Isolation and Characterization of Earthworm Peptides with Neuroprotective Effects in Parkinson’s Disease Models
Abstract
1. Introduction
2. Results
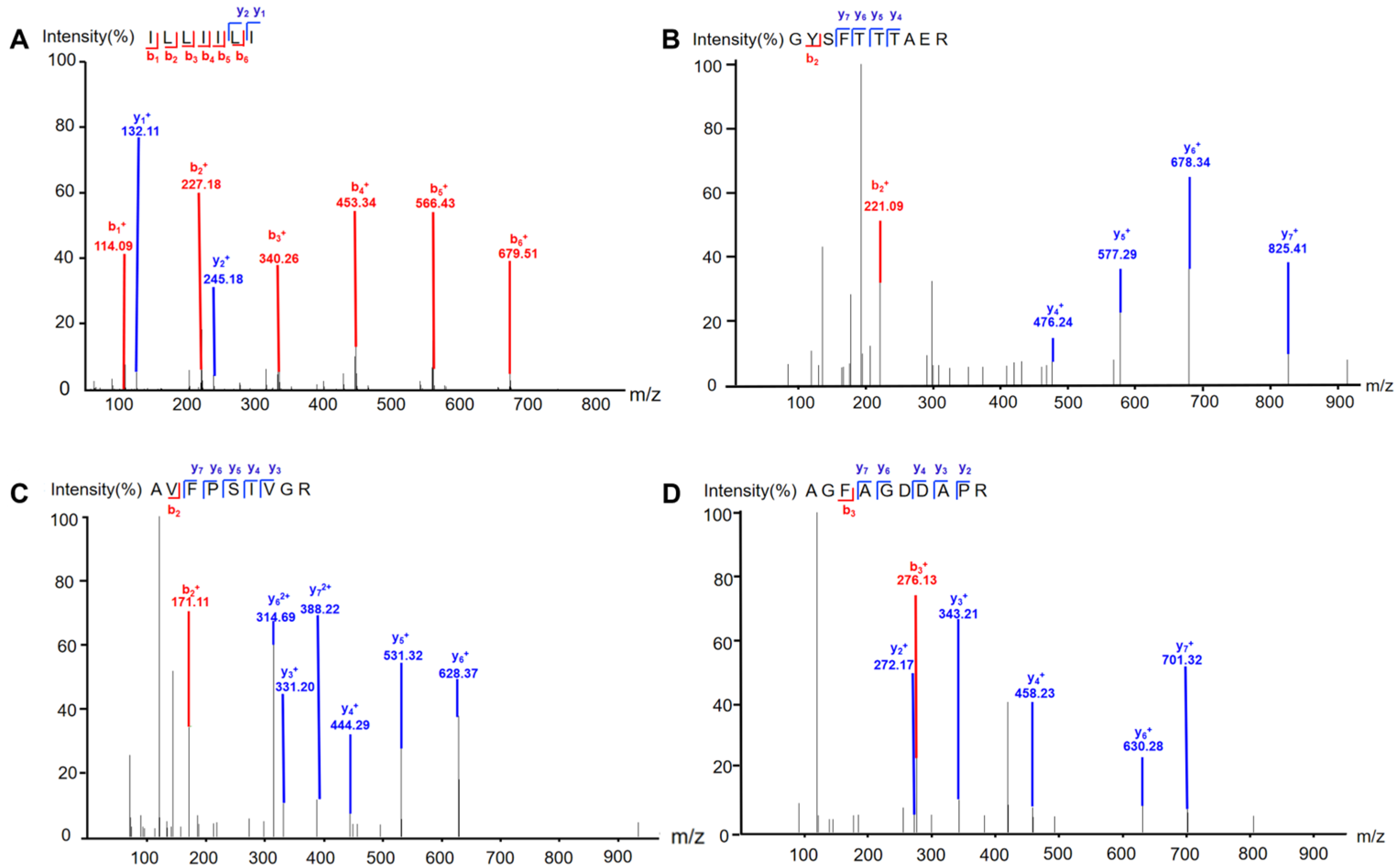
2.1. Isolation and Identification of Earthworm Peptides
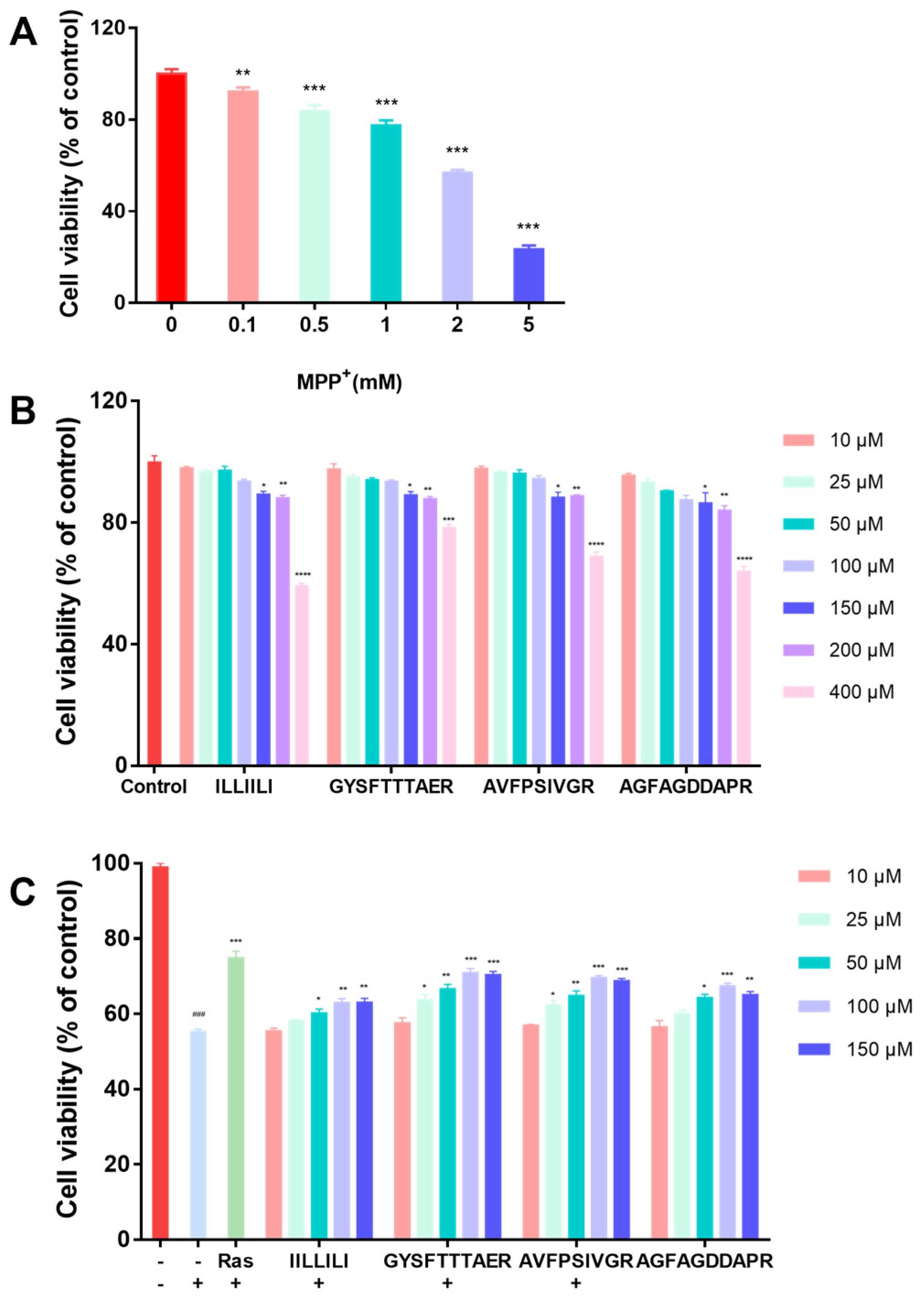
2.2. Neuroprotective Activity Bioassays
2.2.1. Neuroprotective Effects of Peptides in MPP+-Induced Injury of SH-SY5Y Cells
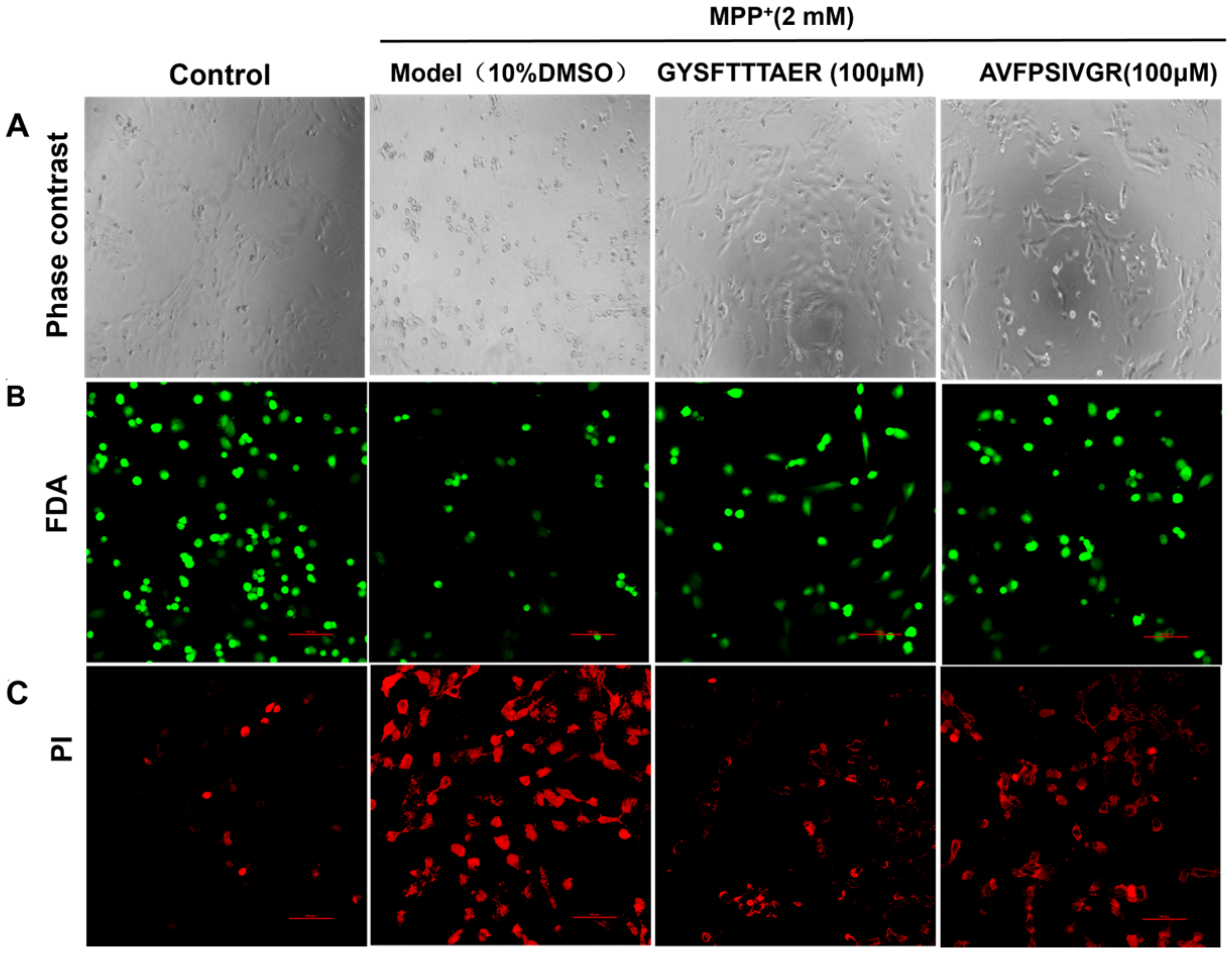
2.2.2. Morphological Analyses of SH-SY5Y Cells
2.3. Effects of Earthworm Peptides on the Mitochondrial Damage and Mitophagy in MPP+-Treated SH-SY5Y Cells
2.4. PINK1 Agonistic Activity of Earthworm Peptides
2.5. Molecular Docking of Peptides and PINK1
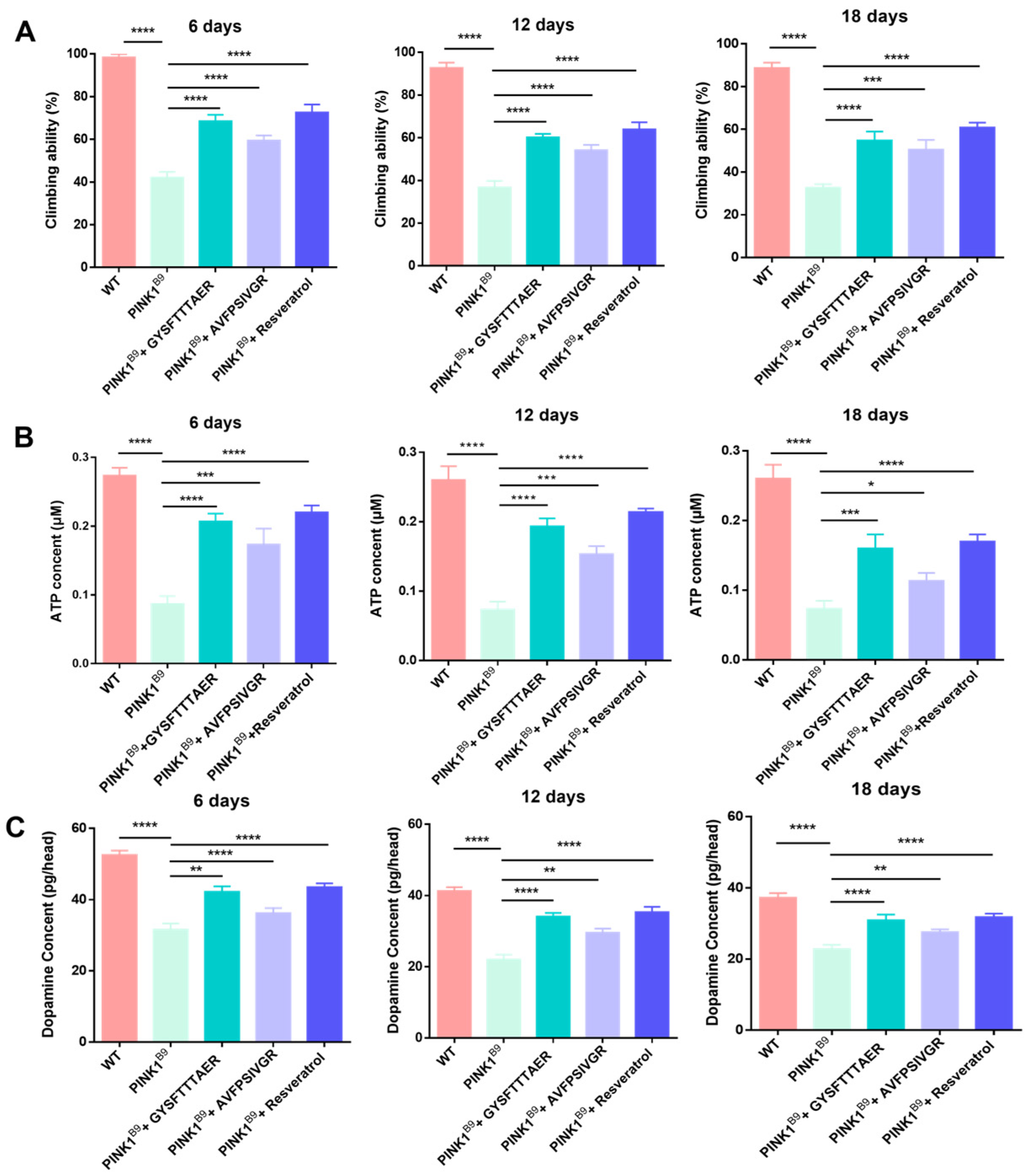
2.6. Neuroprotective Effect of Peptides on PINK1B9 Flies
2.6.1. Lifespan of PINK1B9 Flies Was Extended by Earthworm Peptides
2.6.2. Locomotor Ability of PINK1B9 Flies Was Improved by Earthworm Peptides
2.6.3. ATP Content Increment
2.6.4. Dopamine Content Increment
3. Materials and Methods
3.1. Materials and Reagent
3.2. Isolation and Identification of Earthworm Peptides
3.2.1. Crude Earthworm Extract
3.2.2. Peptide Purification
3.2.3. Identification of the Peptides
3.3. Peptides Synthesis
3.4. Neuroprotective Activity Bioassays
3.4.1. Cell Cultures and Cell Viabilities
3.4.2. Morphological Analyses and Fluorescence Staining of SH-SY5Y Cells
3.5. Measurement of Mitochondrial Membrane Potential
3.6. Quantitative qPCR
3.7. Determination of the Agonistic Activity of PINK1
3.8. Molecular Docking Study
3.8.1. Fly Strains and Husbandry
3.8.2. Lifespan Ability
3.8.3. Climbing Ability
3.8.4. PINK1B9 Flies’ ATP Levels
3.8.5. PINK1B9 Dopamine Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Jiang, H. Nutritive evaluation of earthworms as human food. Future Foods 2017, 10, 128–141. [Google Scholar]
- Fu, Z.R.; Zhang, L.; Liu, X.B.; Zhang, Y.Z.; Zhang, Q.L.; Li, X.M.; Zheng, W.; Sun, L.L.; Tian, J.K. Comparative proteomic analysis of the sun-and freeze-dried earthworm Eisenia fetida with differentially thrombolytic activities. J. Proteomics 2013, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.S.; Zhang, C.L. Research progress on Pheretima and earthworms with related origin. China J. Chin. Mater. Med. 2021, 46, 3298–3302. [Google Scholar]
- Hegemann, J.D.; Zimmermann, M.; Xie, X.; Marahiel, M.A. Lasso peptides: An intriguing class of bacterial natural products. Acc. Chem. Res. 2015, 48, 1909–1919. [Google Scholar] [CrossRef]
- Deng, Z.H.; Gao, S.S.; Xiao, X.; Yin, N.; Ma, S.Y.; Li, W.P.; Li, Y.S. The effect of earthworm extract on mice S180 tumor growth and apoptosis. Biomed. Pharmacother. 2019, 115, 108979. [Google Scholar] [CrossRef]
- Zhu, B.N.; Dong, J.; Gao, X.Y.; He, Y.T.; Sun, H.X. Antiasthmatic effects of Sanglong Pingchuan decoction through inducing a balanced Th1/Th2 immune response. Evid. Based Complement. Altern. Med. 2018, 2018, 2629565. [Google Scholar] [CrossRef]
- He, M.; Xie, W.Q.; Cheng, G.; Li, W.P.; Yu, D.J.; Jin, H.F.; Deng, Z.H.; Li, Y.S. The therapeutic effects of earthworm extract on deep second-degree burn wound healing. Ann. Palliat. Med. 2021, 10, 2869–2879. [Google Scholar] [CrossRef]
- Chang, Y.M.; Chi, W.Y.; Lai, T.Y.; Chen, Y.S.; Tsai, F.J.; Tsai, C.H.; Kuo, W.W.; Cheng, Y.C.; Lin, C.C.; Huang, C.Y. Dilong: Role in peripheral nerve regeneration. Evid. Based Complement. Alternat. Med. 2011, 2011, 380809. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Kuo, W.H.; Lai, T.Y.; Shih, Y.T.; Tsai, F.J.; Tsai, C.H.; Shu, W.T.; Chen, Y.Y.; Chen, Y.S.; Kuo, W.W.; et al. RSC96 schwann cell proliferation and survival induced by dilong through PI3K/Akt sinaling mediated by IGF- I. Evid. Based Complement. Alternat. Med. 2011, 21, 148. [Google Scholar]
- Kim, D.H.; Lee, I.H.; Nam, S.T.; Hong, J.; Zhang, P.; Hwang, J.S.; Seok, H.; Choi, H.; Lee, D.G.; Kim, J.I.; et al. Neurotropic and neuroprotective activities of the earthworm peptide Lumbricusin. Biochem. Biophys. Res. Commun. 2014, 44, 292–297. [Google Scholar] [CrossRef]
- Seo, M.; Lee, J.H.; Baek, M.; Kim, M.A.; Ahn, M.Y.; Kim, S.H.; Yun, E.Y.; Hwang, J.S. A novel role for earthworm peptide Lumbricusin as a regulator of neuroinflammation. Biochem. Biophys. Res. Commun. 2017, 490, 1004–1010. [Google Scholar] [CrossRef]
- Arkinson, C.; Walden, H. Parkin function in Parkinson’s disease. Science 2018, 360, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.V.; Goncharov, A.G.; Babich, O.O.; Larina, V.V.; Orekhov, A.N.; Melnichenko, A.A. Prospects for the development of Pink1 and Parkin activators for the treatment of Parkinson’s Disease. Pharmaceutics 2022, 14, 2514. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.L.; Stoker, T.B.; Greenland, J.C. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis; Exon Publications: Brisbane, Australia, 2018; pp. 3–26. [Google Scholar]
- Kim, K.S. Toward neuroprotective treatments of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3795–3797. [Google Scholar] [CrossRef]
- Vizziello, M.; Borellini, L.; Franco, G.; Ardolino, G. Disruption of mitochondrial homeostasis: The role of PINK1 in Parkinson’s disease. Cells 2021, 10, 3022. [Google Scholar] [CrossRef]
- Hayashida, A.; Li, Y.; Yoshinob, H.; Nakaharac, T.; Yoritakad, A.; Nagarae, Y.; Sakiyamaf, Y.; Kusakag, H.; Takiyamah, Y.; Morimotoi, N.; et al. PINK1 heterozygous mutations in familial Parkinson’s disease. J. Neurol. Sci. 2017, 381, 161–162. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.S.; Liu, R.R.; Mai, Y.Z. Ramelteon ameliorated 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in neuronal cells in a mitochondrial-dependentpathway. Bioengineered 2021, 12, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.W.; Lin, C.C.; Chen, Y.H.; Yang, H.B.; Hung, S.Y. Celastrol inhibits dopaminergic neuronal death of Parkinson’s disease through activating mitophagy. Antioxidants 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, R.R.; Chen, J.Z. Salidroside protects dopaminergic neurons by enhancing PINK1/Parkin-mediated mitophagy. Oxid. Med. Cell Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Dos Santos, M.G.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective Effects of Resveratrol in In vivo and In vitro experimental models of Parkinson’s disease a systematic review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef]
- Dewapriya, P.; Himaya, S.W.A.; Li, Y.X.; Kim, S.K. Tyrosol exerts a protective effect against dopaminergic neuronal cell death in in vitro model of Parkinson’s disease. Food Chem. 2013, 141, 1147–1157. [Google Scholar] [CrossRef]
- Prasertsuksri, P.; Kraokaew, P.; Pranweerapaiboon, K.; Sobhon, P.; Chaithirayanon, K. Neuroprotection of andrographolide against neurotoxin MPP+-induced apoptosis in SH-SY5Y cells via activating mitophagy, autophagy, and antioxidant activities. Int. J. Mol. Sci. 2023, 24, 8528. [Google Scholar] [CrossRef]
- Yang, X.; Pan, W.N.; Xu, G.S.; Chen, L.X. Mitophagy: A crucial modulator in the pathogenesis of chronic diseases. Clin. Chim. Acta. 2020, 502, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Fazi, R.; Tintori, C.; Brai, A.; Botta, L.; Selvaraj, M.; Garbelli, A.; Maga, G.; Botta, M. Homology model-based virtual screening for the identification of human helicase DDX3 inhibitors. J. Chem. Inf. Model. 2015, 55, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Su, C.F.; Jiang, L.; Zhang, X.W.; Iyaswamy, A.; Li, M. Resveratrol in rodent models of Parkinson’s disease: A systematic review of experimental studies. Front. Pharmacol. 2021, 12, 644219. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, A.; Dong, J.; Sigears, A.; Lu, B. Grape skin extract improves muscle function and extends lifespan of a drosophila model of Parkinson’s disease through activation of mitophagy. Exp. Gerontol. 2018, 113, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Inoshita, T.; Meng, H.R.; Shiba-Fukushima, K.; Hara, K.Y.; Sawamura, N.; Hattori, N. Light-driven activation of mitochondrial proton-motive force improves motor behaviors in a Drosophila model of Parkinson’s disease. Commun. Biol. 2019, 2, 424. [Google Scholar] [CrossRef]
- Bayersdorfer, F.; Voigt, A.; Schneuwly, S.; Botella, J.A. Dopamine-dependent neurodegeneration in Drosophila models of familial and sporadic Parkinson’s disease. Neurobiol. Dis. 2010, 40, 113–119. [Google Scholar] [CrossRef]
- Aerts, L.; Craessaerts, K.; De Strooper, B.; Morais, V.A. In vitro comparison of the activity requirements and substrate specificity of human and triboleum castaneum PINK1 orthologues. PloS ONE 2016, 11, 146083. [Google Scholar] [CrossRef]
- Alrashidi, H.; Eaton, S.; Heales, S. Biochemical characterization of proliferative and differentiated SH-SY5Y cell line as a model for Parkinson’s disease. Neurochem. Int. 2021, 145, 105009. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.L.; Liang, J.W.; Ding, Y.Y.; Meng, F.H. An integrated network, RNA sequencing, and experiment pharmacology approach reveals the active component, potential target, and mechanism of Gelsemium elegans in the treatment of colorectal cancer. Front. Oncol. 2020, 10, 616628. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Soya, N.; Truong, L.; Croteau, N.; Lukacs, G.L.; Trempe, J.F. PINK1 autophosphorylation is required for ubiquitin recognition. EMBO. Rep. 2018, 15, 4. [Google Scholar]
- Lee, S.; Zhang, C.; Liu, X. Role of glucose metabolism and ATP in maintaining PINK1 levels during Parkin-mediated mitochondrial damage responses. J. Biol. Chem. 2014, 290, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, M.; Santhi, N.; Bhuvaneswari, V. Pharmacophore and virtual screening of JAK3 inhibitors. Bioinformation 2014, 10, 157–163. [Google Scholar] [CrossRef] [PubMed]



| Peptide | Agonistic Rate (%) 1 | EC50 (μM) 1 |
|---|---|---|
| ILLIILI | 17.16 ± 0.55 | Nd |
| GYSFTTTAER | 52.46 ± 0.96 | 76.77 ± 1.3 |
| AVFPSIVGR | 50.35 ± 0.72 | 88.5 ± 0.97 |
| AGFAGDDAPR | 33.45 ± 0.45 | Nd |
| Resveratrol 2 | 91.45 ± 0.34 | 13.75 ± 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Hu, Y.; Bai, X.; Liao, X. Isolation and Characterization of Earthworm Peptides with Neuroprotective Effects in Parkinson’s Disease Models. Molecules 2025, 30, 1952. https://doi.org/10.3390/molecules30091952
Shi G, Hu Y, Bai X, Liao X. Isolation and Characterization of Earthworm Peptides with Neuroprotective Effects in Parkinson’s Disease Models. Molecules. 2025; 30(9):1952. https://doi.org/10.3390/molecules30091952
Chicago/Turabian StyleShi, Guangyu, Yikao Hu, Xiaolin Bai, and Xun Liao. 2025. "Isolation and Characterization of Earthworm Peptides with Neuroprotective Effects in Parkinson’s Disease Models" Molecules 30, no. 9: 1952. https://doi.org/10.3390/molecules30091952
APA StyleShi, G., Hu, Y., Bai, X., & Liao, X. (2025). Isolation and Characterization of Earthworm Peptides with Neuroprotective Effects in Parkinson’s Disease Models. Molecules, 30(9), 1952. https://doi.org/10.3390/molecules30091952








