Synthesis and Characterisation of Multivariate Metal–Organic Frameworks for Controlled Doxorubicin Absorption and Release
Abstract
1. Introduction
2. Results and Discussion
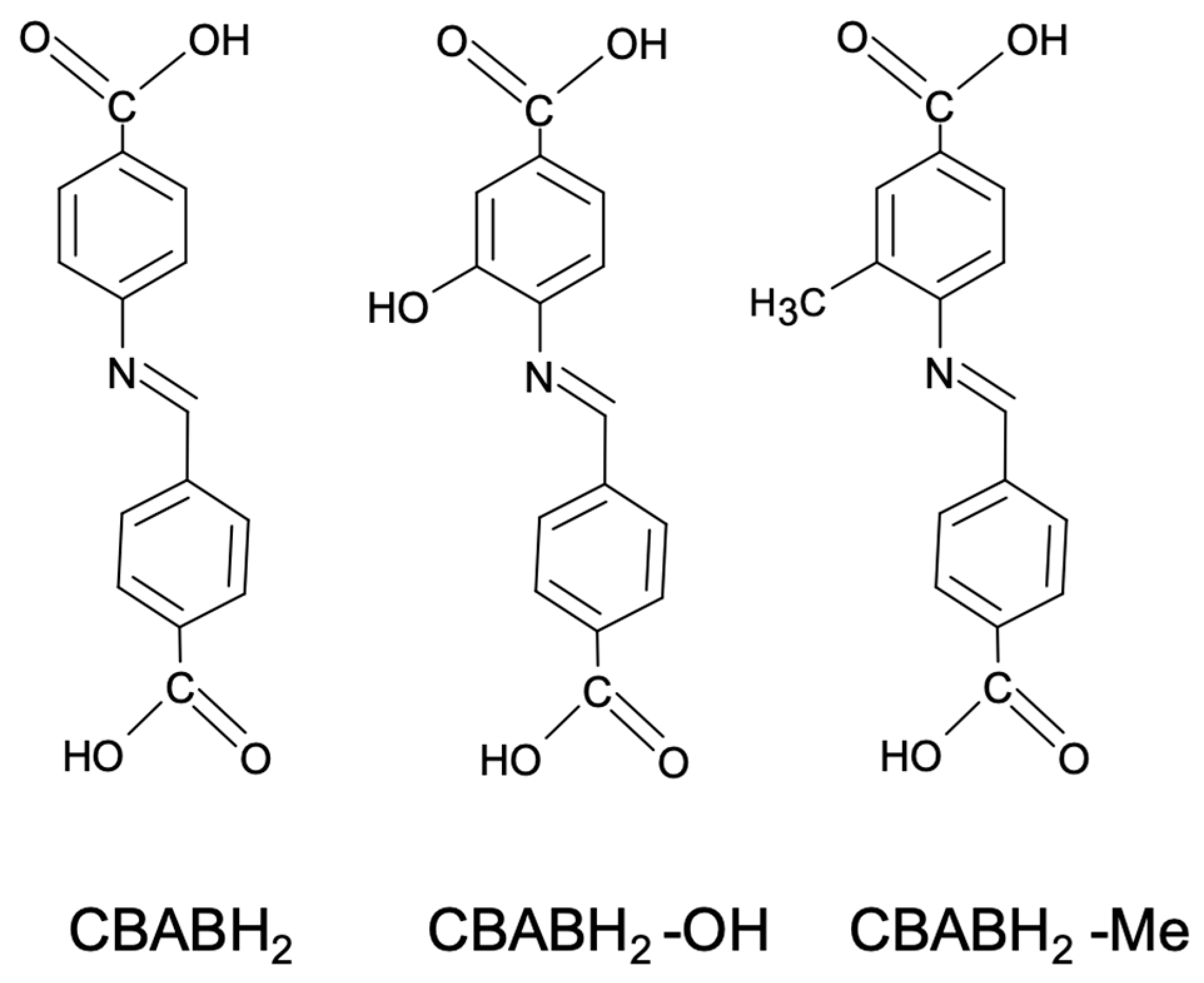
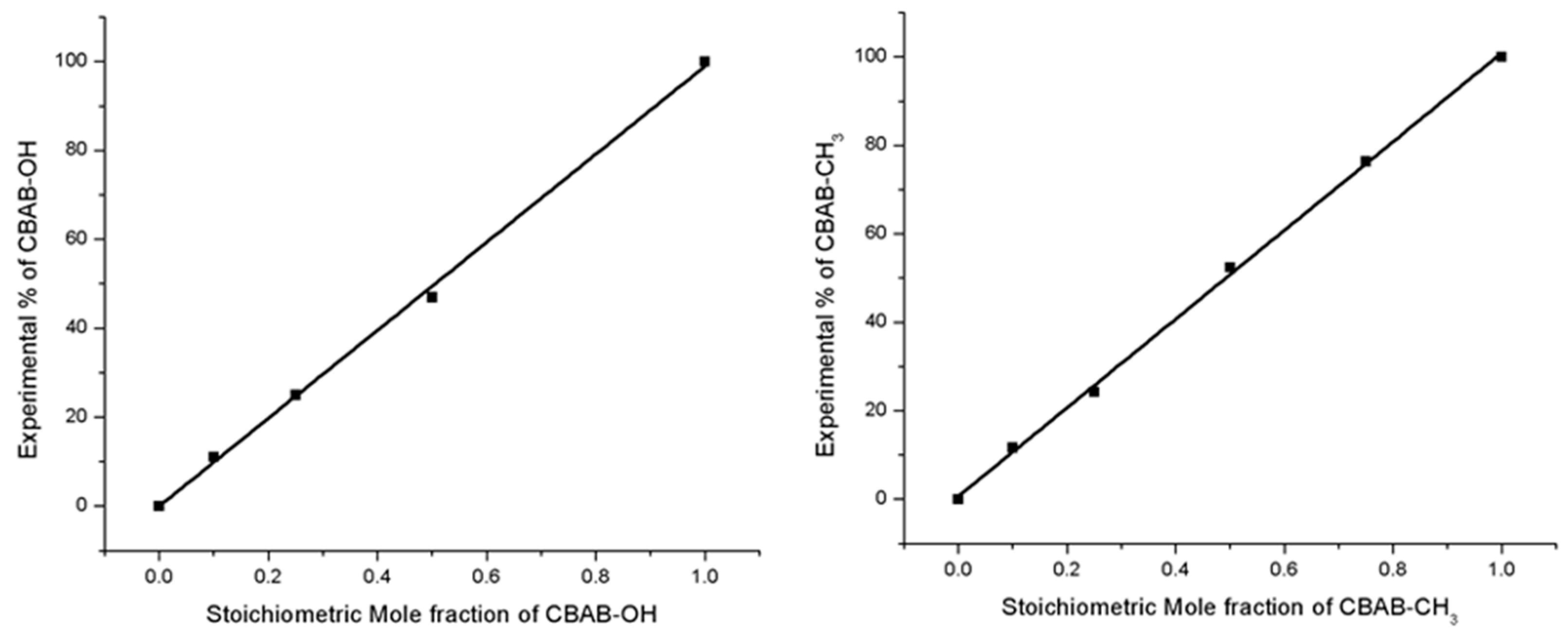
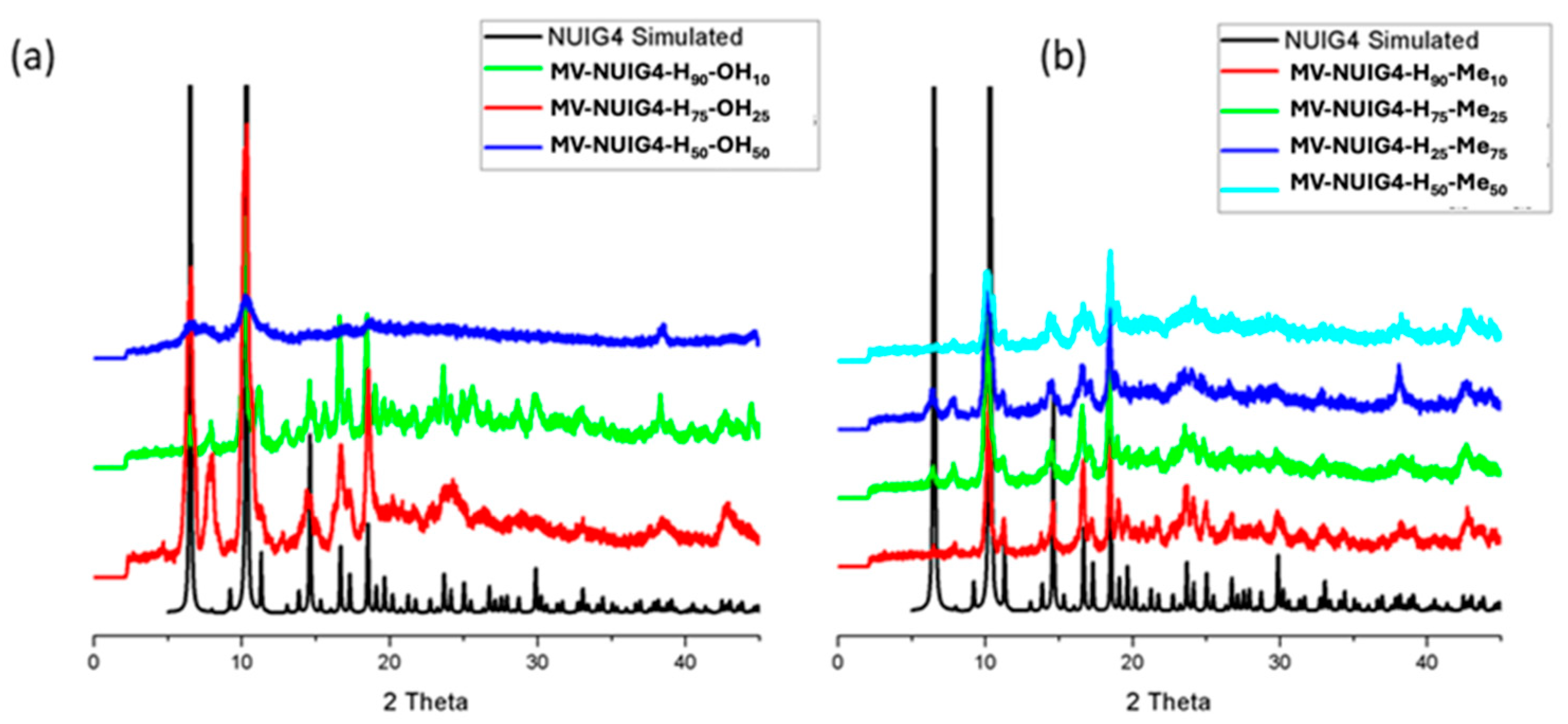
2.1. Synthetic Discussion
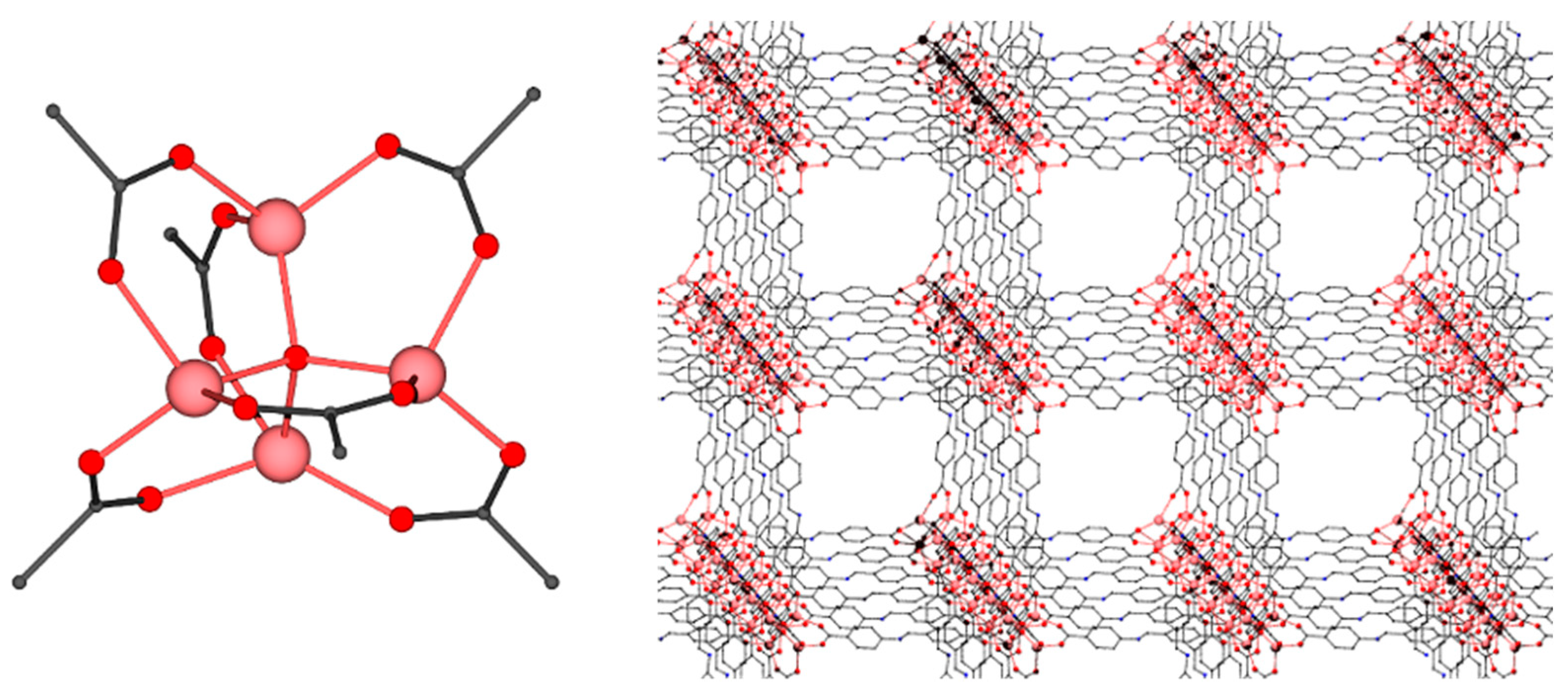
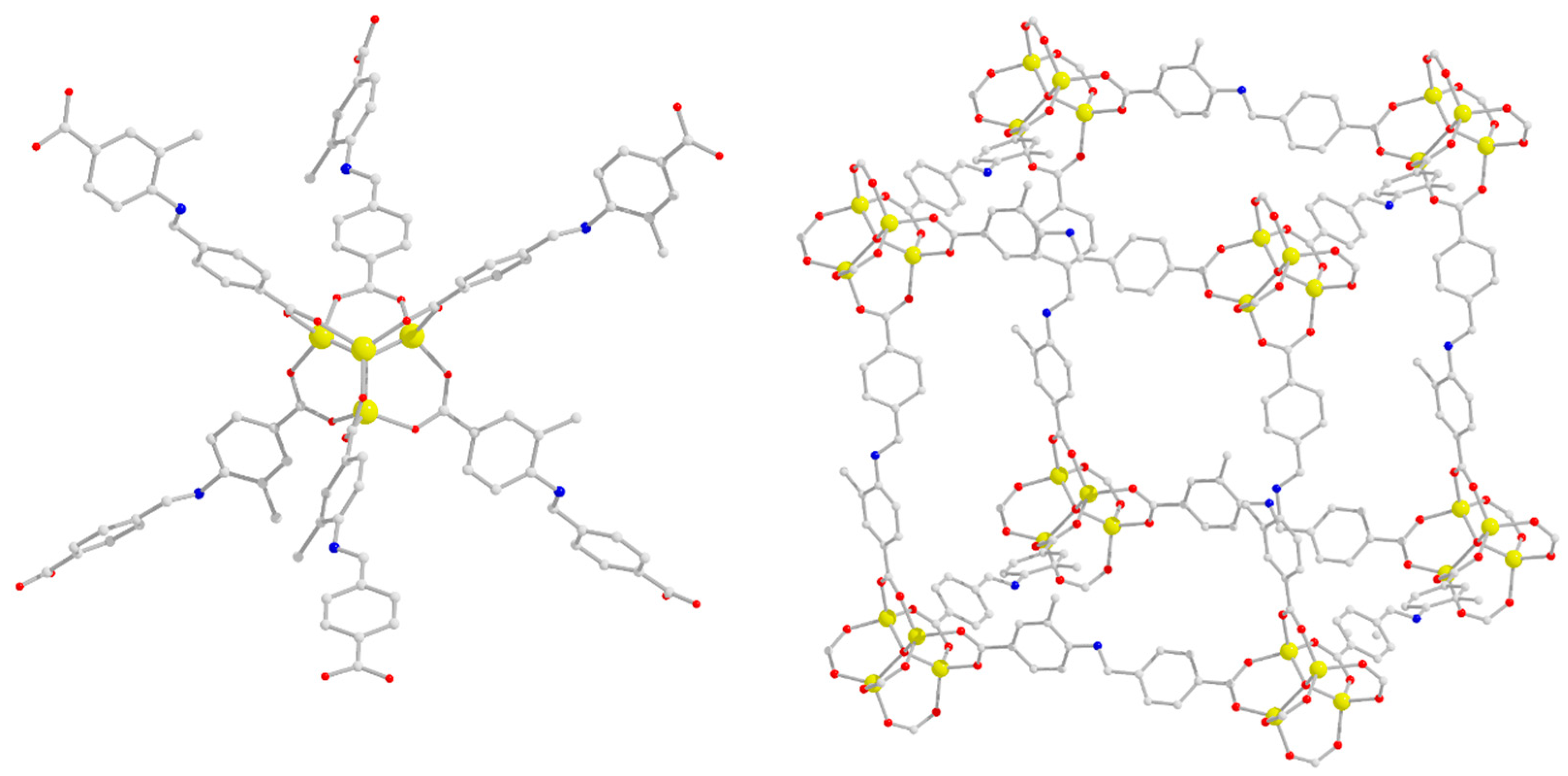
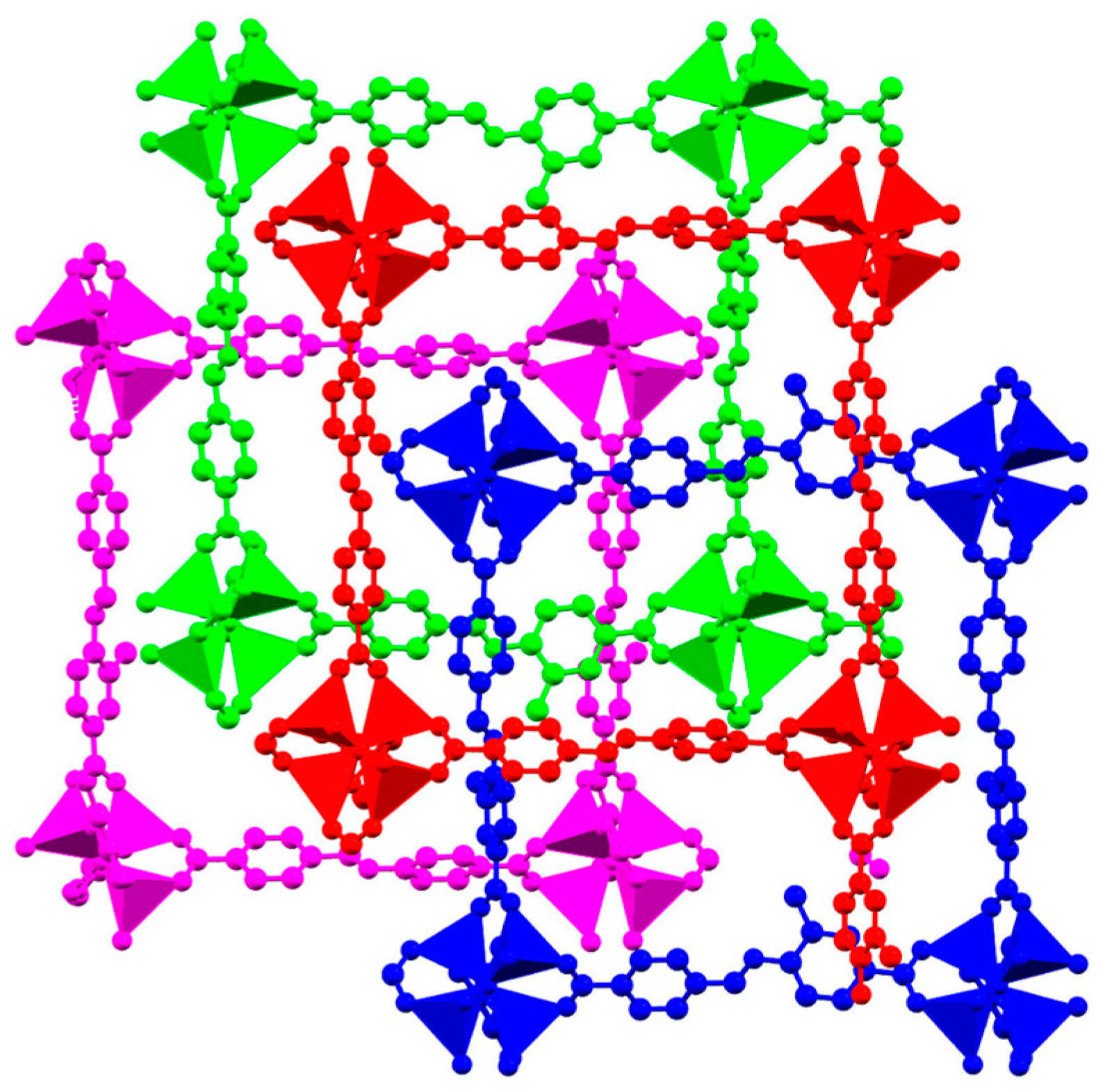
2.2. Description of Structures
2.3. Dox Absorption and Release Studies
3. Materials and Methods
3.1. Materials, and Physical and Spectroscopic Measurements
3.2. Compound Synthesis
3.2.1. Synthesis of NUIG4-Me
3.2.2. Synthesis of MV-NUIG4 Analogues
3.3. X-Ray Crystallography
3.4. Crystal Structure Model Construction
3.5. NMR Studies
3.6. MOF Activation and Drug Loading
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.; Efthymiou, C.G.; Sanii, R.; Patyk-Kazmierczak, E.; Alsharabasy, A.M.; Winterlich, M.; Kumar, N.; Sensharma, D.; Tong, W.; Guerin, S.; et al. NUIG4: A biocompatible pcu metal–organic framework with an exceptional doxorubicin encapsulation capacity. J. Mater. Chem. B 2022, 10, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.J.; Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kumar, S.K. Recent Developments of Metal–Organic Framework-Based Materials for Drug Delivery Applications. ACS Omega 2022, 49, 44507–44519. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 37, 1704303. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O. Modular Chemistry: Secondary Building Units as the Basis of Design for Highly Porous and Robust Metal–Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef]
- Lazaro, I.A.; Chen, X.; Ding, M.; Eskandari, A.; Fairen-Jimenez, D.; Gimenez-Marques, M.; Gref, R.; Lin, W.; Luo, T.; Forgan, R.S. Metal–organic frameworks for biological applications. Nat. Rev. Methods Primers 2024, 4, 42. [Google Scholar] [CrossRef]
- Ahmed, A.; McHugh, D.; Papatriantafyllopoulou, C. Synthesis and Biomedical Applications of Highly Porous Metal–Organic Frameworks. Molecules 2022, 27, 6585. [Google Scholar] [CrossRef]
- Hefayathullah, M.; Singh, S.; Ganesan, V.; Maduraiveeran, G. Metal–organic frameworks as emerging smart nanoplatforms for electrochemical biosensors applications. Adv. Colloid Interface Sci. 2024, 331, 102321. [Google Scholar]
- Chao, Y.; Deng, N.; Zhou, Z. A review of recent advances in metal–organic frameworks materials for zero-energy passive adsorption of chemical pollutants in indoor environments. Sci. Total Environ. 2024, 953, 175926. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ibarlucea, B.; Huang, C.; Dong, R.; Al Aiti, M.; Huang, S.; Cuniberti, G. Multi-metallic MOF based composites for environmental applications: Synergizing metal centers and interactions. Nanoscale Horiz. 2024, 9, 1432–1474. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, H.; Zhou, T.; Chen, X.; Li, W.; Puang, H. Metal–Organic Frameworks and Their Composites for Environmental Applications. Adv. Sci. 2022, 9, e2204141. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.U.; Trukhan, N.; Muller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Metal–organic frameworks—Prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Letwaba, J.; Uyor, U.O.; Mavhungu, M.L.; Achuka, N.O.; Popoola, P.A. A review on MOFs synthesis and effect of their structural characteristics for hydrogen adsorption. RSC Adv. 2024, 14, 14233–14253. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Wang, H.; Zhou, M.; Ke, G.; Zhang, L.; Wu, J.; Gao, Z.; Lu, D. A comprehensive transformer-based approach for high-accuracy gas adsorption predictions in metal–organic frameworks. Nat. Commun. 2024, 15, 1904. [Google Scholar] [CrossRef]
- Karth, N.; Wright, K.R.; Ahmed, A.; Siegel, D.J.; Matzger, A.J. High-throughput discovery of metal–organic frameworks for hydrogen storage. J. Am. Chem. Soc. 2024, 146, 10517–10526. [Google Scholar]
- Jiang, C.; Wang, X.; Ouyang, Y.; Lu, K.; Jiang, W.; Ku, H.; Wei, X.; Wang, Z.; Dai, F.; Sun, D. Recent advances in metal–organic frameworks for gas adsorption/separation. Nanoscale Adv. 2022, 4, 2077–2095. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-based membranes for gas separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Hiraide, S.; Sakanaka, Y.; Kajiro, H.; Kawaguchi, S.; Miyahara, M.T.; Tanaka, H. High-throughput gas separation by flexible metal–organic frameworks with fast gating and thermal management capabilities. Nat. Commun. 2020, 11, 3867. [Google Scholar] [CrossRef] [PubMed]
- Winterlich, M.; Efthymiou, C.G.; Papawassiliou, W.; Carvalho, J.P.; Pell, A.J.; Mayans, J.; Escuer, A.; Carty, M.P.; McArdle, P.; Tylianakis, E.; et al. A biocompatible ZnNa₂-based metal–organic framework with high ibuprofen, nitric oxide and metal uptake capacity. Mater. Adv. 2020, 1, 2248–2260. [Google Scholar] [CrossRef]
- Ahmed, A.; Kelly, A.; Leonard, D.; Saleem, W.; Bezrukov, A.; Efthymiou, C.G.; Zaworotko, M.J.; Tiana, D.; Boyd, A.; Papatriantafyllopoulou, C. Synthesis and characterisation of antimicrobial metal–organic frameworks as multi-drug carriers. Dalton Trans. 2024, 53, 11867–11875. [Google Scholar] [CrossRef]
- Vikal, A.; Maurya, R.; Patel, P.; Paliwal, S.R.; Narang, R.K.; Das Gupta, G.; Das Kurmi, B. Exploring metal–organic frameworks (MOFs) in drug delivery: A concise overview of synthesis approaches, versatile applications, and current challenges. Appl. Mater. Today 2024, 41, 102443. [Google Scholar] [CrossRef]
- Saboorizadeh, B.; Zareh-Dorabei, R.; Safavi, M.; Safarifard, V. Applications of metal–organic frameworks (MOFs) in drug delivery, biosensing, and therapy: A comprehensive review. Langmuir 2024, 40, 22477–22503. [Google Scholar] [CrossRef] [PubMed]
- Khafaga, D.S.R.; El-Morsy, M.T.; Faried, H.; Diab, A.H.; Shehab, S.; Saleh, A.M.; Ali, G.A.M. Metal–organic frameworks in drug delivery: Engineering versatile platforms for therapeutic applications. RSC Adv. 2024, 14, 30201–30229. [Google Scholar] [CrossRef]
- Bunzen, H.; Jirak, D. Nanoscale Metal–Organic Frameworks as Contrast Agents for Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2022, 14, 50445–50460. [Google Scholar] [CrossRef]
- Duman, F.D.; Forgan, R.S. Applications of Nanoscale Metal–Organic Frameworks as Imaging Agents in Biology and Medicine. J. Mater. Chem. B 2021, 9, 3423–3449. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, Y.; Li, D.-S.; Qiu, J.; Xu, X.; Yang, H.Y. A review of metal–organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef]
- Sen, R.; Hazra, D.K.; Mukherjee, M.; Koner, S. Gd26 Cluster Consisting of Distorted Cubane Cores: Synthesis, Structure and Heterogeneous Catalytic Epoxidation of Olefins. Eur. J. Inorg. Chem. 2011, 18, 2826–2831. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Qin, Q.-P.; Zhang, X. A novel Zn(II)-based metal–organic framework as a luminescent sensor for the detection of nitroaromatic explosives. ACS Appl. Mater. Interfaces 2022, 14, 18589–18597. [Google Scholar] [CrossRef]
- Wang, Z.-F.; Li, C.; Qin, Q.-P.; Zhang, S.; Zhang, X. A new Zn(II)-based metal–organic framework for efficient sensing of Fe3+ ions in aqueous solution. ACS Appl. Nano Mater. 2023, 6, 23430–23438. [Google Scholar] [CrossRef]
- Zheng, Y.-T.; Li, S.; Huang, N.-Y.; Li, X.; Xu, Q. Recent advances in metal–organic framework-derived materials for electrocatalytic and photocatalytic CO₂ reduction. Coord. Chem. Rev. 2024, 510, 215858. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Ghoreishi, Z.; Majidi, M.R.; Yoon, Y.; Dizge, N.; Khataee, A. Metal–organic frameworks (MOF)-based sensors for detection of toxic gases: A review of current status and future prospects. Mater. Chem. Phys. 2023, 299, 127512. [Google Scholar] [CrossRef]
- Wu, T.; Gao, X.; Ge, F.; Zheng, H. Metal–organic frameworks (MOFs) as fluorescence sensors: Principles, development and prospects. CrystEngComm 2022, 24, 7881–7901. [Google Scholar] [CrossRef]
- Shen, X.; Tissot, A.; Serre, C. Recent progress on MOF-based optical sensors for VOC sensing. Chem. Sci. 2022, 13, 13978–14007. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, D.; Wu, L.; Yin, X.; Zhang, X.; Patterson, L.H.; Zhang, J. Metal–Organic Frameworks for Gene Therapy and Detection. Adv. Funct. Mater. 2023, 12, 2212277. [Google Scholar] [CrossRef]
- Poddar, A.; Pyreddy, S.; Carraro, F.; Dhakal, S.; Russell, A.; Field, M.R.; Reddy, T.S.; Falcaro, P.; Doherty, C.M.; Shukla, R. ZIF-C for targeted RNA interference and CRISPR/Cas9 based gene editing in prostate cancer. Chem. Commun. 2020, 56, 15406–15409. [Google Scholar] [CrossRef]
- Ringaci, A.; Yaremenko, A.V.; Shevchenko, K.G.; Zvereva, S.D.; Nikitin, M.P. Metal–Organic Frameworks for Simultaneous Gene and Small Molecule Delivery In Vitro and In Vivo. Chem. Eng. J. 2021, 418, 129386. [Google Scholar] [CrossRef]
- Wang, X.; Lan, P.C.; Ma, S. Metal–Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Cent. Sci. 2020, 9, 1497–1506. [Google Scholar] [CrossRef]
- Xia, H.; Li, N.; Zhong, X.; Jiang, Y. Metal–Organic Frameworks: A Potential Platform for Enzyme Immobilization and Related Applications. Front. Bioeng. Biotechnol. 2020, 8, 695. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, W.; Wang, Y.; Su, R.; He, Z. A Facile Strategy for Enzyme Immobilization with Highly Stable Hierarchically Porous Metal–Organic Frameworks. Nanoscale 2017, 9, 17561–17570. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.M.; Alexandre, J.Y.N.H.; Souza, J.E.S.; Neto, J.G.L.; De Souza Junior, P.G.; Rocha, M.V.P.; Dos Santos, J.C.S. The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Zhou, J.; Xing, X.; Li, L. Enhancement of Protein Crystallization Using Nano-Sized Metal–Organic Framework. Crystals 2022, 12, 578. [Google Scholar] [CrossRef]
- Zhou, X.; Zhong, Z.; Xu, N.; Zhong, S. Nanoscale MOF–Protein Composites for Theranostics. Crystals 2023, 13, 1229. [Google Scholar] [CrossRef]
- Leite, J.P.; Rodrigues, D.; Ferreira, S.; Figueira, F.; Almeida Paz, F.A.; Gales, L. Mesoporous Metal–Organic Frameworks as Effective Nucleating Agents in Protein Crystallography. Cryst. Growth Des. 2019, 19, 1610–1615. [Google Scholar] [CrossRef]
- Andre, V.; Da Siva, A.R.F.; Fernandes, A.; Frade, R.; Garcia, C.; Rijo, P.; Antunes, A.M.M.; Rocha, J.; Duarte, M.T. Mg- and Mn-MOFs Boost the Antibiotic Activity of Nalidixic Acid. ACS Appl. Bio Mater. 2019, 2, 2347–2354. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Hasan, M.N.; Bagchi, D.; Altass, H.M.; Morad, M.; Althagafi, I.I.; Hameed, A.M.; Sayqal, A.; Khder, A.R.S.; Asghar, B.H.; et al. Nano-metal–organic frameworks as targeted drug delivery agents to combat antibiotic-resistant bacterial infections. R. Soc. Open Sci. 2020, 7, 200959. [Google Scholar] [CrossRef]
- Helal, A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. Multivariate metal-organic frameworks. Nat. Sci. Rev. 2017, 4, 296–298. [Google Scholar] [CrossRef]
- Deng, H.; Doonan, C.J.; Funukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Telfer, S.G. Multicomponent Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202306341. [Google Scholar] [CrossRef]
- Dutta, A.; Pan, Y.; Liu, J.-Q.; Kumar, A. Multicomponent Isoreticular Metal–Organic Frameworks: Principles, Current Status and Challenges. Coord. Chem. Rev. 2021, 445, 214074. [Google Scholar] [CrossRef]
- Smith, K.T.; Stylianou, K.C. Multivariate metal–organic frameworks generated through post-synthetic modification: Impact and future directions. Dalton Trans. 2023, 52, 16578–16585. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.; Grape, E.S.; Gaggioli, C.A.; Tayal, A.; Dharanipragada, A.; Willhammar, T.; Ken Inge, A.; Zou, X.; Liu, B.; et al. A Tunable Multivariate Metal–Organic Framework as a Platform for Designing Photocatalysts. J. Am. Chem. Soc. 2021, 143, 6333–6338. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yuan, S.; Li, J.-L.; Wang, K.-Y.; Day, G.S.; Zhang, P.; Wang, Y.; Zhou, H.-C. Uncovering two principles of multivariate hierarchical metal–organic framework synthesis via retrosynthetic design. ACS Cent. Sci. 2018, 4, 1719–1726. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, S.; Wang, W.; Feng, L.; Liu, X.; Zhang, X.; Wang, X.; Kang, Z.; Dai, F.; Yuan, D.; et al. Optimizing Multivariate Metal–Organic Frameworks for Efficient C₂H₂/CO₂ Separation. J. Am. Chem. Soc. 2020, 142, 8728–8737. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Yang, C.; Tian, Y.; Tao, Z.; Smith, M.; Zhang, H.; Zhang, L.; Li, L.; Gu, Q.; Zhou, H.-C.; et al. Designing multivariate porphyrin-based metal-organic frameworks with Ni/Co dual-metal atom sites for cooperative NO2 capture and NO retention. Sep. Purif. Technol. 2023, 320, 124080. [Google Scholar] [CrossRef]
- Lustig, W.P.; Shen, Z.; Teat, S.J.; Javed, N.; Velasco, E.; O’Carroll, D.M.; Li, J. Rational design of a high-efficiency, multivariate metal–organic framework phosphor for white LED bulbs. Chem. Sci. 2020, 11, 1814–1824. [Google Scholar] [CrossRef]
- Li, Y.-M.; Yuan, J.; Ren, H.; Ji, C.-Y.; Tao, Y.; Wu, Y.; Chou, L.-Y.; Zhang, Y.-B.; Cheng, L. Fine-Tuning the Micro-Environment to Optimize the Catalytic Activity of Enzymes Immobilized in Multivariate Metal–Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 15378–15390. [Google Scholar] [CrossRef]
- Schrimpf, W.; Jiang, J.; Ji, Z.; Hirschle, P.; Lamb, D.C.; Yaghi, O.M.; Wuttke, S. Chemical diversity in a metal–organic framework revealed by fluorescence lifetime imaging. Nat. Commun. 2018, 9, 1647. [Google Scholar] [CrossRef] [PubMed]
- Abanades Lazaro, I.; Wells, C.J.R.; Forgan, R.S. Multivariate Modulation of the Zr MOF UiO-66 for Defect-Controlled Combination Anticancer Drug Delivery. Angew. Chem. 2020, 132, 5249–5255. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate Metal–Organic Frameworks for Dialing-in the Binding and Programming the Release of Bioactive Molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef]
- Taddei, M.; Tiana, D.; Casati, N.; Van Bokhoven, J.A.; Smit, B.; Ranocchiari, M. Mixed-linker UiO-66: Structure–property relationships revealed by a combination of high-resolution powder X-ray diffraction and density functional theory calculations. Phys. Chem. Chem. Phys. 2017, 19, 1551–1559. [Google Scholar] [CrossRef]
- Karzenmeyer, A.M.; Canivet, J.; Holland, G.; Farrusseng, D. Assessing Chemical Heterogeneity at the Nanoscale in Mixed-Ligand Metal–Organic Frameworks with the PTIR Technique. Angew. Chem. Int. Ed. 2014, 53, 2852–2856. [Google Scholar] [CrossRef]
- Lescouet, T.; Kockrick, E.; Pera-Titus, M.; Aguado, S.; Farrusseng, D. Homogeneity of flexible metal–organic frameworks containing mixed linkers. J. Mater. Chem. 2012, 22, 10287–10293. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Islamoglu, T.; El-Kaderi, H.M. Benzothiazole- and benzoxazole-linked porous polymers for carbon dioxide storage and separation. J. Mater. Chem. A 2017, 5, 258–265. [Google Scholar] [CrossRef]
- Wei, P.-F.; Qi, M.-Z.; Wang, Z.-P.; Ding, S.-Y.; Yu, W.; Liu, Q.; Wang, L.-K.; Wang, H.-Z.; An, W.-K.; Wang, W. Benzoxazole-Linked Ultrastable Covalent Organic Frameworks for Photocatalysis. J. Am. Chem. Soc. 2018, 140, 4623–4631. [Google Scholar] [CrossRef] [PubMed]
- Cusin, L.; Peng, H.; Ciesielski, A.; Samori, S. Chemical Conversion and Locking of the Imine Linkage: Enhancing the Functionality of Covalent Organic Frameworks. Angew. Chem. 2021, 133, 14356–14370. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Boultif, A.; Loueer, D. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J. Appl. Crystallogr. 1991, 24 Pt 6, 987–993. [Google Scholar] [CrossRef]
- David, W.I.F.; Shankland, K.; Van De Streek, J.; Pidcock, E.; Motherwell, W.D.S.; Cole, J.C. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
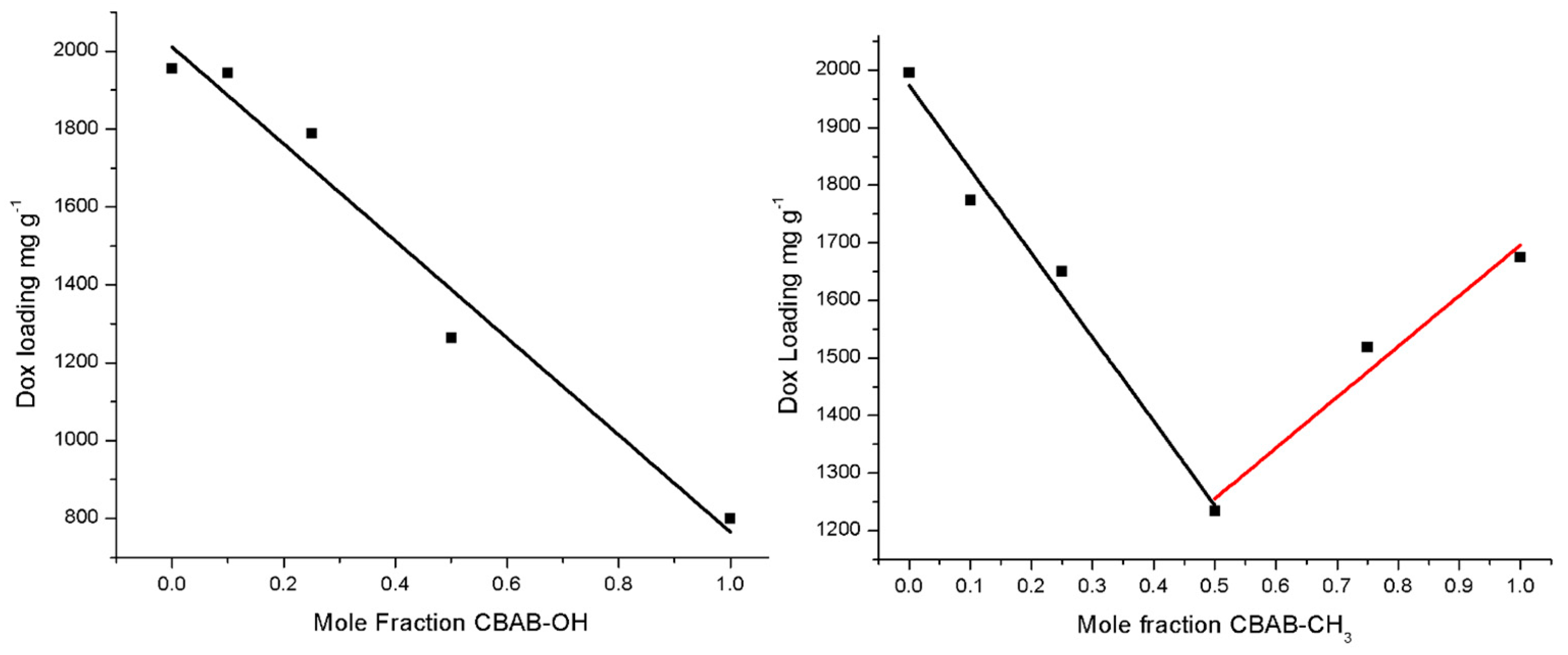

| MV-NUIG4 Analogue | Dox Loading (mg g−1) |
|---|---|
| NUIG4 | 1995 |
| NUIG4-Me | 1675 |
| MV-NUIG4-H90-OH10 | 1944 |
| MV-NUIG4-H75-OH25 | 1788 |
| MV-NUIG4-H50-OH50 | 1264 |
| MV-NUIG4-H90-Me10 | 1773 |
| MV-NUIG4-H75-Me25 | 1650 |
| MV-NUIG4-H50-Me50 | 1234 |
| MV-NUIG4-H25-Me75 | 1518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Bezrukov, A.; Sensharma, D.; O’Malley, C.; Zaworotko, M.J.; Tiana, D.; Papatriantafyllopoulou, C. Synthesis and Characterisation of Multivariate Metal–Organic Frameworks for Controlled Doxorubicin Absorption and Release. Molecules 2025, 30, 1968. https://doi.org/10.3390/molecules30091968
Ahmed A, Bezrukov A, Sensharma D, O’Malley C, Zaworotko MJ, Tiana D, Papatriantafyllopoulou C. Synthesis and Characterisation of Multivariate Metal–Organic Frameworks for Controlled Doxorubicin Absorption and Release. Molecules. 2025; 30(9):1968. https://doi.org/10.3390/molecules30091968
Chicago/Turabian StyleAhmed, Ahmed, Andrey Bezrukov, Debobroto Sensharma, Ciaran O’Malley, Michael J. Zaworotko, Davide Tiana, and Constantina Papatriantafyllopoulou. 2025. "Synthesis and Characterisation of Multivariate Metal–Organic Frameworks for Controlled Doxorubicin Absorption and Release" Molecules 30, no. 9: 1968. https://doi.org/10.3390/molecules30091968
APA StyleAhmed, A., Bezrukov, A., Sensharma, D., O’Malley, C., Zaworotko, M. J., Tiana, D., & Papatriantafyllopoulou, C. (2025). Synthesis and Characterisation of Multivariate Metal–Organic Frameworks for Controlled Doxorubicin Absorption and Release. Molecules, 30(9), 1968. https://doi.org/10.3390/molecules30091968






