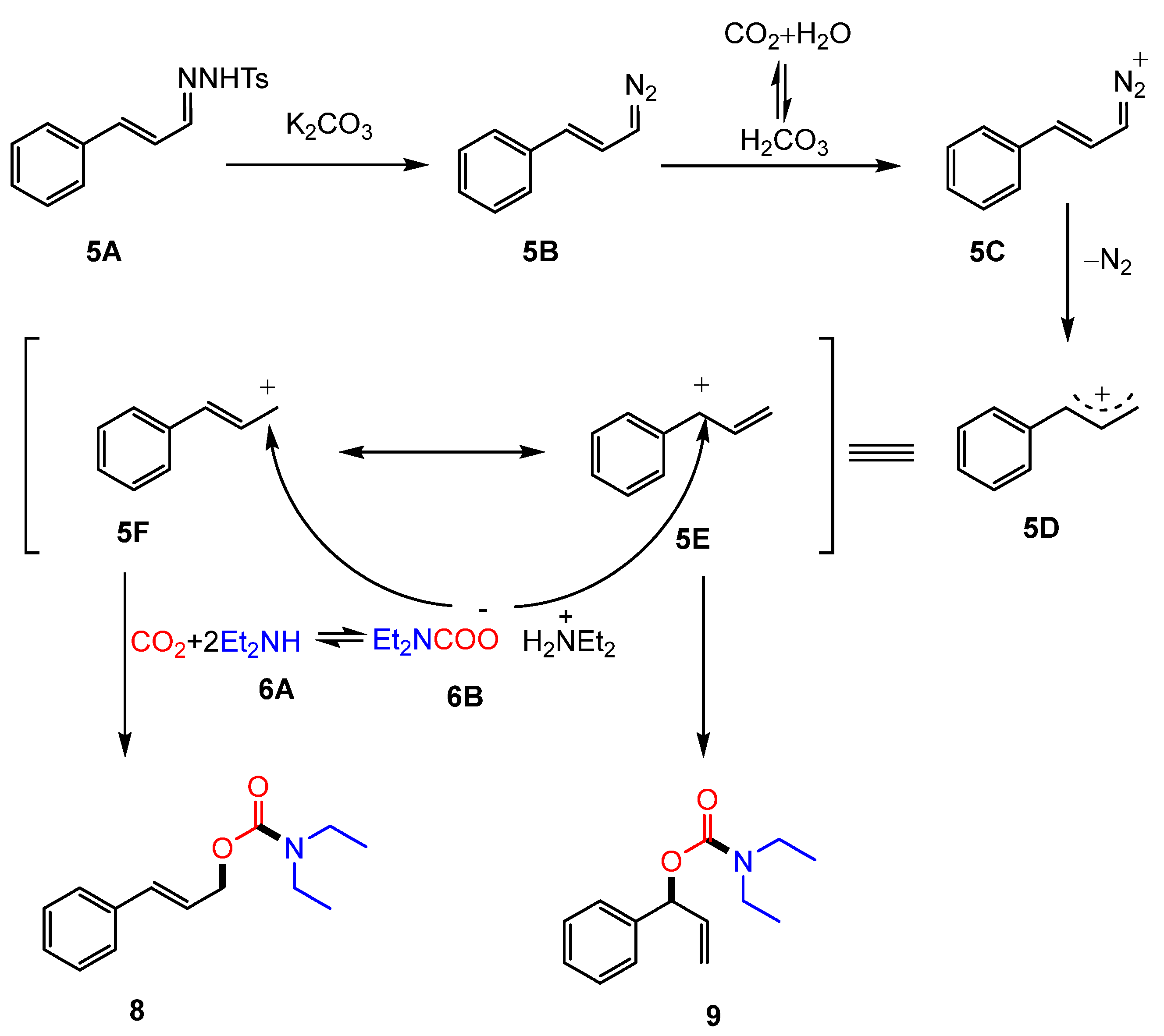
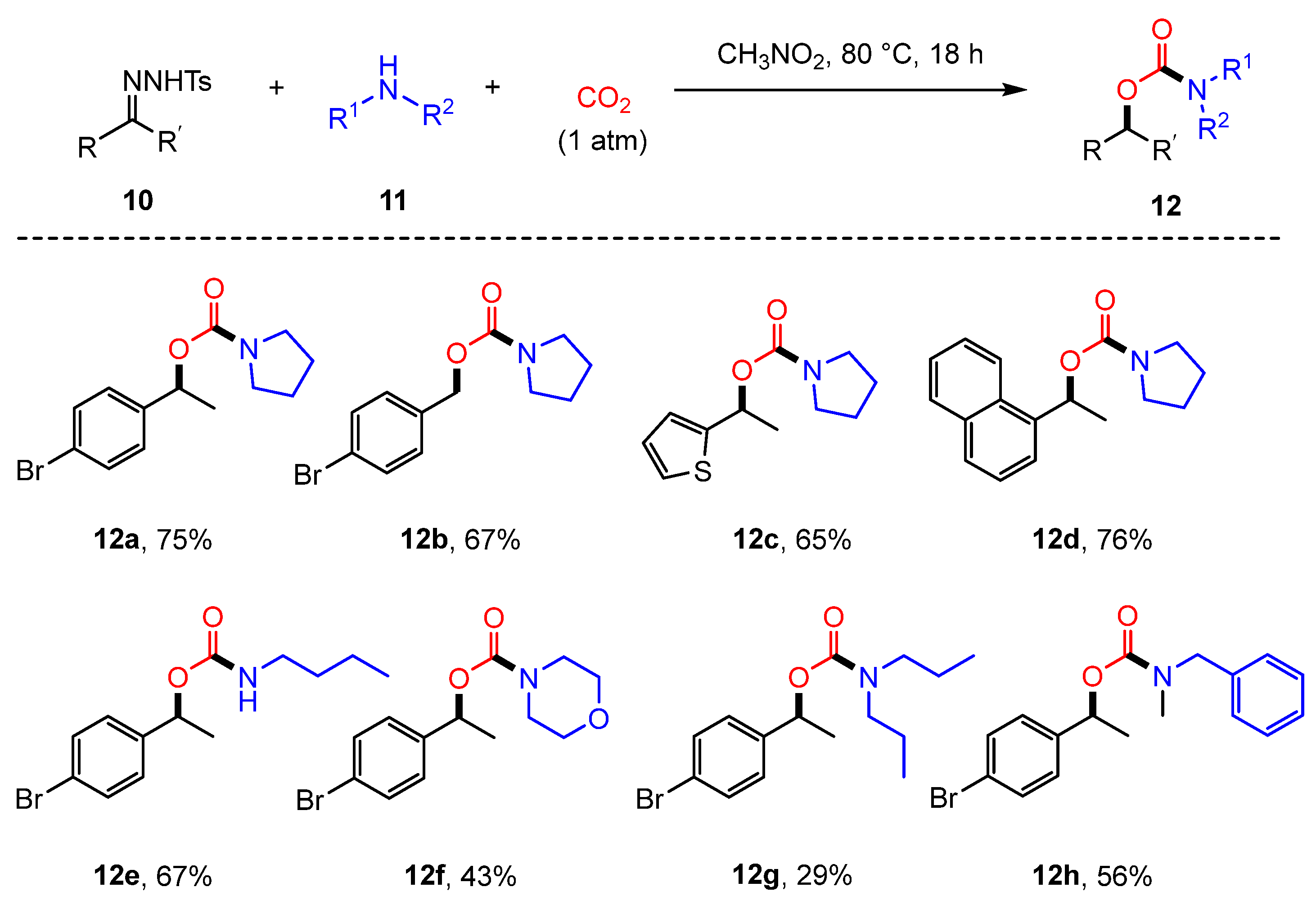
2.1. Coupling of Amines and N-tosylhydrazones with CO2 to Generate Carbamates
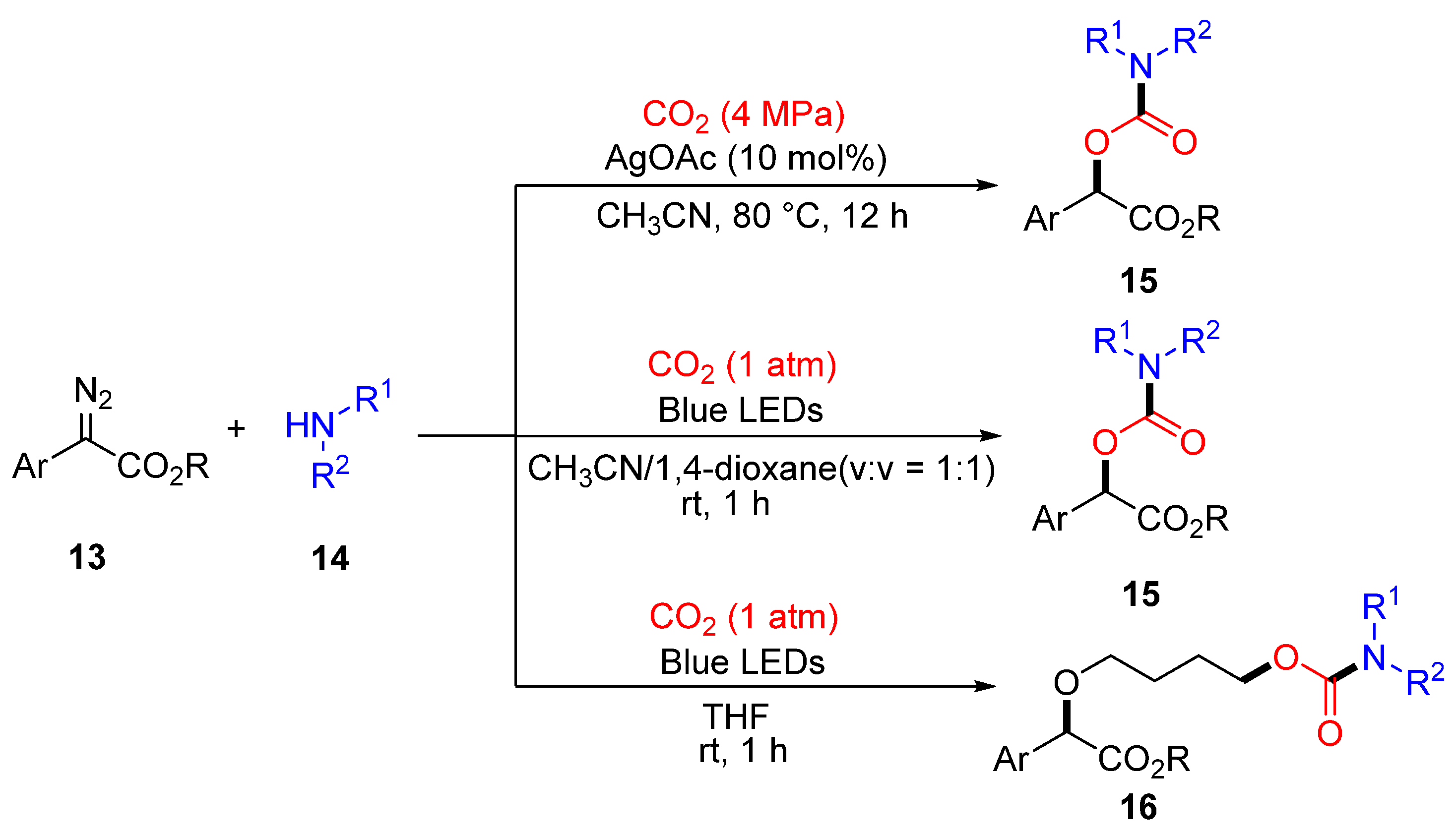
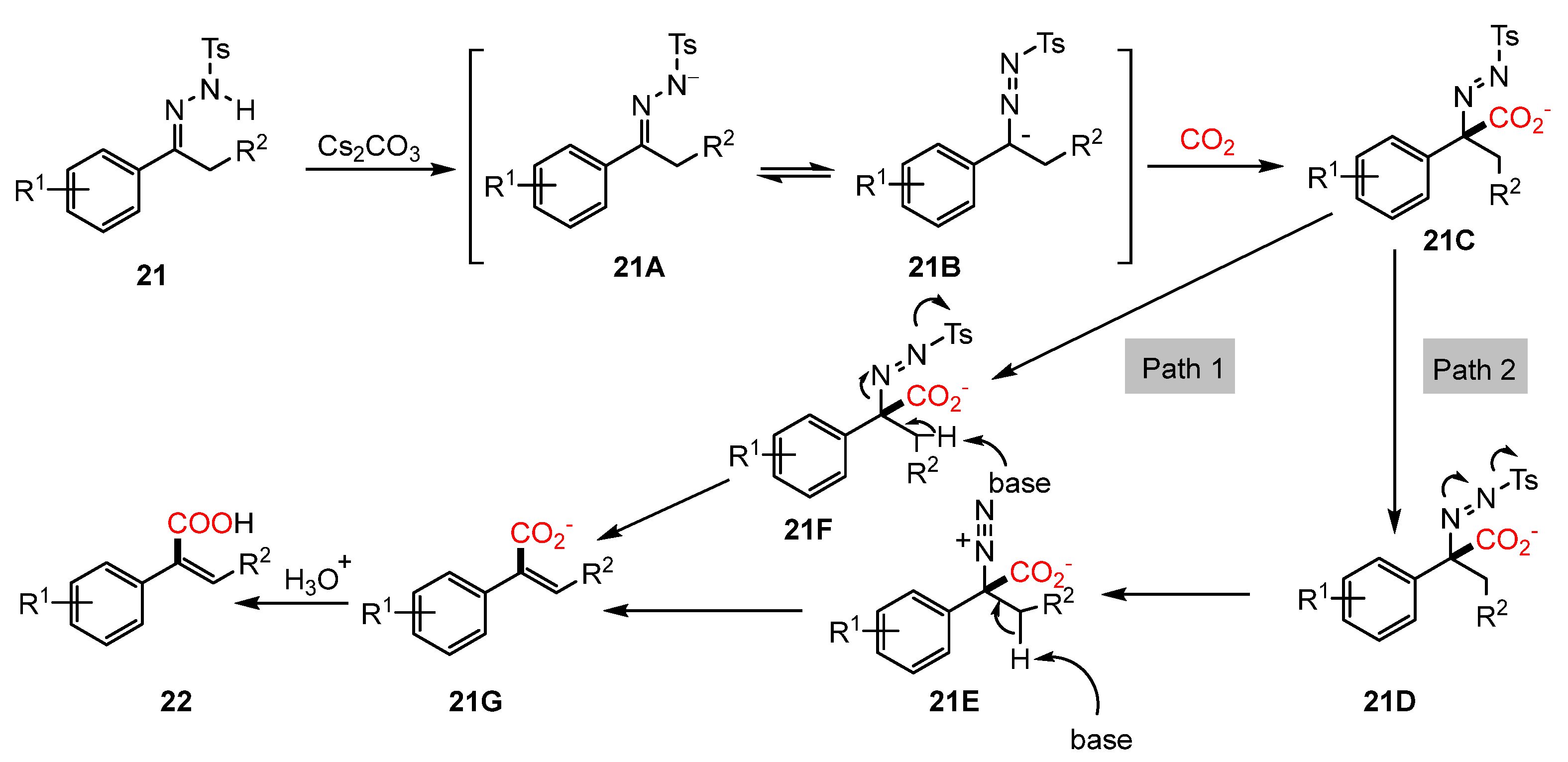
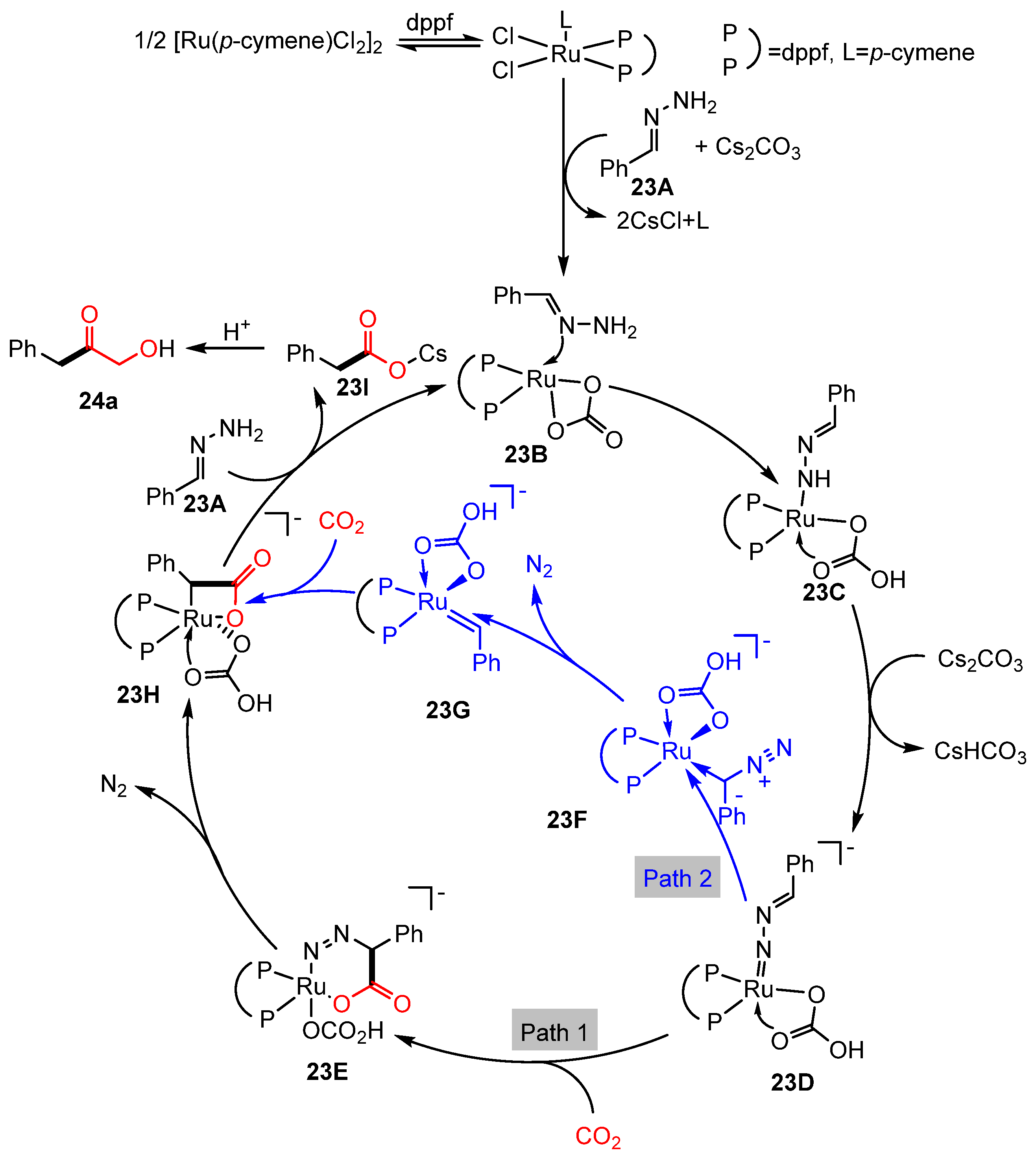
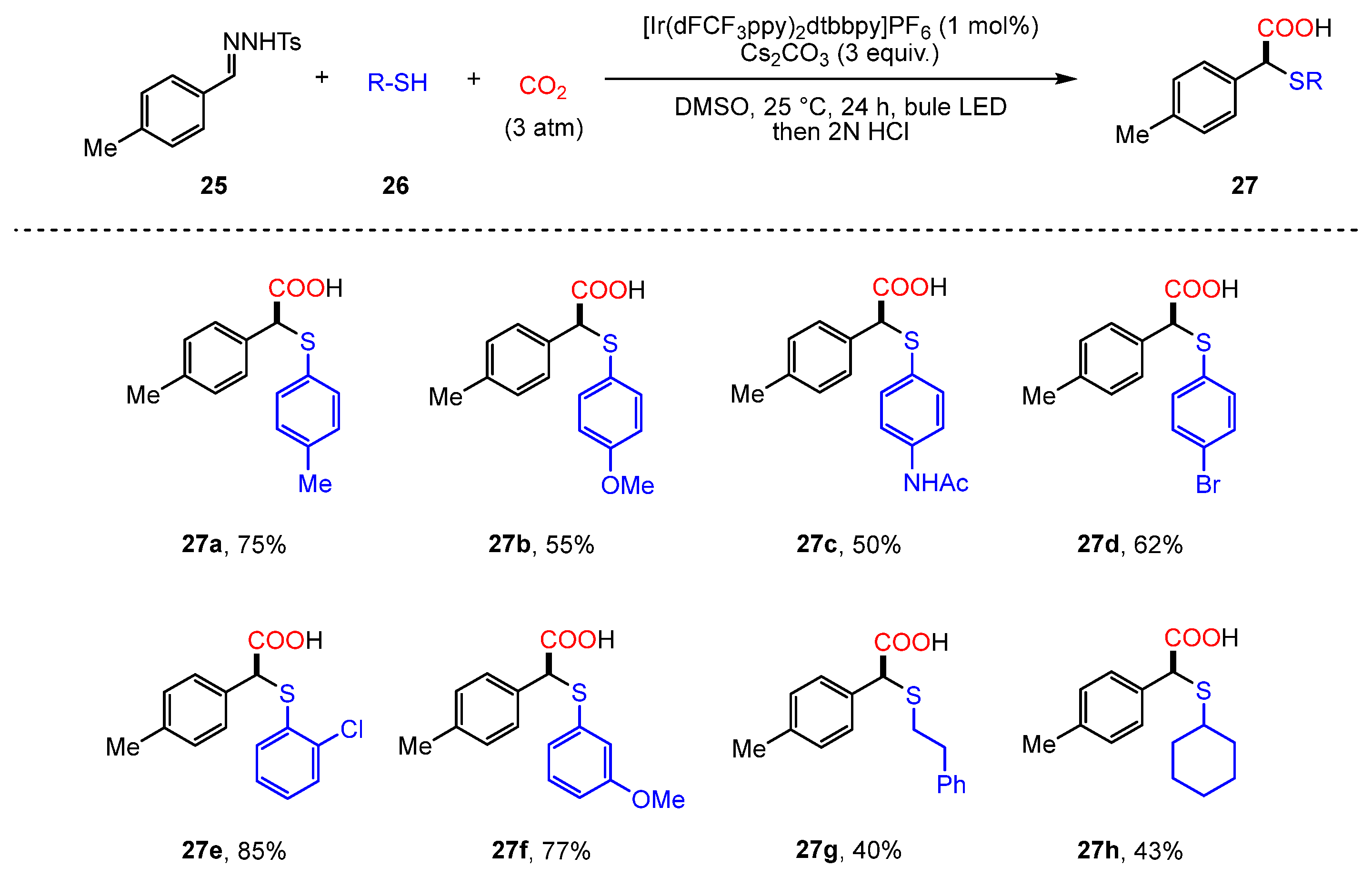
2.2. Carboxylation of Hydrazones/N-Tosylhydrazones with CO2 Through Umpolung
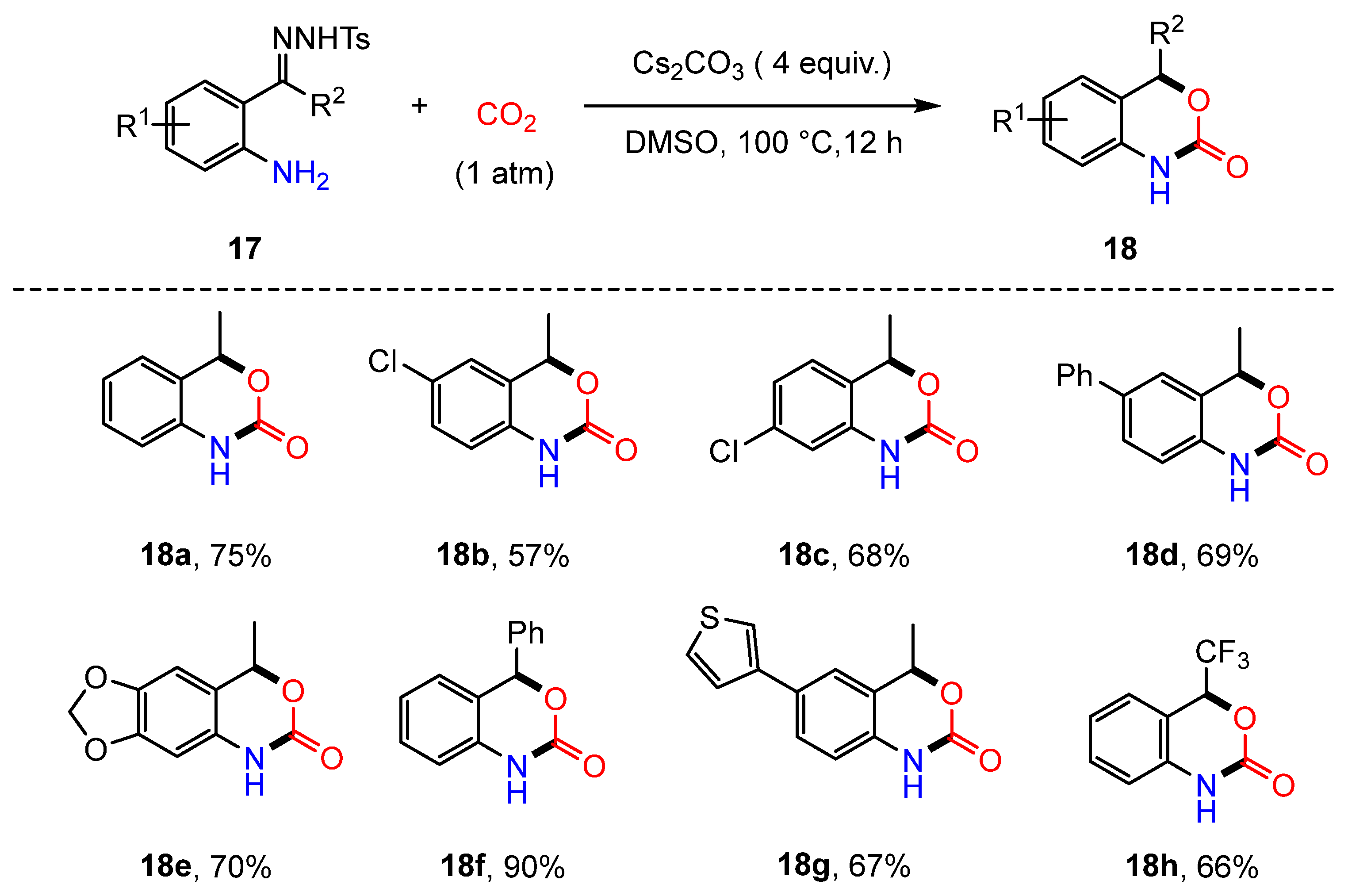
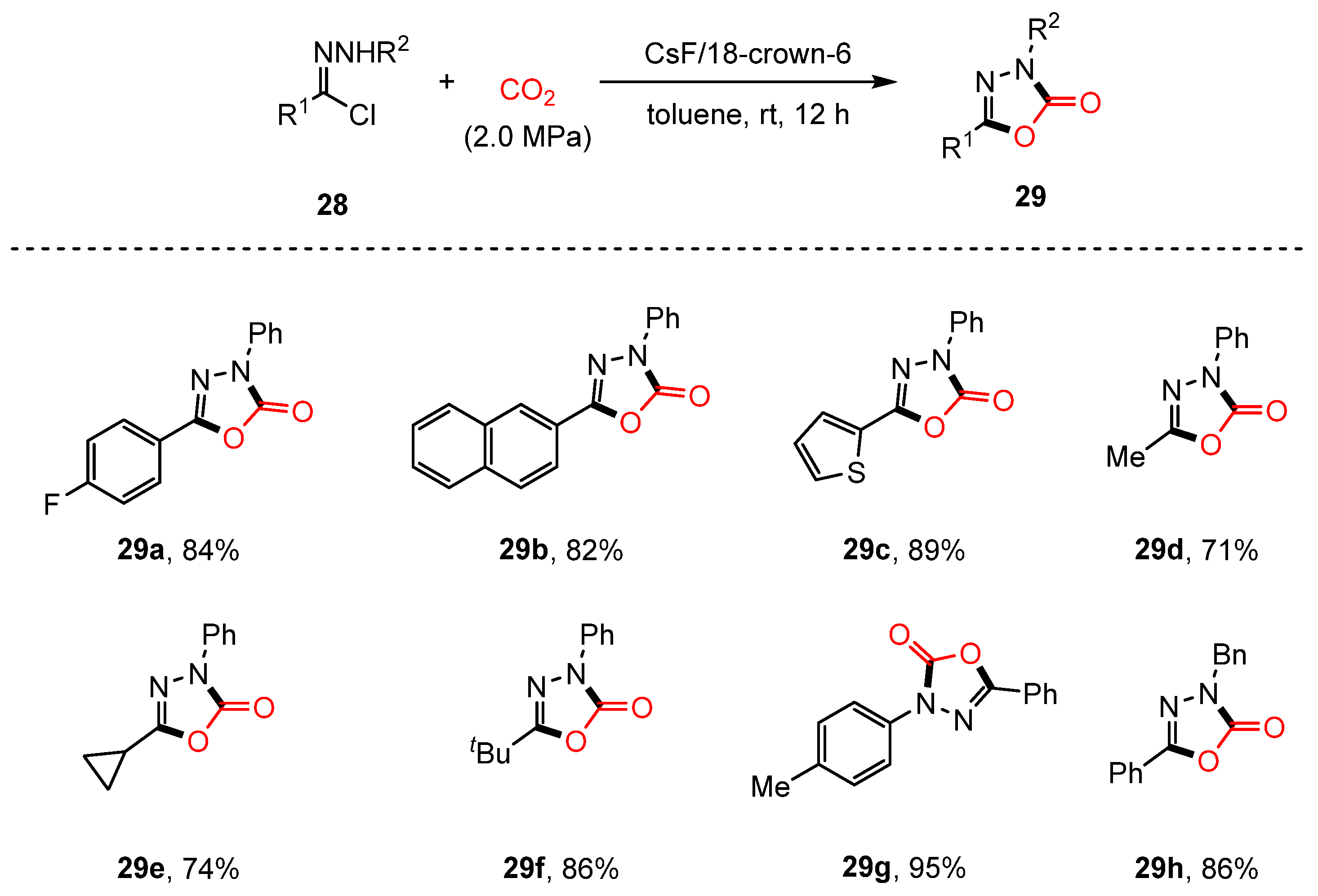
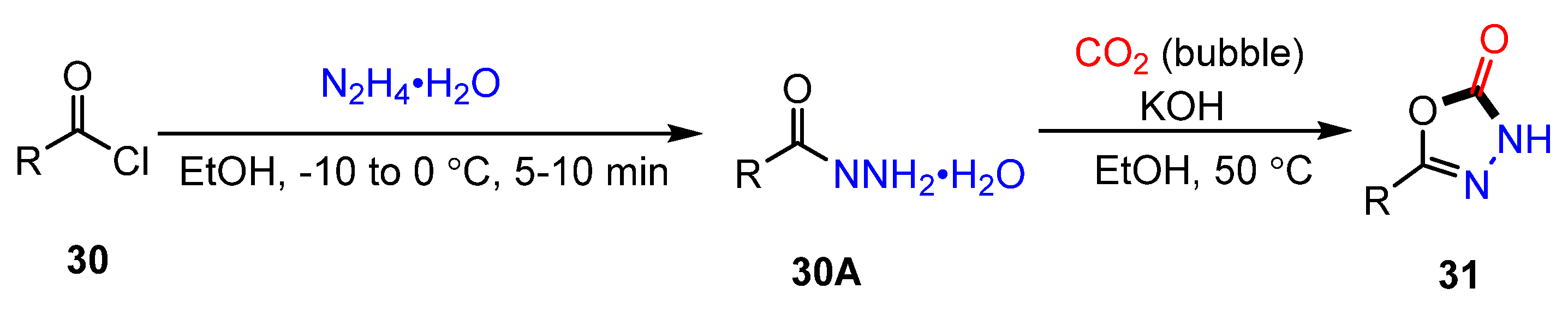
2.3. Cyclization of Hydrazones with CO2
2.4. Lactamization Reaction of N-Tosylhydrazones, 2-Iodoanilines, and CO2
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- He, L.N. Carbon Dioxide Chemistry; Science Press: Beijing, China, 2013; ISBN 978-7-03038333-4. [Google Scholar]
- Aresta, M. (Ed.) Carbon dioxide: Utilization options to reduce its accumulation in the atmosphere. In Carbon Dioxide as Chemical Feedstock; Wiley-VCH: Weinheim, Germany, 2010; pp. 9–13. ISBN 978-3-527-32521-0. [Google Scholar]
- Das, S. (Ed.) CO2 as a Building Block in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2020; pp. 253–285. ISBN 978-3-527-34734-2. [Google Scholar]
- Dufek, E.J.; Lister, T.E.; Stone, S.G.; McIlwain, M.E. Operation of a pressurized system for continuous reduction of CO2. J. Electrochem. Soc. 2012, 159, F514. [Google Scholar] [CrossRef]
- Gong, S.X.; Xie, X.M.; Sun, H.X.; Liu, Y.T.; Li, J.J.; Zhang, Z. Recent Progress on Multi-Component Reactions Involving Nucleophile, Arynes and CO2. Molecules 2024, 29, 3152. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Q.; Liao, L.L.; Ran, C.K.; Ye, J.H.; Yu, D.G. Recent advances in electrochemical carboxylation with CO2. Acc. Chem. Res. 2024, 57, 2728–2745. [Google Scholar] [CrossRef]
- Jia, S.H.; Ma, X.D.; Sun, X.F.; Han, B.X. Electrochemical transformation of CO2 to value-added chemicals and fuels. CCS Chem. 2022, 4, 3213–3229. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.H.; Ju, T.; Liao, L.L.; Huang, H.; Gui, Y.Y.; Zhou, W.J.; Yu, D.G. Visible-light-driven catalytic reductive carboxylation with CO2. ACS Catal. 2020, 10, 10871–10885. [Google Scholar] [CrossRef]
- Wang, S.; Xi, C.J. Recent advances in nucleophile-triggered CO2 -incorporated cyclization leading to heterocycles. Chem. Soc. Rev. 2019, 48, 382–404. [Google Scholar] [CrossRef]
- Song, L.; Jiang, Y.X.; Zhang, Z.; Gui, Y.Y.; Zhou, X.Y.; Yu, D.G. CO2 = CO+[O]: Recent advances in carbonylation of C–H bonds with CO2. Chem. Commun. 2020, 56, 8355–8367. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What should we make with CO2 and how can we make it? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef]
- Tortajada, A.; Juliá-Hernández, F.; Börjesson, M.; Moragas, T.; Martin, R. Transition-metal-catalyzed carboxylation reactions with carbon dioxide. Angew. Chem. Int. Ed. 2018, 57, 15948–15982. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Caillé, F.; Chevalier, A.; Loreau, O.; Horkka, K.; Halldin, C.; Schou, M.; Camus, N.; Kessler, P.; Kuhnast, B.; et al. Late-stage isotopic carbon labeling of pharmaceutically relevant cyclic ureas directly from CO2. Angew. Chem. Int. Ed. 2018, 57, 9744–9748. [Google Scholar] [CrossRef]
- Endrodi, B.; Kecsenovity, E.; Samu, A.; Darvas, F.; Jones, R.V.; Török, V.; Danyi, A.; Janáky, C. Multilayer electrolyzer stack converts carbon dioxide to gas products at high pressure with high efficiency. ACS Energy Lett. 2019, 4, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qi, C.R.; Xiong, W.F.; Jiang, H.F. Recent advances in fixation of CO2 into organic carbamates through multicomponent reaction strategies. Chin. J. Catal. 2022, 43, 1598–1617. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.H.; Takimoto, M.; Hou, Z.M. Carboxylation Reactions with Carbon Dioxide Using N-Heterocyclic Carbene-Copper Catalysts. Chem. Rec. 2020, 20, 494–512. [Google Scholar] [CrossRef] [PubMed]
- Lamaison, S.; Wakerley, D.; Blanchard, J.; Montero, D.; Rousse, G.; Mercier, D.; Rousse, G.; Mercier, D.; Marcus, P.; Taverna, D.; et al. High-current-density CO2-to-CO electroreduction on Ag-alloyed Zn dendrites at elevated pressure. Joule 2020, 4, 395–406. [Google Scholar] [CrossRef]
- Edwards, J.P.; Xu, Y.; Gabardo, C.M.; Dinh, C.T.; Li, J.; Qi, Z.B.; Ozden, A.; Sargent, E.H.; Sinton, D. Efficient electrocatalytic conversion of carbon dioxide in a low-resistance pressurized alkaline electrolyzer. Appl. Energy 2020, 261, 114305. [Google Scholar] [CrossRef]
- Zhang, W.Z.; LV, X.B. Synthesis of carboxylic acids and derivatives using CO2 as carboxylative reagent. Chin. J. Catal. 2012, 33, 745–756. [Google Scholar] [CrossRef]
- Babin, V.; Talbot, A.; Labiche, A.; Destro, G.; Del Vecchio, A.; Elmore, C.S.; Taran, F.; Sallustrau, A.; Audisio, D. Photochemical strategy for carbon isotope exchange with CO2. ACS Catal. 2021, 11, 2968–2976. [Google Scholar] [CrossRef]
- Li, G.; Long, Y.; Li, Z.; Li, S.P.; Zheng, Y.; He, B.H.; Zhou, M.; Hu, Z.Q.; Zhou, M.J.; Hou, Z.H. Reducing the charging voltage of a Zn–air battery to 1.6 V enabled by redox radical-mediated biomass oxidation. ACS Sustain. Chem. Eng. 2023, 11, 8642–8650. [Google Scholar] [CrossRef]
- Zanda, N.; Primitivo, L.; Chaudhari, M.; Kleij, A.W.; Pericàs, M.À. Organocatalytic N-formylation of amines by CO2 in batch and continuous flow. Org. Chem. Front. 2023, 10, 375–381. [Google Scholar] [CrossRef]
- Li, P.F.; Wang, Y.W.; Zhao, H.Y.; Qiu, Y.A. Electroreductive Cross-Coupling Reactions: Carboxylation, Deuteration, and Alkylation. Acc. Chem. Res. 2024, 58, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A.W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.L.; Xie, X.M.; Gao, T.Y.; Qin, J.; Li, J.J.; Chao, F.; Yu, D.G. Iron-promoted carbonylation–rearrangement of α-aminoaryl-tethered alkylidenecyclopropanes with CO2: Facile synthesis of quinolinofurans. Chin. Chem. Lett. 2025, 36, 110056. [Google Scholar] [CrossRef]
- Wolff, L. Chemischen institut der Universität Jena: Methode zum ersatz des sauerstoffatoms der ketone und aldehyde durch wasserstoff. Erste Abhandlung. Justus Liebigs Ann. Chem. 1912, 394, 86–108. [Google Scholar] [CrossRef]
- Wang, H.N.; Dai, X.J.; Li, C.J. Aldehydes as alkyl carbanion equivalents for additions to carbonyl compounds. Nat. Chem. 2017, 9, 374–378. [Google Scholar] [CrossRef]
- Chen, N.; Dai, X.J.; Wang, H.N.; Li, C.J. Umpolung addition of aldehydes to aryl imines. Angew. Chem. Int. Ed. 2017, 129, 6356–6359. [Google Scholar] [CrossRef]
- Wang, S.; König, B. Catalytic generation of carbanions through carbonyl umpolung. Angew. Chem. Int. Ed. 2021, 60, 21624–21634. [Google Scholar] [CrossRef]
- Dai, X.J.; Li, C.C.; Li, C.J. Carbonyl umpolung as an organometallic reagent surrogate. Chem. Soc. Rev. 2021, 50, 10733–10742. [Google Scholar] [CrossRef]
- Zhang, X.L.; Sivaguru, P.; Pan, Y.Z.; Wang, N.; Zhang, W.J.; Bi, X.H. The Carbene Chemistry of N-Sulfonyl Hydrazones: The Past, Present, and Future. Chem. Rev. 2025, 125, 1049–1190. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, J.B. Transition-metal-catalyzed cross-coupling with ketones or aldehydes via N-tosylhydrazones. J. Am. Chem. Soc. 2020, 142, 10592–10605. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.B. N-Tosylhydrazones: Versatile synthons in the construction of cyclic compounds. Chem. Soc. Rev. 2017, 46, 2306–2362. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Zhang, H.B. N-Tosylhydrazones: Versatile reagents for metal-catalyzed and metal-free cross-coupling reactions. Chem. Soc. Rev. 2012, 41, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Arunprasath, D.; Devi Bala, B.; Sekar, G. Luxury of N-Tosylhydrazones in Transition-Metal-Free Transformations. Adv. Synth. Catal. 2019, 361, 1172–1207. [Google Scholar] [CrossRef]
- Chaturvedi, D. Perspectives on the synthesis of organic carbamates. Tetrahedron 2012, 68, 15–45. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.Y.; Fukaya, N.; Choi, J.C. Alkali metal salt as catalyst for direct synthesis of carbamate from carbon dioxide. ACS Sustain. Chem. Eng. 2018, 6, 6675–6681. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Talbot, A.; Caillé, F.; Chevalier, A.; Sallustrau, A.; Loreau, O.; Destr, G.; Taran, F.; Audisio, D. Carbon isotope labeling of carbamates by late-stage [11C], [13C] and [14C] carbon dioxide incorporation. Chem. Commun. 2020, 56, 11677–11680. [Google Scholar] [CrossRef]
- Hair, P.I.; McCormack, P.L.; Curran, M.P. Eszopiclone-A Review of Its Use in the Treatment of Insomnia. Drugs 2008, 68, 1415–1434. [Google Scholar] [CrossRef]
- Wang, S.; Onaran, M.B.; Seto, C.T. Enantioselective Synthesis of 1-Aryltetrahydroisoquinolines. Org. Lett. 2010, 12, 2690–2693. [Google Scholar] [CrossRef]
- Crouzel, C.; Hinnen, F.; Maitre, E. Radiosynthesis of methyl and heptyl [11C] Isocyanates from [11C] phosgene, application to the synthesis of carbamates: [11C] physostygmine and [11C] heptylphysostigmine. Appl. Radiat. Isot. 1995, 46, 167–170. [Google Scholar] [CrossRef]
- Yoshimura, A.; Luedtke, M.W.; Zhdankin, V.V. (Tosylimino)phenyl—λ3—Iodine as a Reagent for the Synthesis of Methyl Carbamates via Hofmann Rearrangement of Aromatic and Aliphatic Carboxamides. J. Org. Chem. 2012, 77, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Ca’, N.D.; Gabriele, B.; Ruffolo, G.; Veltri, L.; Zanetta, T.; Costa, M. Effective Guanidine—Catalyzed Synthesis of Carbonate and Carbamate Derivatives from Propargyl Alcohols and Supercritical Carbon Dioxide. Adv. Synth. Catal. 2011, 353, 133–146. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Shin, S.I.; Nagle, A.S.; Jung, K.W. Efficient Carbamate Synthesis via a Three—Component Coupling of an Amine, CO2, and Alkyl Halides in the Presence of Cs2CO₃ and Tetrabutylammonium Iodide. J. Org. Chem. 2001, 66, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Hooker, J.M.; Reibel, A.T.; Hill, S.M.; Schueller, M.J.; Fowler, J.S. One—Pot, Direct Incorporation of [11C]CO2 into Carbamates. Angew. Chem. Int. Ed. 2009, 48, 3482–3485. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.M.; Zheng, S.C. Enantioselective Domino Reaction of CO2, Amines and Allyl Chlorides under Iridium Catalysis: Formation of Allyl Carbamates. Chem. Commun. 2014, 50, 4455–4458. [Google Scholar] [CrossRef]
- Riemer, D.; Hirasapara, P.; Das, S. Chemoselective Synthesis of Carbamates Using CO2 as Carbon Source. ChemSusChem 2016, 9, 1916–1920. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Mishra, N.; Mishra, V. An Efficient, One-Pot Synthesis of Carbamates from the Corresponding Alcohols Using Mitsunobu’s Reagent. Monatsh. Chem. 2007, 138, 57–60. [Google Scholar] [CrossRef]
- Dinsmore, C.J.; Mercer, S.P. Carboxylation and Mitsunobu Reaction of Amines to Give Carbamates: Retention vs Inversion of Configuration Is Substituent-Dependent. Org. Lett. 2004, 6, 2885–2888. [Google Scholar] [CrossRef]
- Ion, A.; Van Doorslaer, C.; Parvulescu, V.; Jacobs, P.; De Vos, D. Green synthesis of carbamates from CO2, amines and alcohols. Green Chem. 2008, 10, 111–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.H.; Wu, D.S.; Zhou, Y.Q.; Yu, D.G. Synthesis of Oxazolidin-2-ones from Unsaturated Amines with CO2 by Using Homogeneous Catalysis. Chem.-Asian J. 2018, 13, 2292–2306. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Y.; Wang, J.B. Diazo Compounds and N-Tosylhydrazones: Novel Cross-Coupling Partners in Transition-Metal-Catalyzed Reactions. Acc Chem. Res. 2013, 46, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, Y.; Wang, J.B. Catalytic Cascade Reactions Involving Metal Carbene Migratory Insertion. ACS Catal. 2013, 3, 2586–2598. [Google Scholar] [CrossRef]
- Davies, H.W.; Schwarz, M. The Effects of Hydrogen Bonding on the Absorption Spectra of Some Substituted Benzaldehyde Tosylhydrazone Anions. J. Org. Chem. 1965, 30, 1242–1244. [Google Scholar] [CrossRef]
- Xiong, W.F.; Qi, C.R.; He, H.T.; Ouyang, L.; Zhang, M.; Jiang, H.F. Base-Promoted Coupling of Carbon Dioxide, Amines, and N-Tosylhydrazones: A Novel and Versatile Approach to Carbamates. Angew. Chem. Int. Ed. 2015, 54, 3084–3087. [Google Scholar] [CrossRef]
- Hong, J.Y.; Seo, U.R.; Chung, Y.K. Synthesis of carbamates from amines and N-tosylhydrazones under atmospheric pressure of carbon dioxide without an external base. Org. Chem. Front. 2016, 3, 764–767. [Google Scholar] [CrossRef]
- Qi, C.R.; Yan, D.H.; Xiong, W.F.; Jiang, H.F. Silver-Catalyzed Three-Component Coupling of Carbon Dioxide, Amines and α-Diazoesters. Chin. J. Chem. 2018, 36, 399–405. [Google Scholar] [CrossRef]
- Cheng, R.X.; Qi, C.R.; Wang, L.; Xiong, W.F.; Liu, H.J.; Jiang, H.F. Visible light-promoted synthesis of organic carbamates from carbon dioxide under catalyst-and additive-free conditions. Green Chem. 2020, 22, 4890–4895. [Google Scholar] [CrossRef]
- Xiong, H.; Wu, X.P.; Wang, H.P.; Sun, S.; Yu, J.T.; Cheng, J. The Reaction of o-Aminoacetophenone N-Tosylhydrazone and CO2 toward 1,4-Dihydro-2H-3,1-benzoxazin-2-ones. Adv. Synth. Catal. 2019, 361, 3538–3542. [Google Scholar] [CrossRef]
- Seebach, D. Methods of reactivity umpolung. Angew. Chem. Int. Ed. 1979, 18, 239–258. [Google Scholar] [CrossRef]
- Marion, N.; Díez-González, S.; Nolan, S.P. N-heterocyclic carbenes as organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2988–3000. [Google Scholar] [CrossRef]
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Yang, M.H.; Matikonda, S.S.; Altman, R.A. Preparation of fluoroalkenes via the shapiro reaction: Direct access to fluorinated peptidomimetics. Org. Lett. 2013, 15, 3894–3897. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.J.; Morrison, A.J.; Pazicky, M.; Weber, T. Modified Shapiro reactions with bismesitylmagnesium as an efficient base reagent. Org. Lett. 2012, 14, 2250–2253. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, V.; Zhai, H.M.; Hall, D.G. Convenient Preparation of Cycloalkenyl Boronic Acid Pinacol Esters. Synth. Commun. 2008, 38, 3984–3995. [Google Scholar] [CrossRef]
- Adlington, R.M.; Barrett, A.G.M. Recent applications of the Shapiro reaction. Acc. Chem. Res. 1983, 16, 55–59. [Google Scholar] [CrossRef]
- Paquette, L.A.; Fristad, W.E.; Dime, D.S.; Bailey, T.R. Silanes in organic synthesis. 8. Preparation of vinylsilanes from ketones and their regiospecific cyclopentenone annulation. J. Org. Chem. 1980, 45, 3017–3028. [Google Scholar] [CrossRef]
- Chamberlin, A.R.; Stemke, J.E.; Bond, F.T. Vinyllithium reagents from arenesulfonylhydrazones. J. Org. Chem. 2002, 43, 147–154. [Google Scholar] [CrossRef]
- Shapiro, R.H. Alkenes from tosylhydrazones. Org. React. 2004, 23, 405–507. [Google Scholar] [CrossRef]
- Stemke, J.E.; Chamberlin, A.R.; Bond, F.T. A convenient route to vinyllithium reagents. Tetrahedron Lett. 1976, 17, 2947–2950. [Google Scholar] [CrossRef]
- Sun, S.; Yu, J.T.; Jiang, Y.; Cheng, J. Cs2CO₃-promoted carboxylation of N-tosylhydrazones with carbon dioxide toward α-arylacrylic acids. J. Org. Chem. 2015, 80, 2855–2860. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.S.; Zhu, L.; Ye, J.H.; Zhang, Z.; Huang, H.; Zeng, H.Y.; Yu, L.; Yu, D.G. Ruthenium-catalyzed umpolung carboxylation of hydrazones with CO2. Chem. Sci. 2018, 9, 4873–4878. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, B.Y.; Sršen, M.; König, B. Umpolung difunctionalization of carbonyls via visible-light photoredox catalytic radical-carbanion relay. J. Am. Chem. Soc. 2020, 142, 7524–7531. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Zhang, W.Z.; Zhang, N.; Lu, X.B. 1,3-Dipolar cycloaddition of nitrile imine with carbon dioxide: Access to 1,3,4-oxadiazole-2(3H)-ones. J. Org. Chem. 2017, 82, 7637–7642. [Google Scholar] [CrossRef]
- Brahmayya, M.; Dai, S.A.; Suen, S.Y. Synthesis of 5-substituted-3 H-[1, 3, 4]-oxadiazol-2-one derivatives: A carbon dioxide route (CDR). RSC Adv. 2015, 5, 65351–65357. [Google Scholar] [CrossRef]
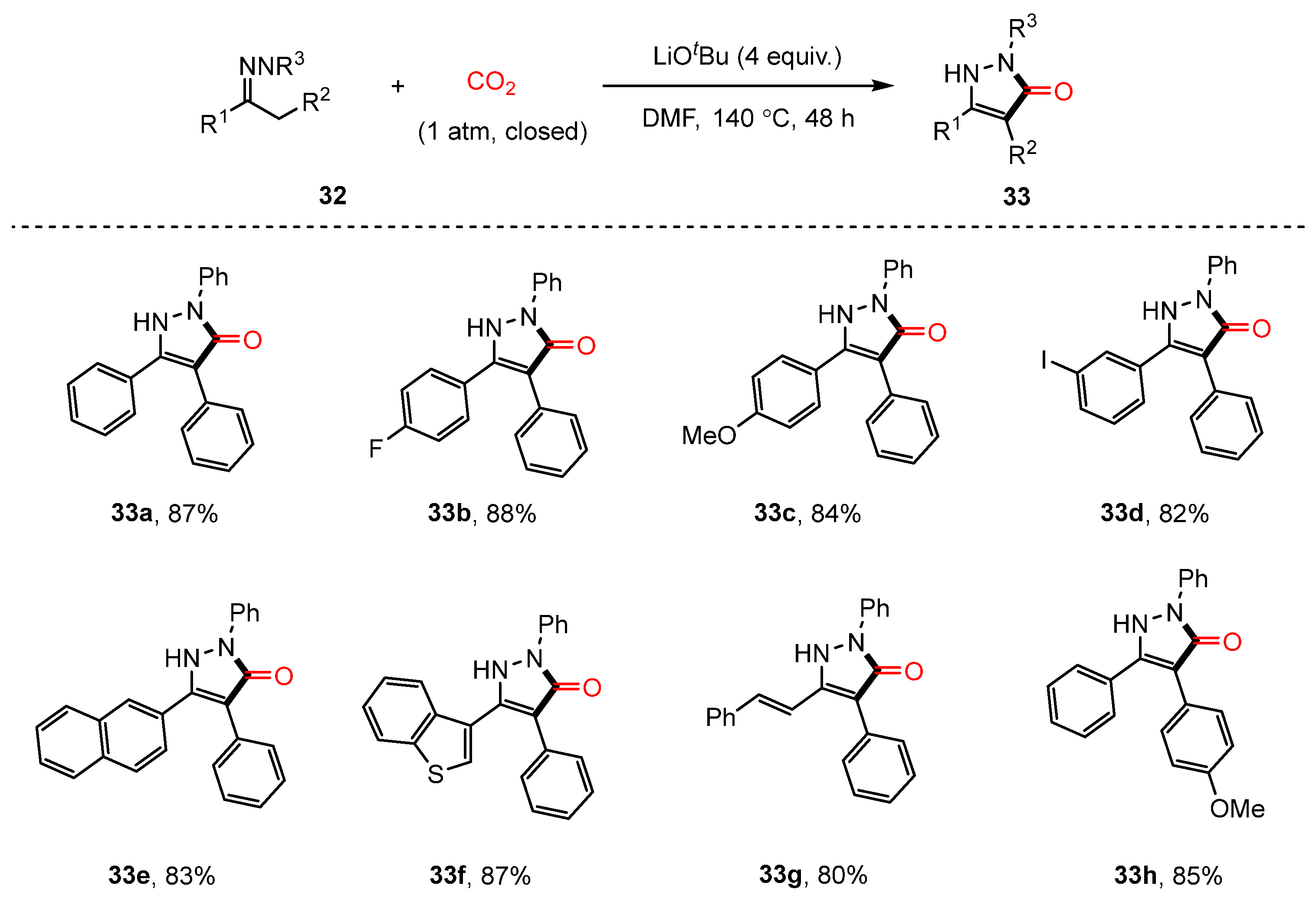
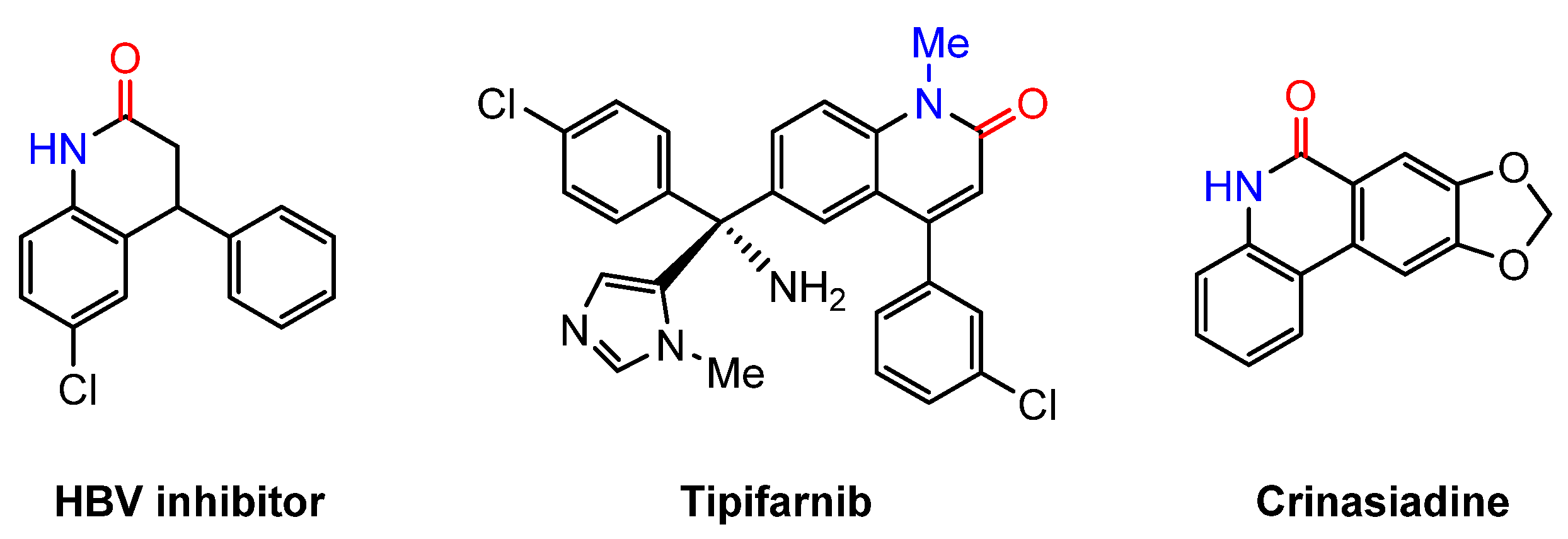
- Wang, K.; Ouyang, J.; Liu, H.; Yin, L.J.; Yang, K.Q.; Lan, L.F.; Hu, Y.H.; Hu, N.F. C(sp3)–H Carbonylative Cyclization of Hydrazones with CO2: Synthesis of Pyrazolone Derivatives. J. Org. Chem. 2024, 89, 18746–18751. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, L.L.; Yan, S.S.; Wang, L.; He, Y.Q.; Ye, J.H.; Li, J.; Zhi, Y.G.; Yu, D.G. Lactamization of sp2 C-H Bonds with CO2: Transition-Metal-Free and Redox-Neutral. Angew. Chem. Int. Ed. 2016, 55, 7068–7072. [Google Scholar] [CrossRef]
- Sun, S.; Hu, W.M.; Gu, N.; Cheng, J. Palladium-Catalyzed Multi-Component Reactions of N-Tosylhydrazones, 2-Iodoanilines and CO2 towards 4-Aryl-2-Quinolinones. Chem. Eur. J. 2016, 22, 18729–18732. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.-X.; Gong, S.-X.; Zhang, H.-Y.; Liu, Y.-T.; Shi, L.-L.; Zhu, Y.-J.; Xie, X.-M.; Li, J.-J.; Wen, J.; Guan, Y.-C.; et al. Research Progress on the Reaction of Carbon Dioxide with Hydrazones and Their Derivatives. Molecules 2025, 30, 1987. https://doi.org/10.3390/molecules30091987
Sun H-X, Gong S-X, Zhang H-Y, Liu Y-T, Shi L-L, Zhu Y-J, Xie X-M, Li J-J, Wen J, Guan Y-C, et al. Research Progress on the Reaction of Carbon Dioxide with Hydrazones and Their Derivatives. Molecules. 2025; 30(9):1987. https://doi.org/10.3390/molecules30091987
Chicago/Turabian StyleSun, Hong-Xia, Shao-Xuan Gong, Hong-Yang Zhang, Yu-Ting Liu, Li-Ling Shi, Yong-Jie Zhu, Xiu-Mei Xie, Jun-Jie Li, Jing Wen, Yong-Chang Guan, and et al. 2025. "Research Progress on the Reaction of Carbon Dioxide with Hydrazones and Their Derivatives" Molecules 30, no. 9: 1987. https://doi.org/10.3390/molecules30091987
APA StyleSun, H.-X., Gong, S.-X., Zhang, H.-Y., Liu, Y.-T., Shi, L.-L., Zhu, Y.-J., Xie, X.-M., Li, J.-J., Wen, J., Guan, Y.-C., Zhang, Z., Zhang, M., & Zhang, Y.-F. (2025). Research Progress on the Reaction of Carbon Dioxide with Hydrazones and Their Derivatives. Molecules, 30(9), 1987. https://doi.org/10.3390/molecules30091987






