Partial Oxidation of CH4 in Plasma: The Effects of Oxidant and Catalyst Addition
Abstract
1. Introduction
2. Results
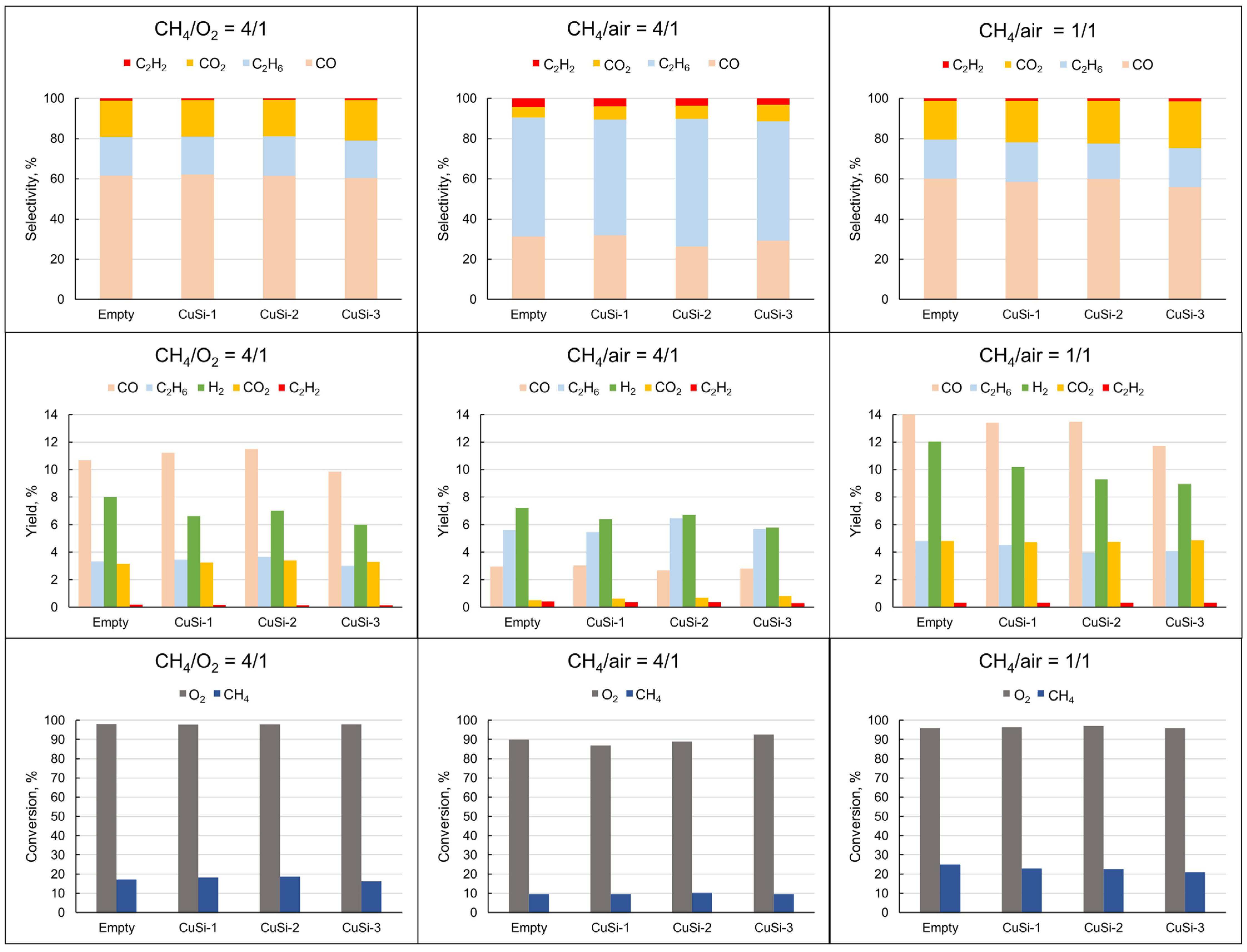
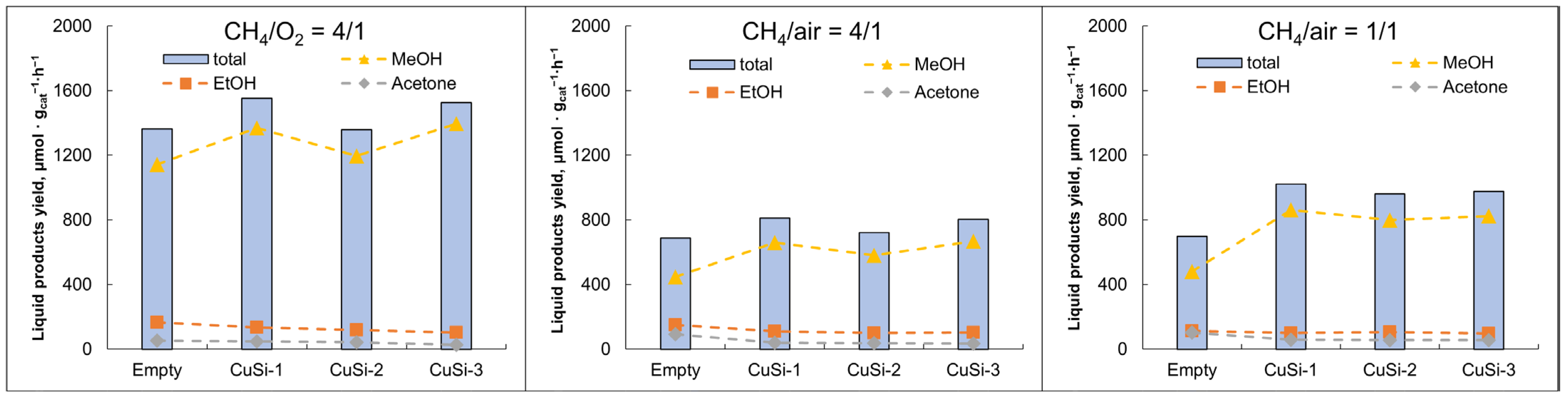
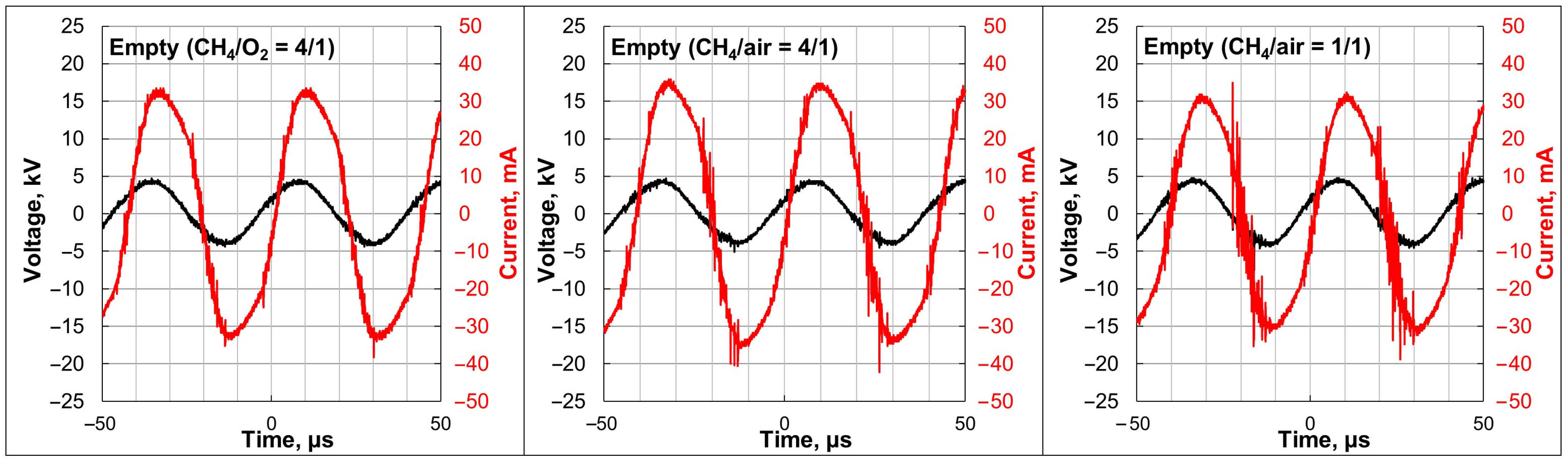
2.1. Plasma-Catalytic Methane Partial Oxidation
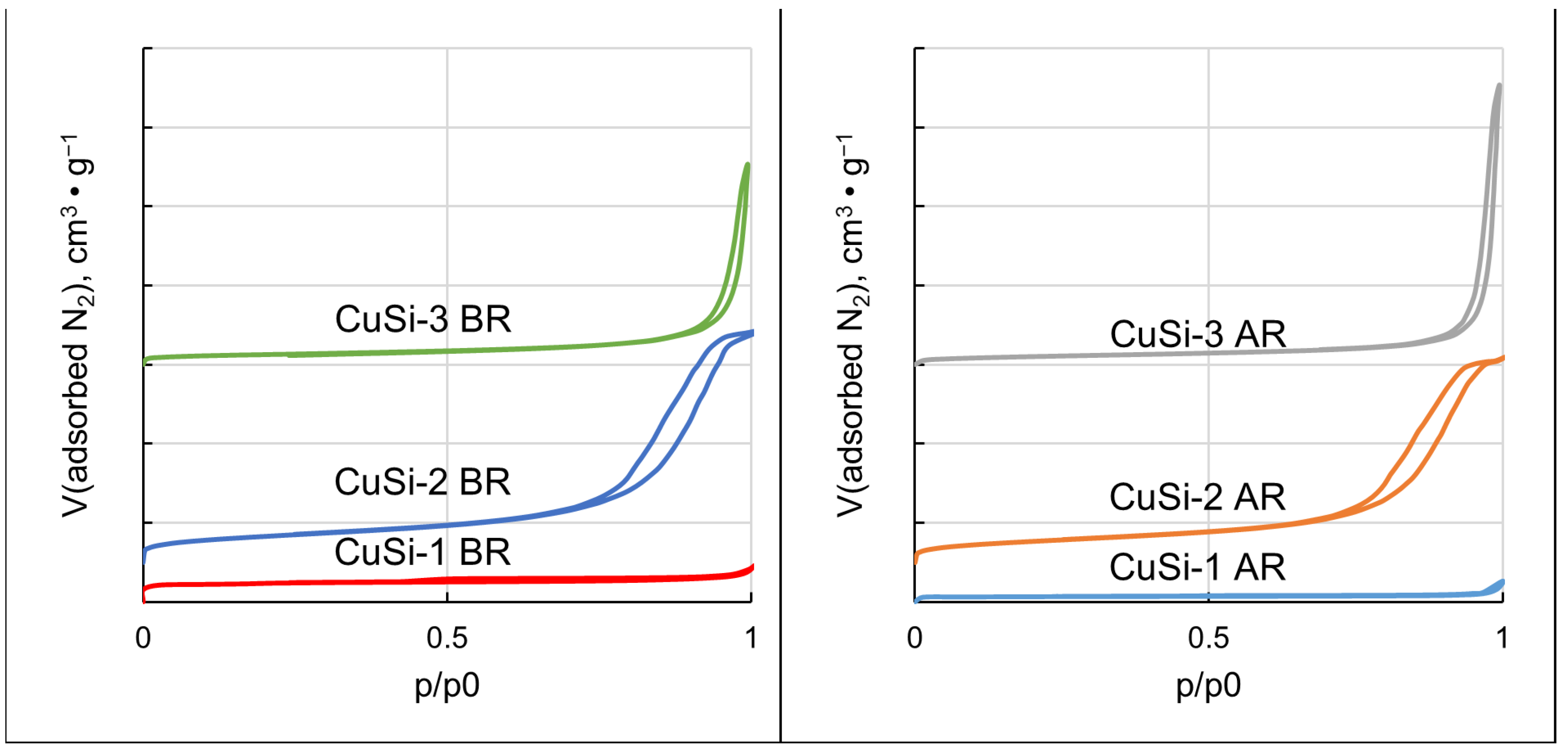
2.2. Catalysts’ Characterization
3. Discussion
3.1. Plasma-Catalytic Methane Partial Oxidation
3.2. Catalysts’ Characterization
4. Materials and Methods
4.1. Catalysts’ Synthesis
4.2. Physico-Chemical Methods
4.3. Plasma-Catalytic Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller |
| DBD | Dielectric barrier discharge |
| TGA | Thermogravimetric analysis |
| TPD | Temperature-programmed desorption |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescent spectroscopy analysis |
References
- Caballero, A.; Pérez, P.J. Methane as Raw Material in Synthetic Chemistry: The Final Frontier. Chem. Soc. Rev. 2013, 42, 8809. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Galvan, M.C.; Mota, N.; Ojeda, M.; Rojas, S.; Navarro, R.M.; Fierro, J.L.G. Direct Methane Conversion Routes to Chemicals and Fuels. Catal. Today 2011, 171, 15–23. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam Reforming of Methane: Current States of Catalyst Design and Process Upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Nesterenko, N.; Medeiros-Costa, I.C.; Clatworthy, E.B.; Cruchade, H.; Konnov, S.V.; Dath, J.-P.; Gilson, J.-P.; Mintova, S. Methane-to-Chemicals: A Pathway to Decarbonization. Natl. Sci. Rev. 2023, 10, nwad116. [Google Scholar] [CrossRef]
- Saeidi, S.; Sápi, A.; Khoja, A.H.; Najari, S.; Ayesha, M.; Kónya, Z.; Asare-Bediako, B.B.; Tatarczuk, A.; Hessel, V.; Keil, F.J.; et al. Evolution Paths from Gray to Turquoise Hydrogen via Catalytic Steam Methane Reforming: Current Challenges and Future Developments. Renew. Sustain. Energy Rev. 2023, 183, 113392. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Direct Conversion Technologies of Methane to Methanol: An Overview. Renew. Sustain. Energy Rev. 2016, 65, 250–261. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Sarangi, P.K.; Bhatia, L.; Singh, A.K.; Shadangi, K.P. Conversion of Methane to Methanol: Technologies and Future Challenges. Biomass. Convers. Biorefin. 2021, 12, 1851–1875. [Google Scholar] [CrossRef]
- Alsudani, F.T.; Saeed, A.N.; Ali, N.S.; Majdi, H.S.; Salih, H.G.; Albayati, T.M.; Saady, N.M.C.; Shakor, Z.M. Fisher–Tropsch Synthesis for Conversion of Methane into Liquid Hydrocarbons through Gas-to-Liquids (GTL) Process: A Review. Methane 2023, 2, 24–43. [Google Scholar] [CrossRef]
- Da Silva, M.J. Synthesis of Methanol from Methane: Challenges and Advances on the Multi-Step (Syngas) and One-Step Routes (DMTM). Fuel. Process. Technol. 2016, 145, 42–61. [Google Scholar] [CrossRef]
- Rajeev, A.; Mohammed, T.P.; George, A.; Sankaralingam, M. Direct Methane to Methanol Conversion: An Overview of Non-Syn Gas Catalytic Strategies. Chem. Rec. 2025, 25, e202400186. [Google Scholar] [CrossRef]
- Periana, R.A.; Taube, D.J.; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. Platinum Catalysts for the High-Yield Oxidation of Methane to a Methanol Derivative. Science 1998, 280, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Conley, B.L.; Tenn, W.J.; Young, K.J.H.; Ganesh, S.K.; Meier, S.K.; Ziatdinov, V.R.; Mironov, O.; Oxgaard, J.; Gonzales, J.; Goddard, W.A.; et al. Design and Study of Homogeneous Catalysts for the Selective, Low Temperature Oxidation of Hydrocarbons. J. Mol. Catal. A Chem. 2006, 251, 8–23. [Google Scholar] [CrossRef]
- Tian, Y.; Piao, L.; Chen, X. Research Progress on the Photocatalytic Activation of Methane to Methanol. Green Chem. 2021, 23, 3526–3541. [Google Scholar] [CrossRef]
- Arminio-Ravelo, J.A.; Escudero-Escribano, M. Strategies toward the Sustainable Electrochemical Oxidation of Methane to Methanol. Curr. Opin. Green Sustain. Chem. 2021, 30, 100489. [Google Scholar] [CrossRef]
- Fathollahi, P.; Farahani, M.; Rad, R.H.; Khani, M.R.; Asadi, A.; Shafiei, M.; Shokri, B. Selective Oxidation of Methane to Methanol by NTP Plasma: The Effect of Power and Oxygen on Conversion and Selectivity. J. Electrost. 2021, 112, 103594. [Google Scholar] [CrossRef]
- Nozaki, T.; Ağıral, A.; Yuzawa, S.; Gardeniers, J.G.E.H.; Okazaki, K. A Single Step Methane Conversion into Synthetic Fuels Using Microplasma Reactor. Chem. Eng. J. 2010, 166, 288–293. [Google Scholar] [CrossRef]
- Blankenship, A.; Artsiusheuski, M.; Sushkevich, V.; Van Bokhoven, J.A. Recent Trends, Current Challenges and Future Prospects for Syngas-Free Methane Partial Oxidation. Nat. Catal. 2023, 6, 748–762. [Google Scholar] [CrossRef]
- Ravi, M.; Ranocchiari, M.; Van Bokhoven, J.A. The Direct Catalytic Oxidation of Methane to Methanol—A Critical Assessment. Angew. Chem. Int. Ed. 2017, 56, 16464–16483. [Google Scholar] [CrossRef]
- Ollegott, K.; Wirth, P.; Oberste-Beulmann, C.; Awakowicz, P.; Muhler, M. Fundamental Properties and Applications of Dielectric Barrier Discharges in Plasma-Catalytic Processes at Atmospheric Pressure. Chem. Ing. Tech. 2020, 92, 1542–1558. [Google Scholar] [CrossRef]
- Baig, S.; Sajjadi, B. Non-Thermal Plasma Enhanced Catalytic Conversion of Methane into Value Added Chemicals and Fuels. J. Energy Chem. 2024, 97, 265–301. [Google Scholar] [CrossRef]
- Maslova, V.; Nastase, R.; Veryasov, G.; Nesterenko, N.; Fourré, E.; Batiot-Dupeyrat, C. Current Status and Challenges of Plasma and Plasma-Catalysis for Methane Coupling: A Review. Prog. Energy Combust. Sci. 2024, 101, 101096. [Google Scholar] [CrossRef]
- Chen, G.; Snyders, R.; Britun, N. CO2 Conversion Using Catalyst-Free and Catalyst-Assisted Plasma-Processes: Recent Progress and Understanding. J. CO2 Util. 2021, 49, 101557. [Google Scholar] [CrossRef]
- Ji, H.; Lin, L.; Chang, K. Plasma-Assisted CO2 Decomposition Catalyzed by CeO2 of Various Morphologies. J. CO2 Util. 2022, 68, 102351. [Google Scholar] [CrossRef]
- Golubev, O.V.; Maximov, A.L. Dielectric Barrier Discharge Plasma Combined with Ce-Ni Mesoporous Catalysts for CO2 Splitting to CO. Plasma. Chem. Plasma. Process. 2024, 44, 2087–2100. [Google Scholar] [CrossRef]
- Michiels, R.; Engelmann, Y.; Bogaerts, A. Plasma Catalysis for CO2 Hydrogenation: Unlocking New Pathways toward CH3OH. J. Phys. Chem. C 2020, 124, 25859–25872. [Google Scholar] [CrossRef]
- Du, J.; Zong, L.; Zhang, S.; Gao, Y.; Dou, L.; Pan, J.; Shao, T. Numerical Investigation on the Heterogeneous Pulsed Dielectric Barrier Discharge Plasma Catalysis for CO2 Hydrogenation at Atmospheric Pressure: Effects of Ni and Cu Catalysts on the Selectivity Conversions to CH4 and CH3OH. Plasma Process. Polym. 2021, 19, 2100111. [Google Scholar] [CrossRef]
- Nozaki, T.; Chen, X.; Kim, D.-Y.; Zhan, C. Combination of DBD and Catalysts for CH4 and CO2 Conversion: Basics and Applications. Plasma Chem. Plasma Process. 2023, 43, 1385–1410. [Google Scholar] [CrossRef]
- Mei, D.; Sun, M.; Liu, S.; Zhang, P.; Fang, Z.; Tu, X. Plasma-Enabled Catalytic Dry Reforming of CH4 into Syngas, Hydrocarbons and Oxygenates: Insight into the Active Metals of γ-Al2O3 Supported Catalysts. J. CO2 Util. 2022, 67, 102307. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma Catalysis: A Brief Tutorial. Plasma Res. Express 2019, 1, 043001. [Google Scholar] [CrossRef]
- Chen, H.; Lee, H.; Chen, S.; Chao, Y.; Chang, M. Review of Plasma Catalysis on Hydrocarbon Reforming for Hydrogen Production—Interaction, Integration, and Prospects. Appl. Cat. B 2008, 85, 1–9. [Google Scholar] [CrossRef]
- Scapinello, M.; Delikonstantis, E.; Stefanidis, G.D. The Panorama of Plasma-Assisted Non-Oxidative Methane Reforming. Chem. Eng. Process 2017, 117, 120–140. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Neyts, E.C.; Bogaerts, A. Influence of the Material Dielectric Constant on Plasma Generation inside Catalyst Pores. J. Phys. Chem. C 2016, 120, 25923–25934. [Google Scholar] [CrossRef]
- Van‘t Veer, K.; Engelmann, Y.; Reniers, F.; Bogaerts, A. Plasma-Catalytic Ammonia Synthesis in a DBD Plasma: Role of Microdischarges and Their Afterglows. J. Phys. Chem. C 2020, 124, 22871–22883. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.-H.; Song, Y.-H.; Choi, W.C.; Park, Y.-K.; Kim, D.H. Synergistic Effect of Non-Thermal Plasma–Catalysis Hybrid System on Methane Complete Oxidation over Pd-Based Catalysts. Chem. Eng. J. 2015, 259, 761–770. [Google Scholar] [CrossRef]
- De Rosa, F.; Hardacre, C.; Graham, W.G.; McCullough, G.; Millington, P.; Hinde, P.; Goguet, A. Comparison between the Thermal and Plasma (NTP) Assisted Palladium Catalyzed Oxidation of CH4 Using AC or Nanopulse Power Supply. Catal. Today 2022, 384–386, 177–186. [Google Scholar] [CrossRef]
- Lv, H.; Liu, X.; Hao, Y.; Yi, Y. Coupling of Dielectric Barrier Discharge and Cu–S-1 Catalyst for Direct Oxidation of Methane to Methanol. Plasma. Chem. Plasma Process 2023, 43, 1963–1978. [Google Scholar] [CrossRef]
- Indarto, A.; Choi, N.J.W.; Lee, N.H.; Song, N.H.K. Methanol Synthesis Over Cu and Cu-Oxide-Containing ZnO/Al2O3 Using Dielectric Barrier Discharge. IEEE Trans. Plasma Sci. 2008, 36, 516–518. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Huang, L.; Lei, L. Post-Plasma Catalysis for Methane Partial Oxidation to Methanol: Role of the Copper-Promoted Iron Oxide Catalyst. Chem. Eng. Technol. 2010, 33, 2073–2081. [Google Scholar] [CrossRef]
- Chawdhury, P.; Wang, Y.; Ray, D.; Mathieu, S.; Wang, N.; Harding, J.; Bin, F.; Tu, X.; Subrahmanyam, C. A Promising Plasma-Catalytic Approach towards Single-Step Methane Conversion to Oxygenates at Room Temperature. Appl. Catal. B Environ. 2021, 284, 119735. [Google Scholar] [CrossRef]
- Zhou, L.M.; Xue, B.; Kogelschatz, U.; Eliasson, B. Partial Oxidation of Methane to Methanol with Oxygen or Air in a Nonequilibrium Discharge Plasma. Plasma. Chem. Plasma Process 1998, 18, 375–393. [Google Scholar] [CrossRef]
- Sandoval-Díaz, L.-E.; Gonzalez-Amaya, J.-A.; Trujillo, C.-A. General aspects of zeolite acidity characterization. Microporous Mesoporous Mater. 2015, 215, 229–243. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces. 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Gorbunov, N.A.; Mel’nikov, A.S. Effect of Molecular Nitrogen on the Electron Mobility in a Mixture of Argon and Optically Excited Sodium Vapor. Tech. Phys. 1999, 44, 361–366. [Google Scholar] [CrossRef]
- Smirnov, S.A.; Titov, V.A.; Shikova, T.G.; Ovtsyn, A.A.; Kadnikov, D.V. Influence of gas products of heterogeneous reactions on parameters of the argon plasma. Prikl. Fiz. 2016, 4, 43–48. [Google Scholar]
- Yi, Y.; Li, S.; Cui, Z.; Hao, Y.; Zhang, Y.; Wang, L.; Liu, P.; Tu, X.; Xu, X.; Guo, H.; et al. Selective Oxidation of CH4 to CH3OH through Plasma Catalysis: Insights from Catalyst Characterization and Chemical Kinetics Modelling. Appl. Catal. B Environ. 2021, 296, 120384. [Google Scholar] [CrossRef]
- Lv, H.; Meng, S.; Cui, Z.; Li, S.; Li, D.; Gao, X.; Guo, H.; Bogaerts, A.; Yi, Y. Plasma-Catalytic Direct Oxidation of Methane to Methanol over Cu-MOR: Revealing the Zeolite-Confined Cu2+ Active Sites. Chem. Eng. J. 2024, 496, 154337. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.-W.; Chen, L.; Lei, L.-C. Direct Oxidation of Methane to Methanol over Cu-Based Catalyst in an AC Dielectric Barrier Discharge. Plasma. Chem. Plasma Proces. 2010, 31, 67–77. [Google Scholar] [CrossRef]
- Indarto, A.; Yang, D.R.; Palgunadi, J.; Choi, J.-W.; Lee, H.; Song, H.K. Partial Oxidation of Methane with Cu–Zn–Al Catalyst in a Dielectric Barrier Discharge. Chem. Eng. Process 2008, 47, 780–786. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Huang, L.; Lei, L. Application of In-Plasma Catalysis and Post-Plasma Catalysis for Methane Partial Oxidation to Methanol over a Fe2O3-CuO/γ-Al2O3 Catalyst. J. Nat. Gas. Chem. 2010, 19, 628–637. [Google Scholar] [CrossRef]
- Jurković, D.L.; Puliyalil, H.; Pohar, A.; Likozar, B. Plasma-activated Methane Partial Oxidation Reaction to Oxygenate Platform Chemicals over Fe, Mo, Pd and Zeolite Catalysts. Int. J. Energy. Res. 2019, 43, 8085–8099. [Google Scholar] [CrossRef]
- Chawdhury, P.; Kumar, D.; Subrahmanyam, C. NTP Reactor for a Single Stage Methane Conversion to Methanol: Influence of Catalyst Addition and Effect of Promoters. Chem. Eng. J. 2019, 372, 638–647. [Google Scholar] [CrossRef]



| Sample | Oxide Content, wt% | |||
|---|---|---|---|---|
| SiO2 | CuO | Na2O | Al2O3 | |
| CuSi-1 | 80 | 12.8 | 4 | 3.2 |
| CuSi-2 | 87.1 | 10.1 | 2.8 | - |
| CuSi-3 | 84.2 | 11.3 | 4.5 | - |
| Sample | Acid Site Concentration, μmol/g | |||
|---|---|---|---|---|
| Weak Sites ~150 °C | Medium Sites ~200–350 °C | Strong Sites ~400 °C | Total (Medium + Strong Sites) | |
| CuSi-1 | 13 | 153 | 4 | 157 |
| CuSi-2 | 12 | 83 | 1 | 84 |
| CuSi-3 | 8 | 43 | 0 | 43 |
| Sample | SBET, m2/g | Vpores, cm3/g | d(pores), nm |
|---|---|---|---|
| CuSi-1 BR | 180 | 0.11 | 1.4 |
| CuSi-1 AR | 50 | 0.05 | 0.8 |
| CuSi-2 BR | 234 | 0.87 | 13 |
| CuSi-2 AR | 190 | 0.79 | 13 |
| CuSi-3 BR | 89 | 0.61 | 52 |
| CuSi-3 AR | 73 | 0.92 | 57 |
| Catalyst Composition | X(CH4), % | CH4/O2 Ratio | Oxygenates’ Distribution | Power, W | Remarks | Ref |
|---|---|---|---|---|---|---|
| Cu/γ-Al2O3 | 12.5 | 5/1 | HCOOH, CH3OH, HCOH, C2H5OH, CH3COOH, CH3C(O)CH3 | 1.8 | The acid sites on the surface increase the C2 oxygenates’ formation | [39] |
| CuO/γ-Al2O3 | 35 | 4/1 | CH3OH | 61 | Mo promoter reduces CO2 selectivity | [48] |
| Mo-CuO/γ-Al2O3 | 31 | |||||
| CuO-ZnO/Al2O3 | 25 | 4/1 | CH3OH, HCOH | 50 | Syngas yield decreased with catalyst addition | [49] |
| Cu-ZnO/Al2O3 | n/d | 5/1 | CH3OH | 60–80 | CuO resulted in higher methanol selectivity than that of CuO | [37] |
| Fe2O3-CuO/ceramic pellet | 25 | 1/1 | CH3OH | 140 | The CuO promoter had no significant effect on methane conversion but enhanced methanol selectivity | [38] |
| Fe2O3-CuO/γ-Al2O3 | 43 | 1/1 | CH3OH | 120 | Higher methanol yield was observed using the “In plasma catalysis” configuration | [50] |
| ZSM-5 | 6 | 4/1 | HCOH, CH3OH | 20 | Formaldehyde was the main product | [51] |
| Cu/MOR | 8 | 4/1 | HCOH, HCOOH CH3OH | 11–14 | Higher loading of Cu decreased methanol selectivity; wetness-impregnated Cu catalysts led to over-oxidation of CH4 to CO2 | [47] |
| Cu-ZSM-5 | 6 | 4/1 | HCOH, HCOOH CH3OH | 15 | Dealumination of the zeolite resulted in methanol yield increase | [36] |
| Cu- ZSM-5 (dealuminated) | 6 | |||||
| Cu/microSiAl | 23 | 4/1 | CH3OH, C2H5OH, CH3C(O)CH3 | 26 | Less methanol yield and more gaseous product yield was observed in the presence of mesoporous silica supported sample | This work |
| Cu/mesoSi | 22.5 | 24 | ||||
| Cu/macroSi | 21 | 22 | ||||
| CuZrAl | 9 | 7.5/1 | HCOH, CH3OH | 1.7 | Promoters enhanced the oxygenate yield due to increased dispersion of Cu particles | [52] |
| CuZnAl | ||||||
| CuMgAl |
| Sample | Support | |
|---|---|---|
| CuSi-1 | Microporous | Purchased Zeolite ZSM-5 |
| CuSi-2 | Mesoporous | Synthesized |
| CuSi-3 | Macroporous | Synthesized |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubev, O.V.; Maximov, A.L. Partial Oxidation of CH4 in Plasma: The Effects of Oxidant and Catalyst Addition. Molecules 2025, 30, 1958. https://doi.org/10.3390/molecules30091958
Golubev OV, Maximov AL. Partial Oxidation of CH4 in Plasma: The Effects of Oxidant and Catalyst Addition. Molecules. 2025; 30(9):1958. https://doi.org/10.3390/molecules30091958
Chicago/Turabian StyleGolubev, Oleg V., and Anton L. Maximov. 2025. "Partial Oxidation of CH4 in Plasma: The Effects of Oxidant and Catalyst Addition" Molecules 30, no. 9: 1958. https://doi.org/10.3390/molecules30091958
APA StyleGolubev, O. V., & Maximov, A. L. (2025). Partial Oxidation of CH4 in Plasma: The Effects of Oxidant and Catalyst Addition. Molecules, 30(9), 1958. https://doi.org/10.3390/molecules30091958








