An “On–Off” AIE-Based Lock-and-Key Fluorescent Probe System for Detection of Fentanyl/Norfentanyl
Abstract
1. Introduction
2. Results
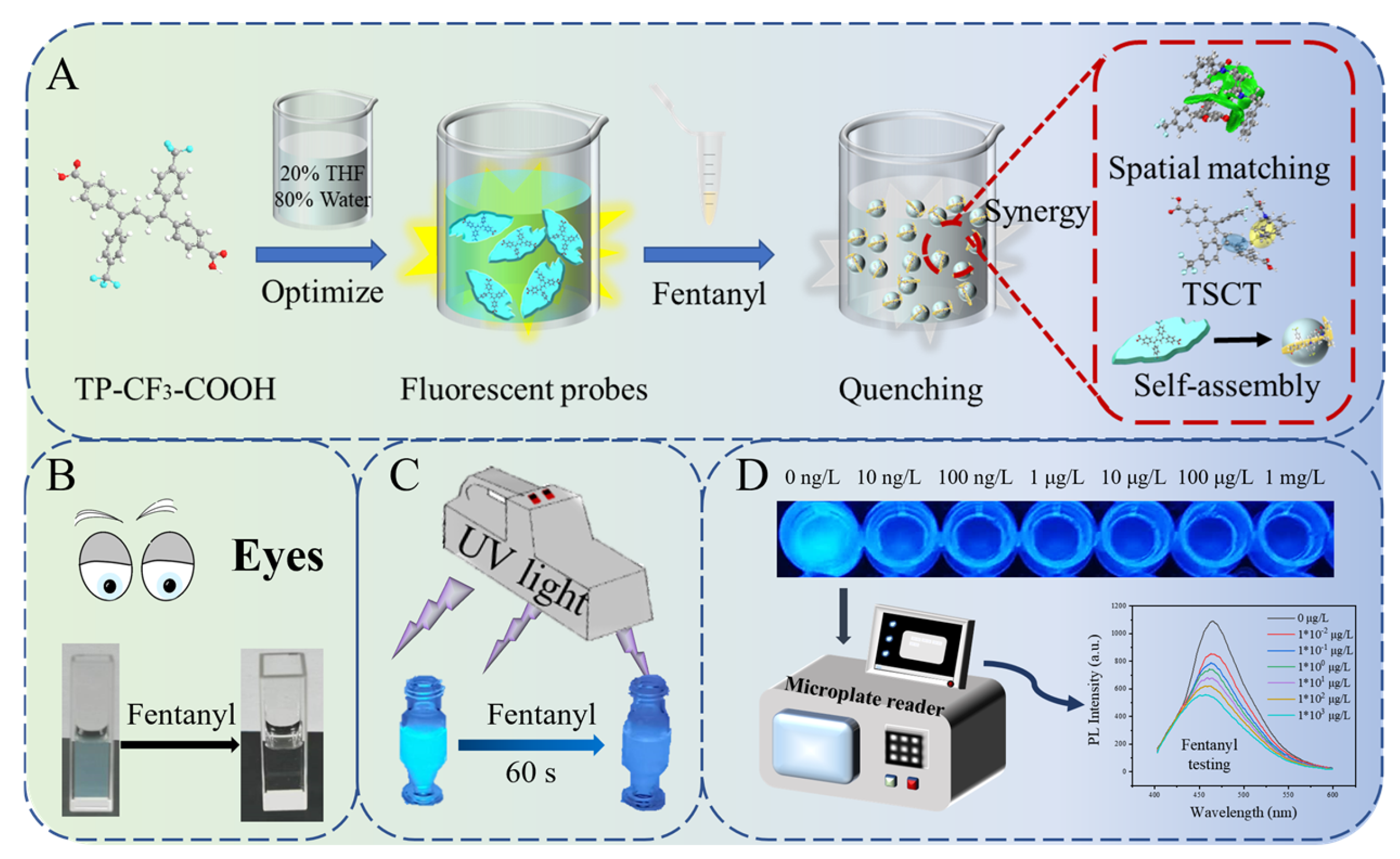
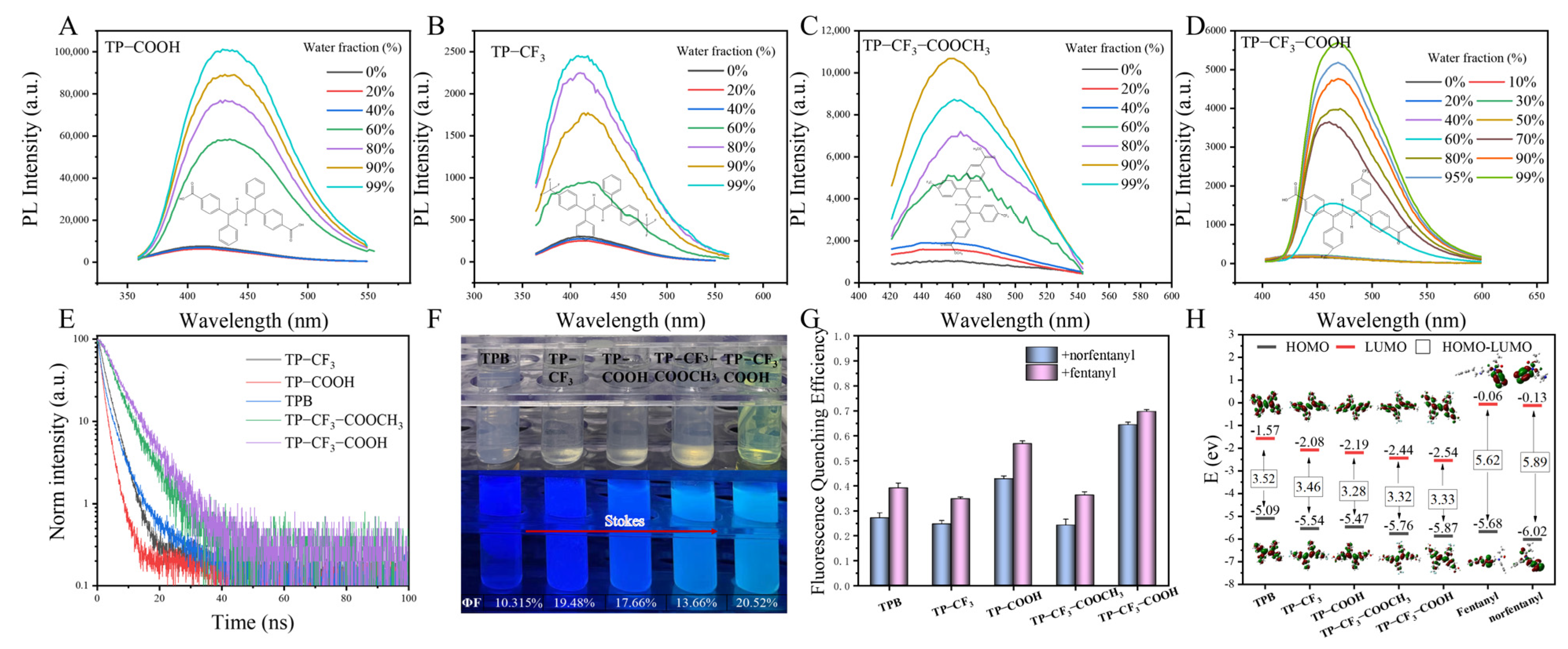
2.1. Synthesis and Characterization
2.2. Probe Recognition Mechanism
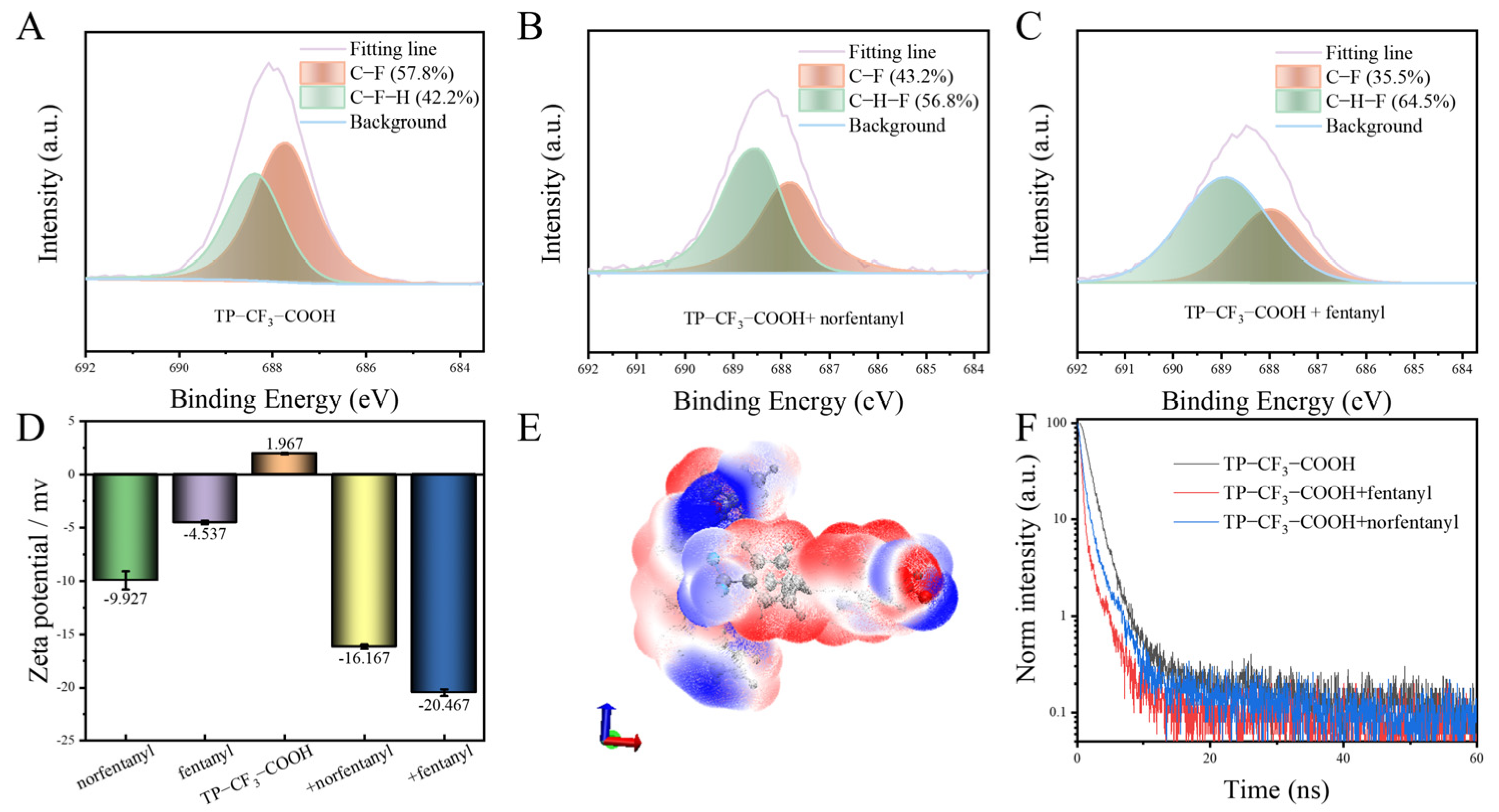
2.3. Multiple Specific Interactions Between Probes and Fentanyl/Norfentanyl
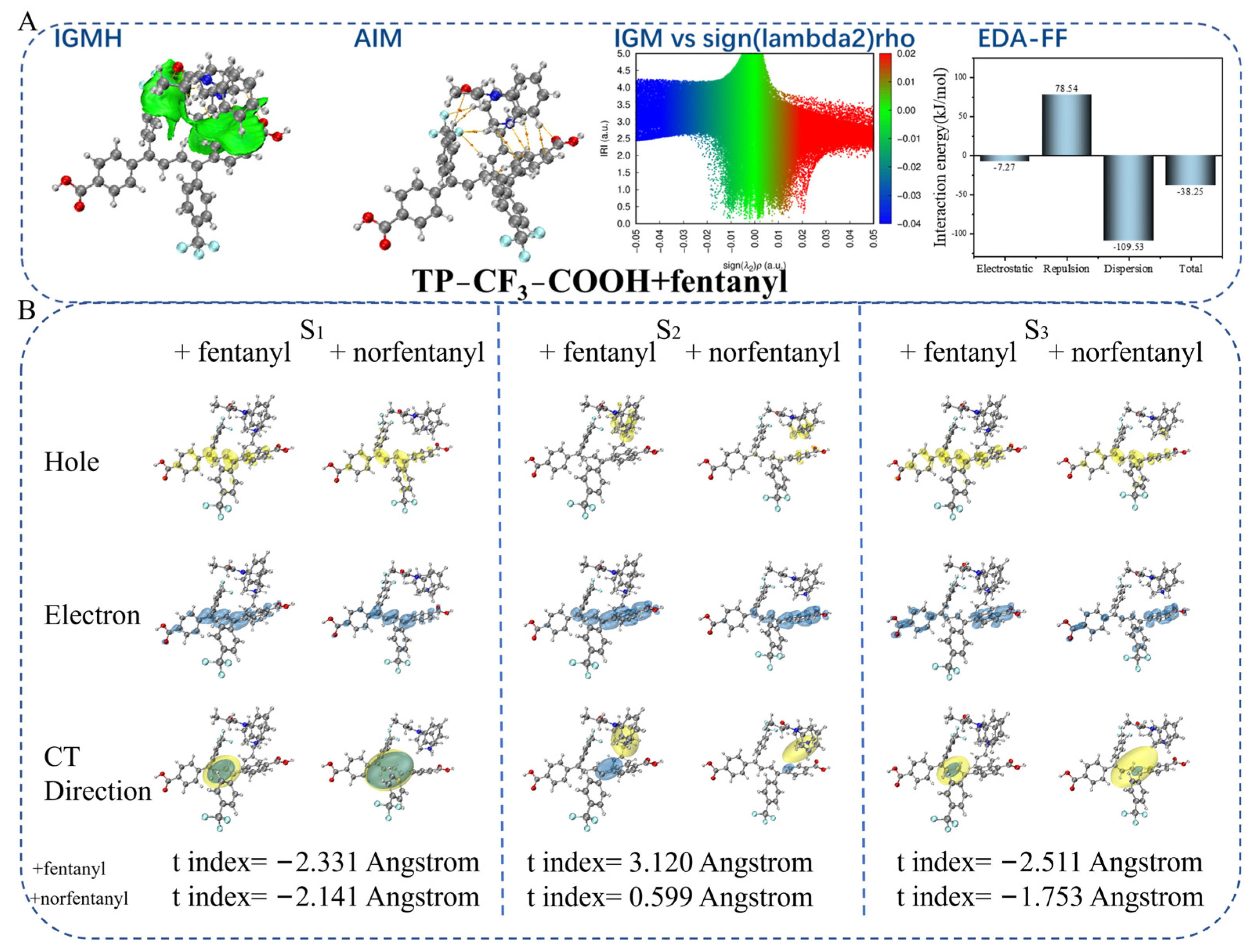
2.4. Theoretical Calculation
2.5. Analysis of Samples
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Probes
3.3. Preparation of Probe and Sample Solution
3.4. Isothermal Titration Calorimetry (ITC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciccarone, D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr. Opin. Psychiatry 2021, 34, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Prekupec, M.P.; Mansky, P.A.; Baumann, M.H. Misuse of novel synthetic opioids: A deadly new trend. J. Addict. Med. 2017, 11, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Faggiani, F.; Bolchi, C.; Pallavicini, M.; Dei Cas, M. Ten years of fentanyl-like drugs: A technical-analytical review. Anal. Sci. 2019, 35, 479–491. [Google Scholar] [CrossRef]
- Kempen, A. World Drug Report 2024. Available online: https://www.unodc.org/unodc/es/data-and-analysis/world-drug-report-2024.html (accessed on 12 January 2025).
- Coffin, P.O.; McMahan, V.M.; Murphy, C. Evidence of pre-mortem opioid use among fentanyl overdose decedents in a safety net healthcare system. J. Urban Health 2022, 99, 865–872. [Google Scholar] [CrossRef]
- de Bruin-Hoegee, M.; Kleiweg, D.; Noort, D.; van Asten, A.C. Chemical attribution of fentanyl: The effect of human metabolism. Forensic Chem. 2021, 24, 100330. [Google Scholar] [CrossRef]
- Tokonami, F.; Kimble, B.; Govendir, M. Pharmacokinetic profile of fentanyl in the koala (Phascolarctos cinereus) after intravenous administration, and absorption via a transdermal patch. Animals 2021, 11, 3550. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Kuczynska, K.; Kosmal, W.; Markiewicz, K.; Adamowicz, P. Carfentanil—From an animal anesthetic to a deadly illicit drug. Forensic Sci. Int. 2021, 320, 110715. [Google Scholar] [CrossRef]
- Kral, A.H.; Lambdin, B.H.; Browne, E.N.; Wenger, L.D.; Bluthenthal, R.N.; Zibbell, J.E.; Davidson, P.J. Transition from injecting opioids to smoking fentanyl in San Francisco, California. Drug Alcohol Depend. 2021, 227, 109003. [Google Scholar] [CrossRef]
- Eger, W.H.; Abramovitz, D.; Bazzi, A.R.; Bórquez, A.; Vera, C.F.; Harvey-Vera, A.; Friedman, J.R.; Strathdee, S.A. Changes in injecting versus smoking heroin, fentanyl, and methamphetamine among people who inject drugs in San Diego, California, 2020–2023. Drug Alcohol Depend. 2024, 259, 111318. [Google Scholar] [CrossRef]
- Clarke, S.E.D.; Kral, A.H.; Zibbell, J.E. Consuming illicit opioids during a drug overdose epidemic: Illicit fentanyls, drug discernment, and the radical transformation of the illicit opioid market. Int. J. Drug Policy 2022, 99, 103467. [Google Scholar] [CrossRef]
- Wilson, N.G.; Raveendran, J.; Docoslis, A. Portable identification of fentanyl analogues in drugs using surface-enhanced Raman scattering. Sens. Actuators B Chem. 2021, 330, 129303. [Google Scholar] [CrossRef]
- Shao, W.; Sorescu, D.C.; Liu, Z.; Star, A. Machine learning discrimination and ultrasensitive detection of fentanyl using gold nanoparticle-decorated carbon nanotube-based field-effect transistor sensors. Small 2024, 20, 2311835. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liu, X.; Xie, Y.; Chen, M.; Zhong, H.; Li, M. Quantitative label-free SERS detection of trace fentanyl in biofluids with a freestanding hydrophobic plasmonic paper biosensor. Anal. Chem. 2023, 95, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, S.O.; Brady, P.N.; Grant, C.; Narcisse, V.F.; Flores, P.R.; Lisse, C.H.; Bwambok, D.K. Electrochemical sensors, biosensors, and optical sensors for the detection of opioids and their analogues: Pharmaceutical, clinical, and forensic applications. Chemosensors 2024, 12, 58. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, J.F.; Xiang, X.Y.; Yu, H.L.; Shao, B.; He, Y. Surfactants directly participate in the molecular recognition for visual and sensitive detection of fentanyl. Sens. Actuators B Chem. 2022, 354, 131215. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, Y. Overcoming interferences in the colorimetric and fluorimetric detection of γ-hydroxybutyrate in spiked beverages. Sens. Actuators B Chem. 2022, 364, 131861. [Google Scholar] [CrossRef]
- Zou, R.; Yu, Y.; Pan, H.; Zhang, P.; Cheng, F.; Zhang, C.; Chen, S.; Chen, J.; Zeng, R. Cross-linking induced emission of polymer micelles for high-contrast visualization level 3 details of latent fingerprints. ACS Appl. Mater. Interfaces 2022, 14, 16746–16754. [Google Scholar] [CrossRef]
- Liu, K.; Hu, D.; He, L.; Wang, Z.; Cheng, P.; Sun, P.; Chen, Y.; Li, D. Cationic conjugated polymer coupled non-conjugated segments for dually enhanced NIR-II fluorescence and lower-temperature photothermal-gas therapy. J. Nanobiotechnol. 2024, 22, 451. [Google Scholar] [CrossRef]
- Filipek, P.; Kałkus, M.; Szlapa-Kula, A.; Filapek, M. Bithiophene-based donor–π–acceptor compounds exhibiting aggregation-induced emission as agents to detect hidden fingerprints and electrochromic materials. Molecules 2024, 29, 5747. [Google Scholar] [CrossRef]
- Shi, D.; Yang, Y.; Tong, L.; Zhang, L.; Yang, F.; Tao, J.; Zhao, M. A novel benzothiazole-based fluorescent AIE probe for the detection of hydrogen peroxide in living cells. Molecules 2024, 29, 5181. [Google Scholar] [CrossRef]
- Wu, Q.L.; Huang, F.Y.; Jiang, Y.Y.; Chen, Y.L.; Jiang, P.E.; Lou, Y.L.; Zheng, Y.; Zheng, L.B. Firefly lantern-inspired AIE-enhanced gold nanocluster microspheres for ultrasensitive detection of foodborne pathogenic bacteria. Sens. Actuators B Chem. 2025, 422, 136584. [Google Scholar] [CrossRef]
- Wu, W.N.; Chen, X.; Liu, S.S.; Bie, H.Y.; Fan, Y.C.; Wang, Y.; Xu, Z.H.; James, T.D. A dual-response fluorescent hemicyanine probe for the detection of mitochondrial hypochlorite and viscosity based on ESIPT/AIE and TICT. Sens. Actuators B Chem. 2025, 423, 136695. [Google Scholar] [CrossRef]
- Chin, K.L.O.; Ong, P.J.; Zhu, Q.; Xu, J.; Chua, M.H. Electrofluorochromic switching of heat-induced cross-linkable multi-styryl-terminated triphenylamine and tetraphenylethylene derivatives. Molecules 2024, 29, 2340. [Google Scholar] [CrossRef]
- Deng, L.; Xiong, J.; Liu, W.; Wu, L.; Hu, H.; Wu, J.; Liu, Y.; Yu, L.; Zhou, Y.; Gao, W.; et al. A novel fluorescence sensor for iodide detection based on the 1,3-diaryl pyrazole unit with AIE and mechanochromic fluorescence behavior. Molecules 2023, 28, 7111. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Alam, P.; Bai, H.; Bhalla, V.; Bryce, M.R.; Cao, M.; Chen, C.; Chen, S.; Chen, X.; et al. Aggregation-Induced Emission (AIE), life and health. ACS Nano 2023, 17, 14347–14405. [Google Scholar] [CrossRef]
- Chen, J.; Xia, J.; Gao, W.-J.; Yu, H.-J.; Zhong, J.-X.; Jia, C.; Qin, Y.-S.; She, Z.; Kuang, D.-B.; Shao, G. Tetraphenylbutadiene-based symmetric 3D hole-transporting materials for perovskite solar cells: A trial trade-off between charge mobility and film morphology. ACS Appl. Mater. Interfaces 2020, 12, 21088–21099. [Google Scholar] [CrossRef]
- Chen, J.W.; Xu, B.; Ouyang, X.Y.; Tang, B.Z.; Cao, Y. Aggregation-induced emission of 1,2,3,4-tetraphenylbutadiene from restricted intramolecular rotation. J. Phys. Chem. A 2004, 108, 7522–7526. [Google Scholar] [CrossRef]
- Zheng, Y.; Kobayashi, Y.; Sekine, T.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Visible chiral discrimination via macroscopic selective assembly. Commun. Chem. 2018, 1, 4. [Google Scholar] [CrossRef]
- Barman, D.; Narang, K.; Parui, R.; Zehra, N.; Khatun, M.N.; Adil, L.R.; Iyer, P.K. Review on recent trends and prospects in π-conjugated luminescent aggregates for biomedical applications. Aggregate 2022, 3, e172. [Google Scholar] [CrossRef]
- Marenco, A.J.; Pillai, R.G.; Harris, K.D.; Chan, N.W.C.; Jemere, A.B. Electrochemical determination of fentanyl using carbon nanofiber-modified electrodes. ACS Omega 2024, 9, 17592–17601. [Google Scholar] [CrossRef]
- Sherard, M.M.; Kaplan, J.S.; Simpson, J.H.; Kittredge, K.W.; Leopold, M.C. Functionalized gold nanoparticles and halogen bonding interactions involving fentanyl and fentanyl derivatives. Nanomaterials 2024, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Deng, Z.; Huang, J.; Wen, X.; Zeng, S. NIR-II responsive upconversion nanoprobe with simultaneously enhanced single-band red luminescence and phase/size control for bioimaging and photodynamic therapy. Adv. Mater. 2023, 35, e2207038. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-L.; Wang, H.; Zhang, J.; Wu, B.; Li, Q.; Chen, J.-Y.; Tang, A.-L.; Lam, J.W.Y.; Zhao, Z.; Yang, S.; et al. Understanding the AIE phenomenon of nonconjugated rhodamine derivatives via aggregation-induced molecular conformation change. Nat. Commun. 2024, 15, 999. [Google Scholar] [CrossRef] [PubMed]
- Goetz, K.P.; Vermeulen, D.; Payne, M.E.; Kloc, C.; McNeil, L.E.; Jurchescu, O.D. Charge-transfer complexes: New perspectives on an old class of compounds. J. Mater. Chem. C 2014, 2, 3065–3076. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; He, B.; He, X.; Zhou, X.E.; Guo, S.; Rao, Q.; Yang, J.; Liu, J.; Zhou, Q.; et al. Molecular recognition of morphine and fentanyl by the human μ-opioid receptor. Cell 2022, 185, 4361–4375. [Google Scholar] [CrossRef]
- Zhao, J.; Elgeti, M.; O’Brien, E.S.; Sar, C.P.; El Daibani, A.; Heng, J.; Sun, X.; White, E.; Che, T.; Hubbell, W.L.; et al. Ligand efficacy modulates conformational dynamics of the µ-opioid receptor. Nature 2024, 629, 474–480. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, J.; Wang, D.; Yan, Y.; Huang, J.; Tang, B.Z. Insights into self-assembly of nonplanar molecules with aggregation-induced emission characteristics. ACS Nano 2022, 16, 20559–20566. [Google Scholar] [CrossRef]
- Ye, Q.; Guo, L.; Wu, D.; Yang, B.; Tao, Y.; Deng, L.; Kong, Y. Covalent functionalization of bovine serum albumin with graphene quantum dots for stereospecific molecular recognition. Anal. Chem. 2019, 91, 11864–11871. [Google Scholar] [CrossRef]
- He, B.; Huang, J.; Zhang, J.; Sung, H.H.Y.; Lam, J.W.Y.; Zhang, Z.; Yan, S.; Wang, D.; Zhang, J.; Tang, B.Z. Corrigendum: Correction to “Novel quinolizine AIE system: Visualization of molecular motion and elaborate tailoring for biological application”. Angew. Chem. Int. Ed. 2024, 63, e202408934. [Google Scholar] [CrossRef]
- Park, M.; Kim, H.S.; Yoon, H.; Kim, J.; Lee, S.; Yoo, S.; Jeon, S. Controllable singlet–triplet energy splitting of graphene quantum dots through oxidation: From phosphorescence to TADF. Adv. Mater. 2020, 32, e2000936. [Google Scholar] [CrossRef]
- Xu, X.; Mo, L.; Li, Y.; Pan, X.; Hu, G.; Lei, B.; Zhang, X.; Zheng, M.; Zhuang, J.; Liu, Y.; et al. Construction of carbon dots with color-tunable aggregation-induced emission by nitrogen-induced intramolecular charge transfer. Adv. Mater. 2021, 33, 2104872. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Peng, J.; Yang, S.; Hu, R.; Jiang, X. A donor–acceptor cross-conjugated phenazine macrocycle with a large Stokes shift for sensing transition metal ions with “turn-on” fluorescence. J. Mater. Chem. C 2024, 12, 10127. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Comprehensive Computational Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 240–264. [Google Scholar] [CrossRef]
- Wang, R.; Song, K.Y.; Yang, L.X.; Sun, Y.; Sun, X.X.; Hu, Y. Multiple applications of chiral self-assembled H8–BINOL nanoprobes with AIE-ESIPT characteristics: Chiral switching and pH visualization detection. ACS Appl. Mater. Interfaces 2024, 16, 70783–70794. [Google Scholar] [CrossRef]
- Nan, Q.; Ping, X.; Baohua, S.; Xianyi, Z.; Yan, S.; Song, F.Y. Application of a validated UHPLC-MS/MS method for 28 fentanyl-analogue and novel synthetic opioids in whole blood in authentic forensic cases. J. Chromatogr. B 2019, 1124, 82–99. [Google Scholar] [CrossRef]
- Nazdrajic, E.; Rickert, D.A.; Pawliszyn, J. Rapid analysis of fentanyl and fentanyl analogues from whole blood using SPME coupled to the microfluidic open interface. Anal. Chem. 2024, 96, 821–827. [Google Scholar] [CrossRef]
- Conrado, T.T.; Pedao, E.R.; Valdir, S.F.; Katia, M.; Lucca, B.G. Sensitive, integrated, mass-produced, portable and low-cost electrochemical 3D-printed sensing set (SIMPLE-3D-SenS): A promising analytical tool for forensic applications. Sens. Actuators B Chem. 2025, 427, 137215. [Google Scholar] [CrossRef]
- Gao, Y.J.; Shirinichi, F.; Audrey, H.; Runyao, Z.; Wang, Y.C. A supramolecular–quantum dot system for broad-spectrum detection of fentanyl analogues. Small 2024, 21, 2407702. [Google Scholar] [CrossRef]
- Hu, G.Y.; Li, H.X.; Liu, F. A biosensor based on nanocomposite of g-C3N4 and polyaniline for detection of fentanyl as a doping agent in sports. Alex. Eng. J. 2024, 87, 515–523. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.; Gu, S.; Zhou, T.; Zhao, C.; Guo, Y.; Feng, X.; Tong, B.; Bing, J.; Shi, J.; et al. Mechanochromic behavior of aryl-substituted buta-1,3-diene derivatives with aggregation enhanced emission. Chem. A Eur. J. 2014, 20, 8856–8861. [Google Scholar] [CrossRef]
- Guo, Y.X.; Feng, X.; Han, T.Y.; Wang, S.; Lin, Z.G.; Dong, Y.P.; Wang, B. Tuning the luminescence of metal-organic frameworks for detection of energetic heterocyclic compounds. J. Am. Chem. Soc. 2014, 2, 1520–5126. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhi, J.; Zhang, L.; Qi, Y.; Sun, J.; Jin, Y.; Yin, J.; Yao, K.; Shao, B. An “On–Off” AIE-Based Lock-and-Key Fluorescent Probe System for Detection of Fentanyl/Norfentanyl. Molecules 2025, 30, 1985. https://doi.org/10.3390/molecules30091985
Sun J, Zhi J, Zhang L, Qi Y, Sun J, Jin Y, Yin J, Yao K, Shao B. An “On–Off” AIE-Based Lock-and-Key Fluorescent Probe System for Detection of Fentanyl/Norfentanyl. Molecules. 2025; 30(9):1985. https://doi.org/10.3390/molecules30091985
Chicago/Turabian StyleSun, Jing, Junge Zhi, Li Zhang, Yan Qi, Jiefang Sun, Yushen Jin, Jie Yin, Kai Yao, and Bing Shao. 2025. "An “On–Off” AIE-Based Lock-and-Key Fluorescent Probe System for Detection of Fentanyl/Norfentanyl" Molecules 30, no. 9: 1985. https://doi.org/10.3390/molecules30091985
APA StyleSun, J., Zhi, J., Zhang, L., Qi, Y., Sun, J., Jin, Y., Yin, J., Yao, K., & Shao, B. (2025). An “On–Off” AIE-Based Lock-and-Key Fluorescent Probe System for Detection of Fentanyl/Norfentanyl. Molecules, 30(9), 1985. https://doi.org/10.3390/molecules30091985






