A Study on the Separation of Nitric Acid and Acetic Acid from Simulated Reprocessing Waste by TBP Extraction
Abstract
1. Introduction
2. Results and Discussion
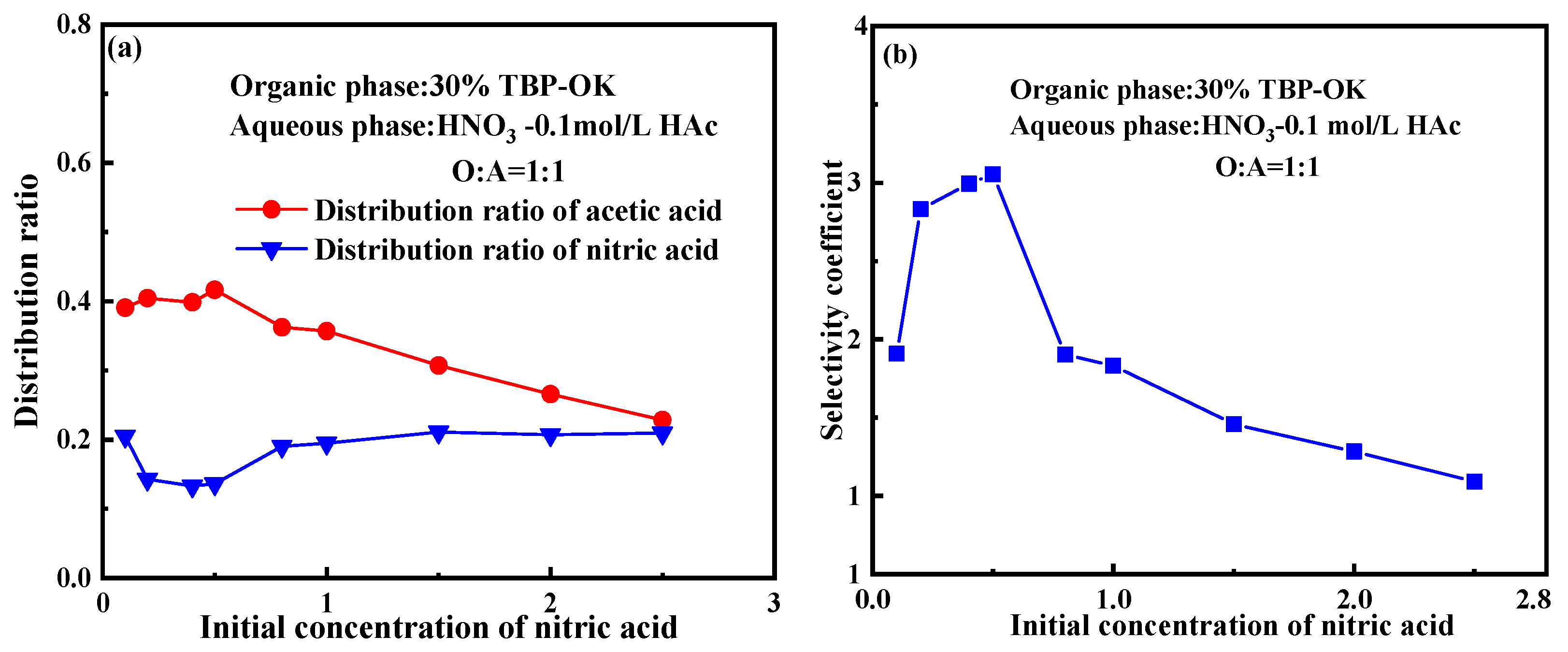
2.1. Effect of Initial Nitric Acid Concentration
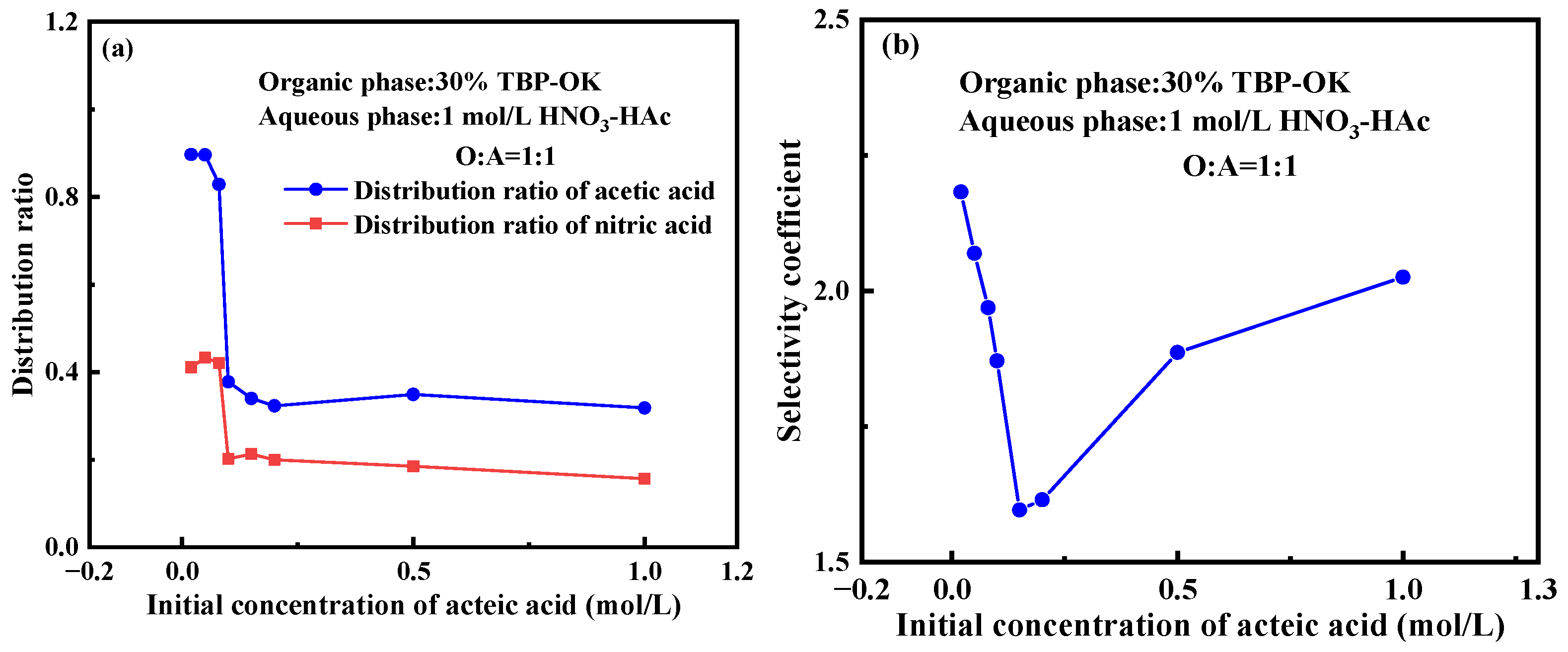
2.2. Effect of Initial Acetic Acid Concentration
2.3. Effect of Phase Ratio
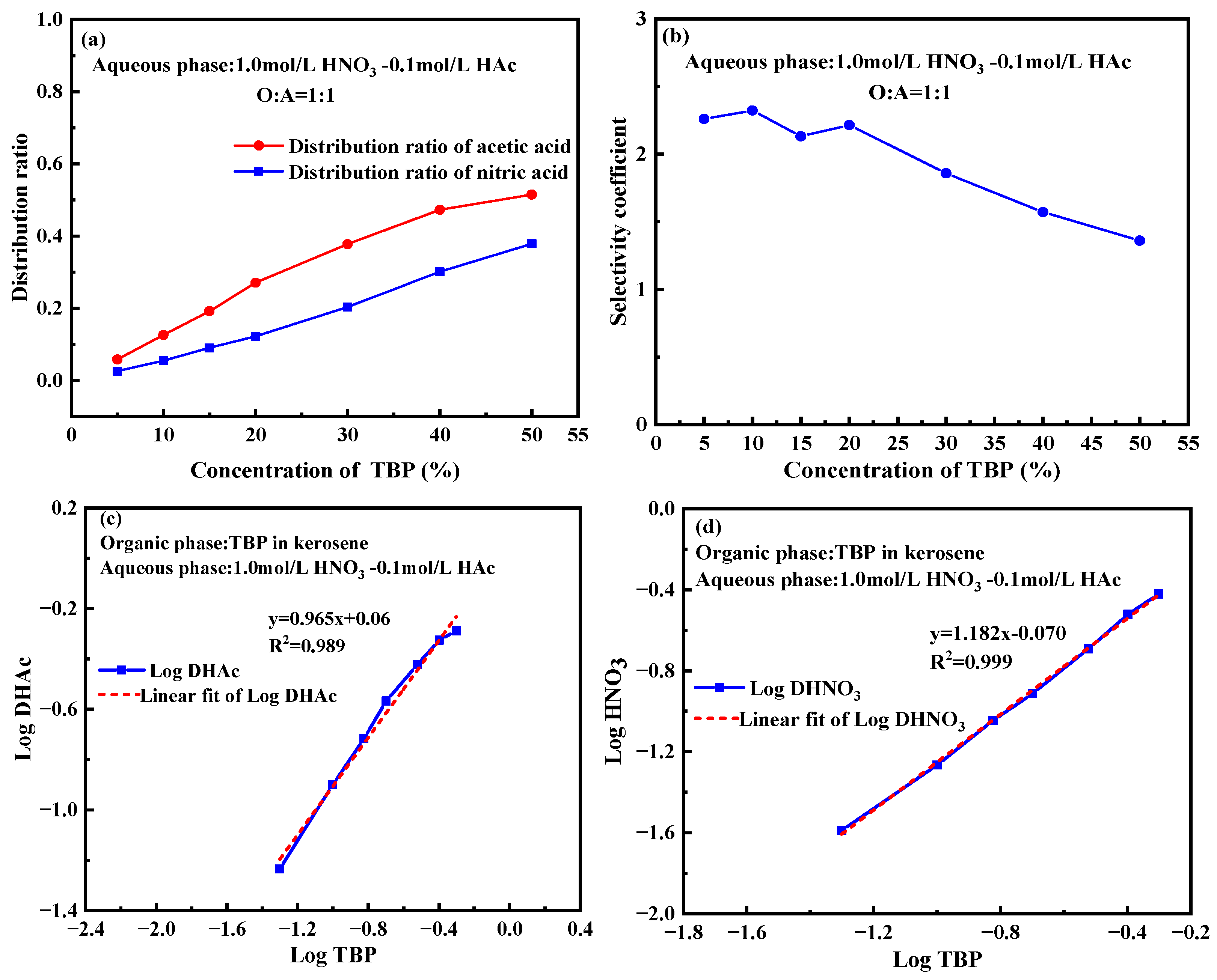
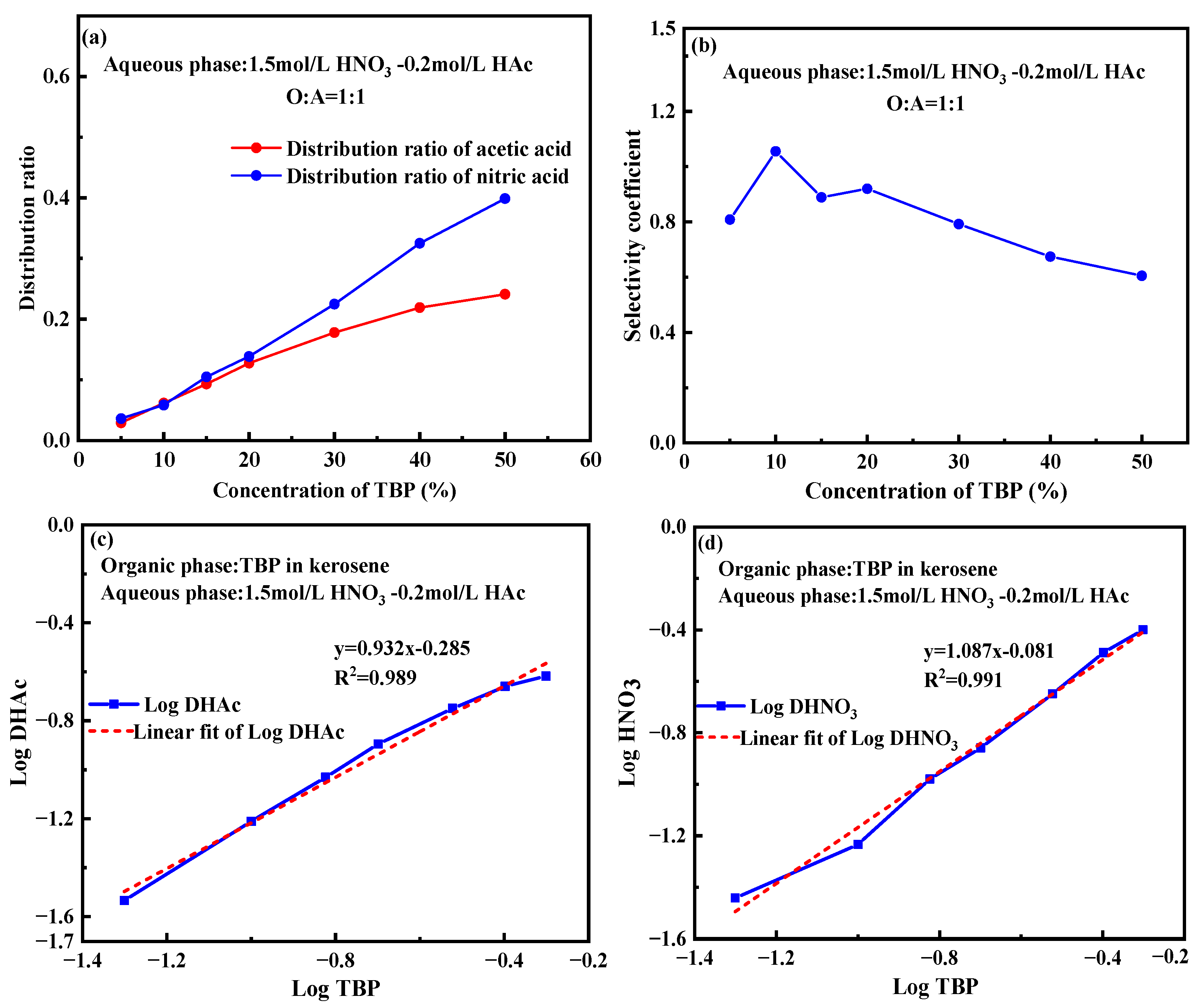
2.4. Effect of TBP Concentration
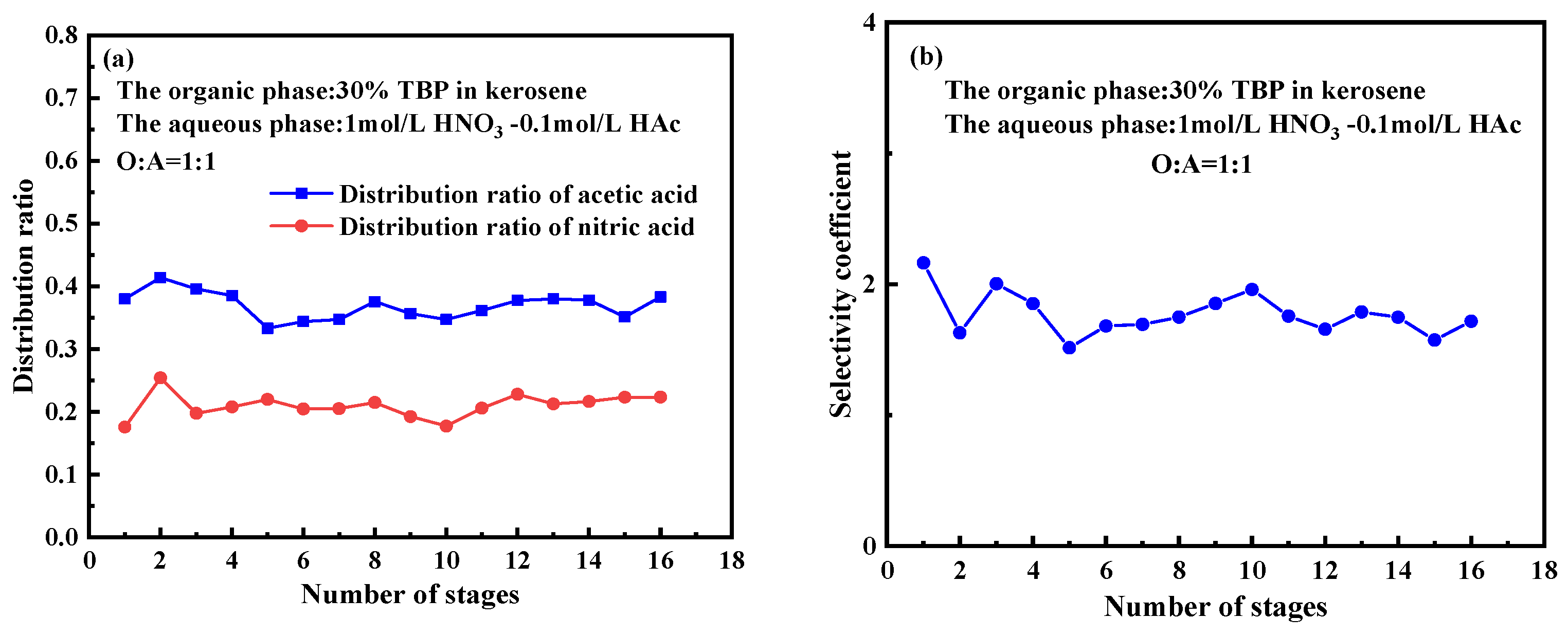
2.5. Extraction Performance and Mass Transfer in Bench-Scale Extraction
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Single-Stage Extraction
3.2.2. Bench-Scale Extraction Experiment
3.3. Analytical Methods
4. Conclusions
- Selective extraction behavior: TBP preferentially extracts acetic acid over nitric acid, especially in the low acid concentration range (HNO₃ < 1 mol/L, HAc < 0.2 mol/L). The selectivity coefficient decreases from 3.05 in a 0.5 mol/L HNO3-0.1 mol/L HAc system to 2.18 in a 1 mol/L HNO3-0.2 mol/L HAc system.
- Complex formation and equilibrium: TBP forms stable 1:1 complexes with both nitric acid and acetic acid, with equilibrium constants of approximately 0.85 under typical operating conditions, specifically in a 1.0 mol/L HNO3-0.1 mol/L HAc system.
- Effect of TBP concentration and phase ratio: Increasing TBP concentration enhances nitric acid extraction, while adjusting the phase ratio improves acetic acid separation. Under 1mol/L HNO3-0.1mol/L HAc system, a higher phase ratio (5:1) increases the selectivity coefficient to 1.9, while 5% TBP-OK further improves it to 2.27.
- Process optimization: A 16-stage countercurrent extraction simulation confirms that optimizing TBP concentration and phase ratio effectively enhances the separation of nitric acid and acetic acid, providing a foundation for process improvement in spent fuel reprocessing.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| AHA | acetohydroxamic acid |
| the concentration of acetic acid in the organic phase | |
| the concentration of acetic acid in the aqueous phase | |
| concentration of nitric acid or acetic acid in both phases at equilibrium, mol/L | |
| concentration measured during the experiment, mol/L | |
| concentration of nitric acid or acetic acid in the organic phase, mol/L | |
| concentration of nitric acid or acetic acid in the aqueous phase, mol/L | |
| the concentration difference between the organic phase and aqueous phase, mol/L | |
| the distribution ratio of nitric acid | |
| the distribution ratio of acetic acid | |
| acetic acid | |
| concentration of nitric acid in the organic phase, mol/L | |
| concentration of nitric acid in the aqueous phase, mol/L | |
| concentration of hydrogen ions in the aqueous phase, mol/L | |
| the purification coefficient | |
| K | overall mass transfer coefficient |
| concentration of nitrate ions in the aqueous phase | |
| the mass transfer rate, mol/s | |
| Q | volumetric flow rate, ml/min |
| the selectivity coefficient | |
| tributyl phosphate | |
| TBP-OK | tributyl phosphate in organic kerosene |
| concentration of the TBP-HNO3 complex in the organic phase, mol/L | |
| concentration of free TBP in the organic phase |
References
- Ekins, P. Step changes for decarbonising the energy system: Research needs for renewables, energy efficiency and nuclear power. Energy Policy 2004, 32, 1891–1904. [Google Scholar] [CrossRef]
- Francis, A.J. Characteristics of nuclear and fossil energy wastes. Experientia 1990, 46, 794–796. [Google Scholar]
- Milliken, J.A.; Joseck, F.; Wang, M.; Yuzugullu, E. The advanced energy initiative. J. Power Sources 2007, 172, 121–131. [Google Scholar]
- Buchao, X.U.; Yanfei, Z.; Ming, H. Analysis on development modes and routes of nuclear energy in the context of low carbon economy. Resour. Sci. 2010, 32, 2186–2191. [Google Scholar]
- Kurniawan, T.A.; Othman, M.H.D.; Singh, D.; Avtar, R.; Hwang, G.H.; Setiadi, T.; Lo, W.H. Technological solutions for long-term storage of partially used nuclear waste: A critical review. Ann. Nucl. Energy 2022, 166, 108736. [Google Scholar] [CrossRef]
- Salvatores, M.; Palmiotti, G. Radioactive waste partitioning and transmutation within advanced fuel cycles: Achievements and challenges. Prog. Part. Nucl. Phys. 2011, 66, 144–166. [Google Scholar]
- Kuzmin, V.I.; Kuzmin, D.V.; Gudkova, N.V.; Kalyakin, S.N.; Mulagaleeva, M.A.; Alekseenko, V.N.; Aksyutin, P.V.; Bartseva, Y.V.; Ivanov, A.V.; Kryuchek, N.M. Autocatalytic decomposition of tributyl phosphate in the spent extractant of the PUREX process for safe disposal of radioactive impurities. Hydrometallurgy 2022, 213, 105909. [Google Scholar] [CrossRef]
- Saab, M.; Réal, F.; Šulka, M.; Cantrel, L.; Virot, F.; Vallet, V. Facing the challenge of predicting the standard formation enthalpies of n-butyl-phosphate species with ab initio methods. J. Chem. Phys. 2017, 146, 244312. [Google Scholar]
- Chen, H.; Taylor, R.; Woodhead, D.; Sarsfield, M.; Whittaker, D.; Carrott, M.; Keywood, B.; Taylor, K.; Jobson, M.; Masters, A. Experimental testing and process simulation of flowsheets for the co-separation of uranium and plutonium using acetohydroxamic acid as a complexing agent. Prog. Nucl. Energy 2024, 176, 105352. [Google Scholar] [CrossRef]
- Herbst, R.; Baron, P.; Nilsson, M. Standard and advanced separation: PUREX processes for nuclear fuel reprocessing. In Reviews in Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Woodhead Publishing: Swaton, UK, 2011; pp. 141–175. [Google Scholar]
- Shuangchen, M.; Jin, C.; Gongda, C.; Weijing, Y.; Sijie, Z. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew. Sustain. Energy Rev. 2016, 58, 1143–1151. [Google Scholar]
- Digiano, F.A.; Scaramelli, A.B. Wastewater treatment: Physical and chemical methods. J. Water Pollut. Control Fed. 1974, 46, 1109–1121. [Google Scholar] [PubMed]
- Agarwal, C.; Pandey, A.K. Remediation and recycling of inorganic acids and their green alternatives for sustainable industrial chemical processes. Environ. Sci. Adv. 2023, 2, 1306–1339. [Google Scholar]
- Zhao, X.; Liu, Z.; Zhao, J.; Kang, T.; Yan, C.; Ju, C.; Ma, L.; Zhang, X.; Wang, Y.; Wu, Y. Highly efficient molecular film for inhibiting volatilization of hazardous nitric acid. Environ. Res. 2024, 246, 118151. [Google Scholar]
- Cantwell, F.F.; Losier, M. Chapter 11 Liquid-liquid extraction. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 37, pp. 297–340. [Google Scholar]
- Mazzola, P.G.; Lopes, A.M.; Hasmann, F.A.; Jozala, A.F.; Penna, T.C.; Magalhaes, P.O.; Pessoa, A., Jr. Liquid-liquid extraction of biomolecules: An overview and update of the main techniques. J. Chem. Technol. Biotechnol. 2008, 83, 143–157. [Google Scholar]
- Rodrigues, G.D.; da Silva, M.D.C.H.; da Silva, L.H.M. Liquid-liquid extraction of metal ions without use of organic solvent. Sep. Purif. Technol. 2008, 62, 687–693. [Google Scholar]
- Moreira, V.R.; Lebron, Y.A.R.; Gontijo, D.; Amaral, M.C.S. One-step recycling of mineral acid from concentrated gold mining wastewater by high-temperature liquid-liquid extraction. Sep. Purif. Technol. 2022, 286, 120447. [Google Scholar]
- Chadni, M.; Moussa, M.; Athès, V.; Allais, F.; Ioannou, I. Membrane contactors-assisted liquid-liquid extraction of biomolecules from biorefinery liquid streams: A case study on organic acids. Sep. Purif. Technol. 2023, 317, 123927. [Google Scholar] [CrossRef]
- Ehsan, R.; Thomas, B.; Kersten, S.R.A.; Van, H.A.G.J.; Boelo, S. Liquid-liquid extraction-based process concepts for recovery of carboxylic acids from aqueous streams evaluated for dilute streams. Chem. Eng. Res. Des. 2018, 137, 510–533. [Google Scholar]
- Shin, C.-H.; Kim, J.-Y.; Kim, J.-Y.; Kim, H.-S.; Lee, H.-S.; Mohapatra, D.; Ahn, J.-W.; Ahn, J.-G.; Bae, W. Recovery of nitric acid from waste etching solution using solvent extraction. J. Hazard. Mater. 2009, 163, 729–734. [Google Scholar] [CrossRef]
- Karunanithi, S.; Kapoor, A.; Kumar, P.S.; Balasubramanian, S.; Rangasamy, G. Solvent extraction of acetic acid from aqueous solutions: A review. Sep. Sci. Technol. 2023, 58, 1985–2007. [Google Scholar]
- Chen, F.; Wang, X.; Liu, W.; Liang, B.; Yue, H.; Li, C. Selective extraction of nitric and acetic acids from etching waste acid using N235 and MIBK mixtures. Sep. Purif. Technol. 2016, 169, 50–58. [Google Scholar]
- Shin, C.-H.; Kim, J.-Y.; Kim, J.-Y.; Kim, H.-S.; Lee, H.-S.; Mohapatra, D.; Ahn, J.-W.; Ahn, J.-G.; Bae, W. A solvent extraction approach to recover acetic acid from mixed waste acids produced during semiconductor wafer process. J. Hazard. Mater. 2009, 162, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Wongsawa, T.; Koonsang, T.; Kunthakudee, N.; Prapasawat, T.; Maneeintr, K.; Pancharoen, U. The experimental investigations on viscosity, surface tension, interfacial tension and solubility of the binary and ternary systems for tributyl phosphate (TBP) extractant in various organic solvents with water: Thermodynamic NRTL model and molecular interaction approach. J. Mol. Liq. 2018, 251, 229–237. [Google Scholar]
- Zhou, Z.; Qin, W.; Fei, W. Extraction equilibria of lithium with tributyl phosphate in three diluents. J. Chem. Eng. Data 2011, 56, 3518–3522. [Google Scholar]
- Shi, C.; Duan, D.; Jia, Y.; Jing, Y. A highly efficient solvent system containing ionic liquid in tributyl phosphate for lithium ion extraction. J. Mol. Liq. 2014, 200, 191–195. [Google Scholar]
- Phillips, W.D.; Milne, N. Ashless phosphorus–containing lubricating oil additives. In Reviews in Lubricant Additives; CRC Press: Boca Raton, FL, USA, 2017; pp. 157–196. [Google Scholar]
- Yang, X.; Yu, G.; Xu, L.; Wang, J. Degradation of the mixed organic solvents of tributyl phosphate and n-dodecane by heterogeneous Fenton-like oxidation using nanoscale zero-valent iron as the catalyst. Chemosphere 2022, 292, 133449. [Google Scholar]
- Ma, J.; Wang, K.; Li, M.; Liu, T.; Yang, C. Technological process for treating radioactive TBP/OK spent solvents. J. Phys. Conf. Ser. 2024, 2713, 012043. [Google Scholar]
- Carrot, M.; Gregson, C.; Taylor, R. Neptunium extraction and stability in the GANEX solvent: 0.2 M TODGA/0.5 M DMDOHEMA/kerosene. Solvent Extr. Ion. Exch. 2013, 31, 463–482. [Google Scholar] [CrossRef]
- Pathak, P.; Kumari, N.; Prabhu, D.; Manchanda, V. Redox behavior of neptunium (V) in tributyl phosphate and N, N-dihexyl octanamide extractants dissolved in n-dodecane. J. Solut. Chem. 2012, 41, 410–421. [Google Scholar] [CrossRef]
- Shakya, A.K.; Jaiswal, Y.; Pal, S.L.; Srivastava, S. Reactive extraction of acetic acid by using tri-butyl-phosphate with different diluents. Chem. Data Collect. 2022, 39, 100855. [Google Scholar]
- Cheng, G.; Li, Y.; Cao, Y.; Zhang, Z. A novel method for the desulfurization of medium–high sulfur coking coal. Fuel 2023, 335, 126988. [Google Scholar]
- Cheng, G.; Li, Y.; Cao, Y.; Wang, X.; Li, E.; Guo, Y.; Lau, E.V. New insights on the understanding of sulfur-containing coal flotation desulfurization. Minerals 2024, 14, 981. [Google Scholar] [CrossRef]
- Pratap, B.; Kumar, S.; Nand, S.; Azad, I.; Bharagava, R.N.; Ferreira, L.F.R.; Dutta, V. Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety. Chemosphere 2023, 313, 137547. [Google Scholar]
- Wright, A.; Paviet-Hartmann, P. Review of physical and chemical properties of tributyl phosphate/diluent/nitric acid systems. Sep. Sci. Technol. 2010, 45, 1753–1762. [Google Scholar]
- Shen, X.; Han, K.; Ma, L.; Gao, M.; Xu, X.; Luo, J. Nano-Ag-forest based surface enhanced Raman spectroscopy (SERS) of confined acetic acid. Colloids Surf. A 2018, 547, 126–133. [Google Scholar]
- Lucas, H.; Petitet, J.-P. High pressure Raman spectroscopy of nitric acid. J. Phys. Chem. A 1999, 103, 8952–8958. [Google Scholar]
- Kertes, A.; King, C.J. Extraction chemistry of fermentation product carboxylic acids. Biotechnol. Bioeng. 1986, 28, 269–282. [Google Scholar]
- Marti, M.E. Solvent modification effect on the physical and chemical extraction of acetic acid. Sep. Sci. Technol. 2016, 51, 1806–1816. [Google Scholar]
- Tamada, J.A.; Kertes, A.S.; King, C.J. Extraction of carboxylic acids with amine extractants. 1. Equilibria and law of mass action modeling. Ind. Eng. Chem. Res. 1990, 29, 1319–1326. [Google Scholar]
- Rydberg, J.; Choppin, G.R.; Musikas, C.; Sekine, T. Solvent extraction equilibria. In Reviews in Solvent Extraction Principles and Practice, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2004; pp. 121–217. [Google Scholar]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar]
- Karita, S.; Kaneta, T. Acid–base titrations using microfluidic paper-based analytical devices. Anal. Chem. 2014, 86, 12108–12114. [Google Scholar] [PubMed]
- Patil, A.R.; Shastri, L.A.; Tilakraj, T.; Inamdar, S.R.; Shastri, S.L.; Hebbar, N.U.; Pawar, V.; Sunagar, V.A. Synthesis and characterization of acid-base indicator: Exploring pH sensor, photophysical, thermal applications and theoretical study. J. Mol. Struct. 2022, 1254, 132363. [Google Scholar]
- Sadler, G.D.; Murphy, P.A. pH and titratable acidity. Food Anal. 2010, 4, 219–238. [Google Scholar]
- Bell, K.; Geist, A.; McLachlan, F.; Modolo, G.; Taylor, R.; Wilden, A. Nitric acid extraction into TODGA. Procedia Chem. 2012, 7, 152–159. [Google Scholar]


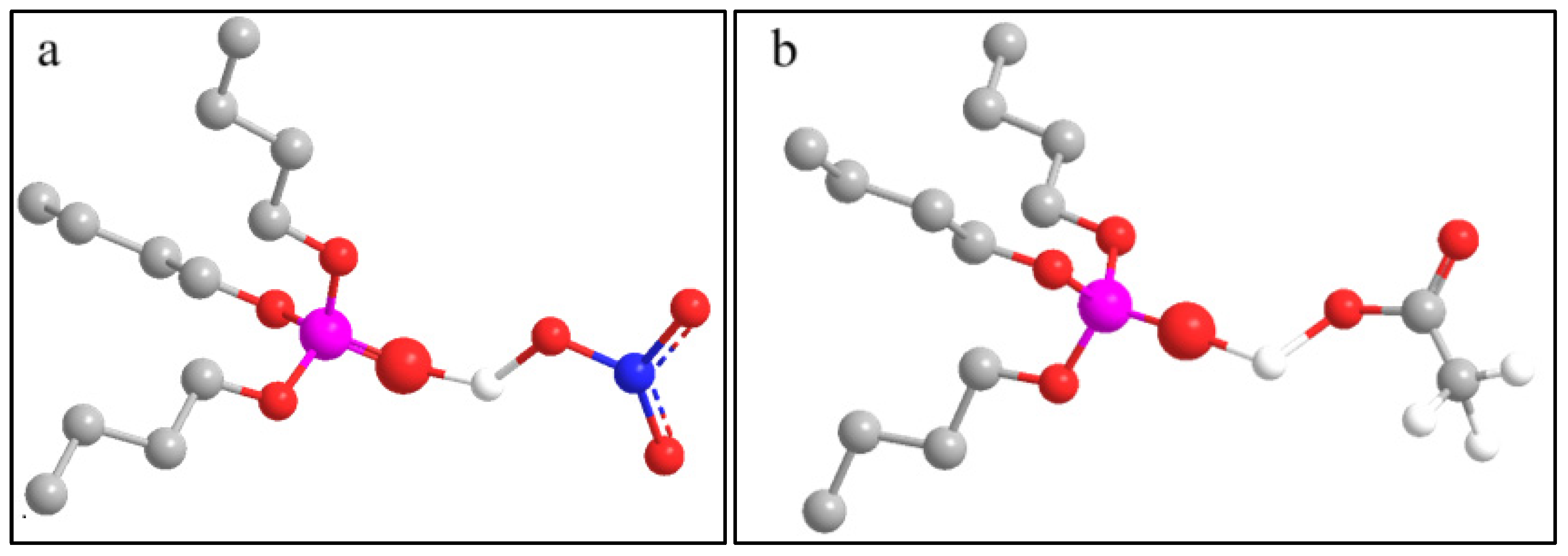


| Stages | (mol/L) | (mol/L) | (mol/s) | (mol/s) |
|---|---|---|---|---|
| 1 | 0.0404 | 0.731 | 1.68 × 10−6 | 3.05 × 10−5 |
| 2 | 0.0499 | 0.7449 | 2.08 × 10−6 | 3.10 × 10−5 |
| 3 | 0.055 | 0.852 | 2.29 × 10−6 | 3.55 × 10−5 |
| 4 | 0.0578 | 0.8222 | 2.41 × 10−6 | 3.43 × 10−5 |
| 5 | 0.0695 | 0.8178 | 2.90 × 10−6 | 3.41 × 10−5 |
| 6 | 0.0691 | 0.8255 | 2.88 × 10−6 | 3.44 × 10−5 |
| 7 | 0.0681 | 0.8043 | 2.84 × 10−6 | 3.35 × 10−5 |
| 8 | 0.0645 | 0.812 | 2.69 × 10−6 | 3.38 × 10−5 |
| 9 | 0.0691 | 0.8286 | 2.88 × 10−6 | 3.45 × 10−5 |
| 10 | 0.0656 | 0.8742 | 2.73 × 10−6 | 3.64 × 10−5 |
| 11 | 0.0675 | 0.8183 | 2.81 × 10−6 | 3.41 × 10−5 |
| 12 | 0.0676 | 0.7754 | 2.82 × 10−6 | 3.23 × 10−5 |
| 13 | 0.0679 | 0.8198 | 2.83 × 10−6 | 3.42 × 10−5 |
| 14 | 0.0668 | 0.8092 | 2.78 × 10−6 | 3.37 × 10−5 |
| 15 | 0.0702 | 0.8135 | 2.93 × 10−6 | 3.39 × 10−5 |
| 16 | −0.3009 | 0.8243 | −1.25 × 10−5 | 3.43 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Ge, X.; He, T.; Li, B.; Li, T.; Wang, H.; Yuan, Z.; Yang, Q.; Yan, T. A Study on the Separation of Nitric Acid and Acetic Acid from Simulated Reprocessing Waste by TBP Extraction. Molecules 2025, 30, 1814. https://doi.org/10.3390/molecules30081814
Lv H, Ge X, He T, Li B, Li T, Wang H, Yuan Z, Yang Q, Yan T. A Study on the Separation of Nitric Acid and Acetic Acid from Simulated Reprocessing Waste by TBP Extraction. Molecules. 2025; 30(8):1814. https://doi.org/10.3390/molecules30081814
Chicago/Turabian StyleLv, Hongbin, Xiao Ge, Tiansheng He, Baole Li, Tianchi Li, Hui Wang, Zhongwei Yuan, Qi Yang, and Taihong Yan. 2025. "A Study on the Separation of Nitric Acid and Acetic Acid from Simulated Reprocessing Waste by TBP Extraction" Molecules 30, no. 8: 1814. https://doi.org/10.3390/molecules30081814
APA StyleLv, H., Ge, X., He, T., Li, B., Li, T., Wang, H., Yuan, Z., Yang, Q., & Yan, T. (2025). A Study on the Separation of Nitric Acid and Acetic Acid from Simulated Reprocessing Waste by TBP Extraction. Molecules, 30(8), 1814. https://doi.org/10.3390/molecules30081814






