Abstract
Flammability is a significant challenge in polymer-based strain sensing applications. In addition, the existing intrinsic flame retardant is not elastic at room temperature, which may potentially damage the flexible equipment. This study presents a series of flame-retardant ionic conductive elastomers (ICEs) (denoted as PCAIPx) containing phosphorus from phytic acid (PA) and nitrogen from choline chloride (ChCl) with multiple hydrogen bonds synthesized using a simple and efficient one-pot UV-initiated radical copolymerization of a polymerizable deep eutectic solvent (PDES). The limiting oxygen index (LOI) value increased from 24.1% for the pure PCAI without PA to 38.3% for PCAIP7.5. The SEM analysis of the residual char shows that the formation of the dense and continuous char layer effectively worked as a shield, preventing further decomposition of the undecomposed polymer inside while hindering the transmission of heat and mass and isolating the oxygen required for combustion. The hydrogen bonds’ cross-linked structure and phosphorus-containing elastomer demonstrate a superior elasticity (elongation at break of up to 2109%), durability, and tear resistance and excellent adhesive properties. Application of PCAIPX in strain sensors showed that the elastomer has excellent cyclic stability and exhibited repeatable and stable resistance change signals in response to repetitive bending motions of the wrist, fingers, elbow, and knee. Consequently, this study provides a simple strategy for the development of a flame-retardant ICE which can effectively reduce fire hazards and potentially be applied in other fire-risk fields such as personal protection, firefighting, and sports equipment.
1. Introduction
Polymeric materials, renowned for their superior performance, are extensively utilized across industrial and domestic applications. However, their innate flammability also renders them a common fuel source in contemporary fires, particularly within the contexts of architectural and urban conflagrations [1,2,3,4]. The combustion behavior of these materials is characterized by unique complexities, encompassing thermal behavior alterations, pyrolytic chemical reaction sequences, and the combustion process itself [5,6,7,8,9]. Under fire conditions, polymers frequently exhibit distinctive changes such as melting, foaming, expansion, and contraction, which significantly influence the pyrolysis, ignition, and combustion processes [10,11,12,13,14,15]. Moreover, the pyrolytic reaction mechanisms of polymers may be altered by various factors present in fires, including high heat fluxes [16,17,18,19,20]. Despite the extensive research conducted by scholars worldwide on the combustion behavior of polymers in fires, the interplay of chemical reactions, mass transfer, and heat transfer poses significant challenges and opportunities in efforts to mitigate the fire risks associated with polymers through flame-retardant treatments [21,22,23,24,25,26,27,28,29].
The mechanisms of flame retardancy are typically categorized into gas-phase flame retardation, condensed-phase flame retardation, and heat exchange interruption mechanisms [12,19]. The heat exchange interruption mechanism primarily operates through physical means, utilizing the endothermic degradation of chemical substances to reduce temperature. The gas-phase flame retardation mechanism is chemical in nature, functioning by generating an increased volume of non-combustible gases to dilute the oxygen concentration, thereby preventing the combustion of materials. Similarly, the condensed-phase flame retardation mechanism is chemical based, involving the production of non-combustible gases and char by chemical substances, which form a physical barrier to impede heat and mass transfer between the gas and condensed phases. Flame retardants, which are substances used to impede the ignition or spread of combustion in materials, can be broadly classified into several categories based on their chemical composition and mechanisms of action. These include halogenated flame retardants, silicone-based flame retardants [5], phosphorus-based flame retardants [10], nitrogen-based flame retardants [20], and inorganic flame retardants [13]. Notably, halogenated flame retardants have been progressively phased out due to the release of toxic gases (HCl or HBr) upon combustion. There is a growing demand for flame retardancy that surpasses the capabilities of single-element agents, leading to a significant interest in the development of multi-element synergistic flame-retardant systems. These systems, which integrate two or more components, aim to achieve a more effective fire-resistant outcome than the sum of their individual effects, offering superior comprehensive performance [5,9,10].
In recent years, bio-based flame retardancy has attracted extensive attention from researchers, and a series of bio-based flame-retardant materials has been prepared [30,31,32]. Phytic acid (PA) is a bio-based molecule which is mainly found in the seeds, roots, and stems of plants, and is a natural resource that can be recycled. PA has a high content of the flame-retardant element phosphorus (with 6 negatively charged phosphate groups connected to 12 hydroxyl groups that can be hydrolyzed into hydrogen ions) and a highly symmetrical molecular structure. Under acidic conditions, it can chelate with metal cations that can catalyze the formation of carbon, and generate stable and non-hydrolytic complexes, which have very excellent flame-retardant potential [18,33]. Because phytic acid has excellent physiological and chemical properties, it has been widely used as an important organophosphorus additive in food, medicine, and other fields; however, the time spent on formal research on its application in the field of flame retardants is relatively short, and its huge potential is still waiting for people to tap. You et al. presented an intrinsic flame-retardant bio-based elastic phytic acid polyurethane (PUPA) with PA and diglycerol synthesized using simple and efficient one-pot polycondensation [33]. The cross-linked structure and polar phosphorus-containing segments of PUPA are fabricated into PUPA-TENG, demonstrating a superior elasticity, flame retardancy, impact resistance, and dielectric constant. Consequently, this study provides a simple strategy for tailoring TENGs toward environmentally friendly and secure power generators and electronics, which can effectively reduce fire hazards and potentially be applied in other fire-risk fields such as personal protection, firefighting, and new energy. Zhang et al. prepared a new type of flame-retardant polyelectrolyte complex (PEC) by using PA and polyethylenimide (PEI) [18]. It was found that, when 20 wt% PA, PEI, and PEC were added to PP matrix, their oxygen indices reached 19.8 vol%, 18.9 vol%, and 25.1 vol%, respectively. The introduction of PEC significantly improved the carbonization degree of PP during combustion and effectively controlled the generation of combustible gas during combustion, achieving an excellent flame-retardant effect.
An elastomer is a kind of polymer with excellent mechanical properties, certain strength, and other characteristics which is widely used in electrical conductors, human body functional equipment, and other applications. However, with the frequent occurrence of fire and other accidents, flame-retardant elastomers are the inevitable direction of our future development [34,35,36,37,38,39,40,41,42,43]. Among them, ionic conductive elastomers (ICEs) are unique materials within elastomers that possess excellent electrical conductivity. Benefiting from their good flexible properties, the applications of ICEs in flexible sensing have witnessed rapid development [44,45,46,47]. In a recent piece of research, we prepared an ICE material using the copolymerization of a ternary polymerizable deep eutectic solvent (PDES) containing choline chloride (ChCl), hydroxyethyl acrylate, and itaconic acid (IA). The resulting ICE exhibited excellent mechanical properties and demonstrated good sensing characteristics when applied in strain sensors [48]. The objective of this study is to develop a flame-retardant ICE (PCAIPX) with multiple hydrogen bonds derived from ChCl and PA with adjustable chemical structures and phosphorus content by controlling the adding of the monomer and PA. The hydrogen bonds’ cross-linked structure and phosphorus-containing elastomer are flame retardant and demonstrate superior elasticity, durability, and tear resistance and excellent adhesive properties and performance. Therefore, PCAIPX has significant potential as an easy-to-use, safe, and environmentally friendly strain sensing material which can be applied in firefighting and personnel protection equipment.
2. Results and Discussion
As shown in Scheme 1, the AA, IA, ChCl, and PA components were mixed at 90 °C to obtain a transparent PDES named CAIPX (X = 5, 7.5, 10, 12.5, indicating the weight ratio of PA), which was then added to a minimum photoinitiator and exposed to UV irradiation to yield the ICE materials named PCAIPX. In the PCAIPX materials, the various components are rich in hydrogen bonds, which endow them with favorable mechanical properties and adhesion. Additionally, the nitrogen-containing component ChCl and the phosphorus-containing component PA can impart certain flame-retardant behavior to the material.
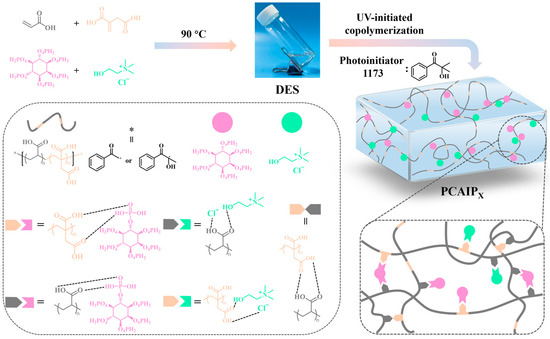
Scheme 1.
Schematic diagram of the synthesis of a flame-retardant ICE with multiple hydrogen bonds.
2.1. Structure Characterization of CAIPX and PCAIPX
Four phosphorus–nitrogen-containing CAIPX PDESs with adjustable chemical structures and phosphorus content are synthesized. The chemical structures of typical CAIPX and PCAIPX were verified using FT-IR (Figure 1). It can be clearly seen that the carbon–carbon double bond disappeared at 1600 cm−1 after the polymerization, while the peak of carboxyl at 2965 cm−1 shifted to 2927 cm−1 and the peak of carbonyl shifted to 1718 cm−1 from 1709 cm−1 after polymerization, proving the successful synthesis of the polymer [25,49,50].
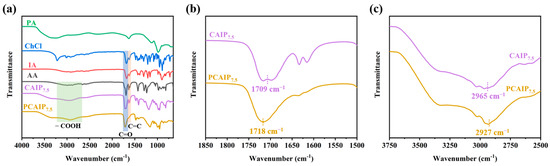
Figure 1.
(a) FTIR spectra of PA, ChCl, IA, AA, CAPI7.5, and PCAPI7.5, (b,c) the enlarged FTIR figures of CAPI7.5 and PCAPI7.5.
The thermal properties of PCAI without PA and PCAIPX with different PA contents were characterized via TGA and DSC techniques and the results are shown in Figure 2. The initial decomposition temperature (Ti), maximum thermal decomposition temperature (Tmax), and residual weight of PCAI were 208.7 °C, 282 °C, and 10.4%. With the addition of PA, the Ti of PCAIPX exhibited a slightly decreasing trend. Specifically, the Ti of PCAIP5 decreased to 205.8 °C, that of PCAIP7.5 decreased to 204.5 °C, that of PCAIP10 decreased to 203.6 °C, and that of PCAIP12.5 decreased to 203.1 °C. Similarly, the Tmax of PCAIP5 decreased to 272.3 °C, for PCAIP7.5, it was 272.4 °C, for PCAIP10, it reached 272.3 °C, and, for PCAIP12.5, it decreased to 272.5 °C. However, the residual weight increased from 10.4% of the PCAI to 21.6%, 22.6%, 24.26%, and 27.0% of PCAIP5, PCAIP7.5, PCAIP10, and PCAIP12.5, respectively, illustrating that the adding of PA increased the carbonization of PCAIPX. Additionally, the Ti of the resultant PCAIPX gradually decreased with the increase in PA. At the same time, the residual weight gradually increased, which was mainly related to the increase in P content. The presence of minor peaks preceding Tmax in the DTG curve proved the occurrence of ester exchange reactions (Figure 2b). For the residual mass at 282 °C, the adding of PA significantly increased the residual mass of PCAIPX in comparison with PCAI, which signified that the PA in PCAIPX possessed the capability to catalyze the char formation of PCAI during thermal degradation, thus holding promise for enhancing the charring ability of PCAIPX. Figure 2c shows the DSC curves of PCAI and PCAIPX. For PCAI, the Tg reached 26.7 °C. Figure 2c reveals a discernible decline in the Tg of PCAIPX upon the incorporation of PA; the Tg of PCAIP5, PCAIP7.5, PCAIP10, and PCAIP12.5 signally decreased to 22.2, 20.6, 16.9, and 4.3 °C, respectively. This may be attributed to the increased water content and weakened intermolecular interactions that accompany the rise in phytic acid levels, which, in turn, facilitate greater molecular mobility.
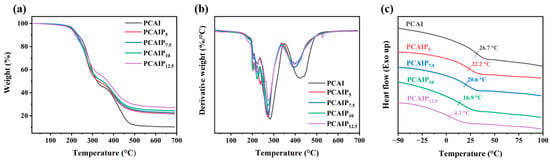
Figure 2.
(a) TGA, (b) DTG, and (c) DSC curves of PCAI and PCAIPx.
2.2. Flame-Retardant Property
The limiting oxygen index (LOI) serves as a pivotal parameter extensively employed in industry to assess the flame resistance of plastics. It is inferred that the LOI values correlate closely with the P content. The PCAI and PCAIPX samples were tested for UL-94 and LOI to evaluate the flame retardancy and the testing results are presented in Figure 3a and Table 1. The LOI value of pure PCAI was only 25.03%, which shows a certain flame retardancy property due to the presence of N-containing ChCl. The values of the LOI increased from 25.03% for PCAI to 31.83%, 40.43%, 52.03%, and 54.57% for PCAIP5, PCAIP7.5, PCAIP10, and PCAIP12.5, respectively. Compared with PCAI, the LOI value of PCAIP12.5 increased by approximately 118%. The LOI values of PCAIPX obviously increased with the loading of PA, effectively suppressing its combustion.
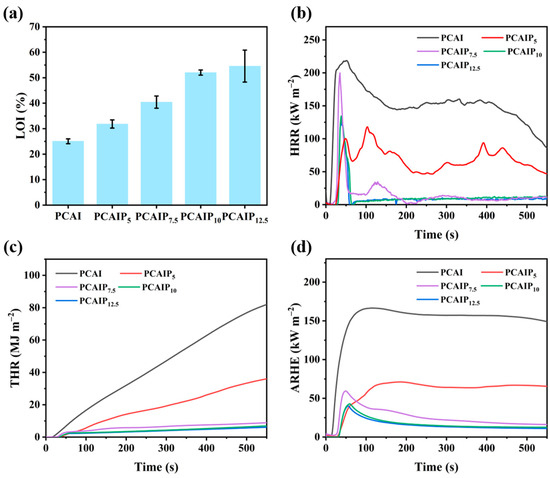
Figure 3.
(a) LOI values of PCAI and PCAIPX. (b) HRR, (c) THR, and (d) ARHE curves of PCAI and PCAIPX from CCT.

Table 1.
Flame retardancy of PCAI and PCAIPX.
Cone calorimetry tests (CCT) were conducted to further elucidate the influence of PA on the combustion characteristics of PCAIPX. Unlike the LOI determination and UL-94 tests, which provided limited information, CCT offered a comprehensive suite of data regarding combustion behavior, enabling a holistic evaluation of the thermal performance and fire hazard potential of PCAIPX. The heat release rate (HRR), total heat release (THR), and average rate of heat emission (ARHE) curves for PCAIPX are presented in Figure 3b–d, with detailed data summarized in Table 2. PCAI exhibited a pHRR of 225.9 kW/m2. Upon incorporation of PA, the pHRR decreased to 199.9 kW/m2 for PCAIP5, indicating that the addition of 5 wt% PA effectively reduced the pHRR. Furthermore, an increase in PA content led to a more pronounced reduction in pHRR, suggesting that a higher PA content enhances the thermal inhibitory capacity of PCAIPX. However, the rate of decline in pHRR diminished with further increases in PA content. Concurrently, the THR and pARHE values decreased with the addition of PA (see Figure 3c,d), highlighting the significant role of the P-containing structure of PA in reducing the heat release of PCAI. As shown in Table 2, the TTI of PCAIPX gradually increased with increasing PA content, rising from 19 s for PCAI to 34 s for PCAIP5. Overall, the incorporation of PA effectively suppressed the combustion heat release of PCAI, thereby enhancing its flame retardancy. This improvement is attributed to the phosphorus within the PCAIPX structure, which generates phosphoric acid and polyphosphoric acid compounds during combustion. These compounds promote the dehydration and carbonization of PCAIP, leading to the formation of a stable char layer. Additionally, the presence of nitrogen within the char layer enhances its thermal stability and mechanical strength [10,19]. During combustion, nitrogen forms nitrogen–carbon bonds, reinforcing the cross-linking structure of the char layer and reducing its susceptibility to decomposition at elevated temperatures. This char layer acts as a barrier to oxygen and heat, minimizing contact between the material’s interior and the flame, thereby decelerating the combustion rate. Moreover, as the PA content in the PCAIPX structure increases, its flame-inhibiting, charring, and barrier effects become more pronounced. This suggests that higher phosphorus content in the PCAIPX structure is more conducive to enhancing char formation and improving the quality of residual char in PCAI.

Table 2.
Detailed results of PCAI and PCAIPX from CCT.
The flame-retardant mechanism of PA in the condensed phase of PCAIPX was investigated by examining the morphology of the residual char following CCT. Digital photographs of the exterior of the residual char and SEM images of the PCAIPX samples are presented in Figure 4. Examination of the exterior surface of the residual char revealed that the pure PCAI exhibited a fragmented char layer (Figure 4a1), with numerous pores and a thin char height of less than 0.5 cm, such that the underlying tin foil was partially visible. In contrast, as the PA content increased, the char layer became progressively denser and taller (Figure 4b1–e1), with a height approaching 2.4 cm for the PCAIPX sample. This enhancement in char density and height was attributed to the expansion effect and catalytic char-forming properties of PA. SEM analysis further revealed that, while the surfaces of the samples were smooth (Figure 4a2), the char layer of pure PCAI contained a higher density of pores (Figure 4a3). Conversely, the introduction of PA resulted in a denser and more continuous char layer with fewer pores (Figure 4b3–e3). The formation of this robust, dense char layer effectively acted as a barrier, preventing further decomposition of the underlying resin, impeding the transfer of heat and mass, and isolating the oxygen necessary for combustion. The XPS curves of PCAIP7.5 before and after combustion are shown in Figure 4f1–g3. The C1s spectra before and after combustion both show three peaks, corresponding to C=O, C-O and C-C, respectively. Their peak area ratio changes from 1:0.52:0.20 before combustion to 1:0.27:0.14 after combustion, indicating that more carbonized layers are formed after combustion, which is beneficial to the flame-retardant effect. Two peaks can be observed in the O1s spectrum; the peaks at 532.2 eV and 533.6 eV belong to C-O-, C-O-C and C=O, P=O, respectively, and the peak area ratio decreases from 1:0.36 before combustion to 1:0.58. In Figure 4f3,g3, two peaks can be observed at 133.25 eV and 134.12 eV, corresponding to P-O-C and P=O, respectively. The peak area ratio of P=O to P-O increases from 0.52:1 before combustion to 1:0.29. This is because the P=O bond has a high bond energy and is relatively stable. Under the high-temperature environment of combustion, the phosphorus-containing PCAIP7.5 undergoes thermal decomposition, and chemical bonds such as the P-O-C bond may be broken to form intermediate products such as phosphoric acid, metaphosphoric acid, and polyphosphoric acid. These phosphorus-containing compounds will further dehydrate to form substances such as polyphosphoric acid with a strong dehydrating effect, which promotes the formation of a carbonized layer on the surface of the combustible. In this process, phosphorus atoms combine with oxygen atoms to form P=O bonds, existing in the form of phosphoric anhydride and other forms, thus playing a flame-retardant role. This result is consistent with the increase in the area ratio of C=O and P=O.
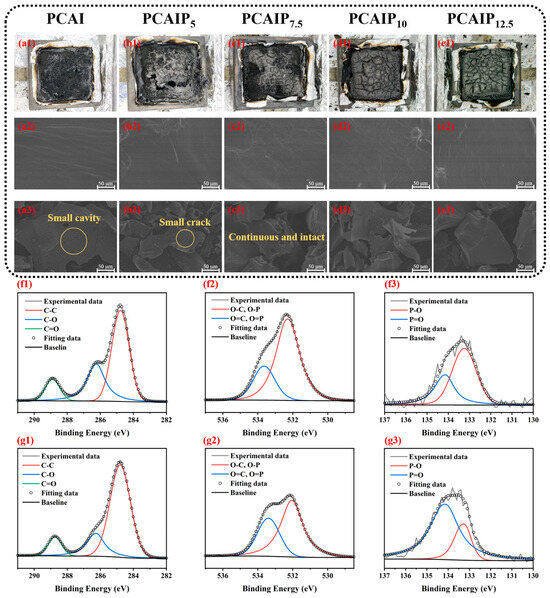
Figure 4.
(a1–e1) Digital images of char residues. SEM images of the pristine samples (a2–e2) as well as of char residues (a3–e3) after CCT of PCAI and PCAIPx. XPS of the pristine samples (f1–f3) and of char residues (g1–g3) after CCT of PCAIPx.
Based on the results obtained from CCT, SEM, and XPS analyses, the proposed flame-retardant mechanism of PCAIPX are as follows: PCAIPX exerts its flame-retarding effects in both the gas and condensed phases. During the gas-phase combustion process, certain P-containing compounds decomposed from PA are capable of scavenging H and OH radicals in the combustion region. This radical-trapping action effectively inhibits the chain-reaction of burning, thereby suppressing the combustion process. PCAIPX, upon thermal decomposition, generates non-flammable gases such as NH3, H2O, and NO2. These gases act to dilute the concentration of combustible gases in the vicinity of the combustion zone. By reducing the proportion of combustible gases, the likelihood and intensity of combustion are decreased. The polyphosphoric/phosphoric acid, which is derived from the pyrolysis of PA in PCAIPX, plays a crucial role in the condensed-phase flame-retardant mechanism. It promotes the carbonization of the PCAIPX elastomer. As a result, the quality and compactness of the char layer formed on the surface of the PCAIPX elastomer are enhanced. This high-quality char residue serves as a physical barrier, impeding the transmission of heat from the flame to the internal elastomer. Additionally, it prevents the release of combustible gases from the substrate, thus avoiding the continued decomposition of the inner material and further suppressing the combustion process [4,9,18,36].
2.3. Mechanical Property
Figure 5a displays the stress–strain curves of PCAIPX elastomers with different PA contents. As the PA content increases, the tensile strength gradually decreases, while the elongation at break increases. This trend is attributed to the increased water content and weakened intermolecular interactions associated with higher PA levels, which facilitate greater molecular mobility. Specifically, the elastomer with a PA content of 7.5% achieves a tensile strength of 0.6 MPa and an elongation at break of 2109%. Figure 5b presents the stress–strain curves of PCAIP7.5 elastomers with varying IA contents. It is evident from the figure that the introduction of a small amount of IA enhances the mechanical property of elastomers. For instance, the tensile strength of PCAIP7.5-0.1 reaches 0.2 MPa, with an elongation at break of 2387%. Meanwhile, PCAIP7.5-0.3 exhibits a tensile strength of 0.6 MPa and an elongation at break of 2180%, which are substantially higher than those of PCAIP7.5-0 (0.1 MPa). As shown in Figure 5c, the toughness of the elastomer increases from 3.7 MJ/m2 for PCAIP7.5-0 to 7.1 MJ/m2 for PCAIP7.5-0.3, while the elastic modulus rises from 0.6 kPa to 2.6 kPa with increasing IA content. This enhancement is attributed to the strong hydrogen bonding interactions between the carboxyl groups of IA and the polymer chains. Furthermore, Figure 5d indicates that the toughness of the elastomers decreases with increasing PA content. However, PCAIP7.5 demonstrates the best toughness, as PA can also form hydrogen bonds with the polymer backbone, thereby contributing to its excellent mechanical properties. Nevertheless, as the PA content further increases, the water content rises, leading to weakened intermolecular interactions and enhanced molecular mobility, which ultimately results in reduced mechanical properties. Therefore, PCAIP7.5-0.3 was selected for subsequent applications and tests in this study.
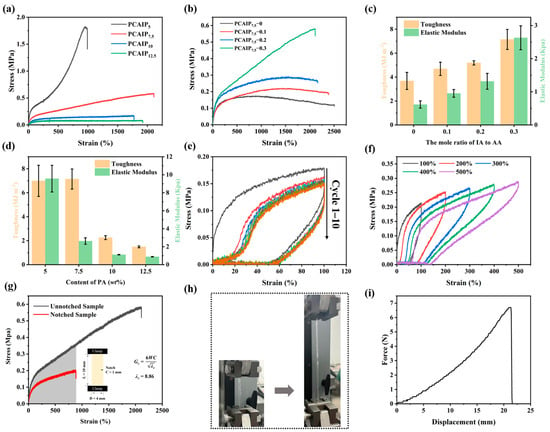
Figure 5.
(a) Stress–strain curves of PCAIPx elastomers with different PA contents. (b) Stress–strain curves of PCAIP7.5 elastomers with different IA contents. (c) Young’s modulus and toughness of PCAIP7.5 elastomers with different IA contents. (d) Young’s modulus and toughness of PCAIPx elastomers with different PA contents. (e) Stress–strain curves of PCAIP7.5-0.3 subjected to cyclic loading–unloading tests. (f) Stress–strain curves of PCAIP7.5 upon being stretched to 100% strain without intervals. (g) Stress–strain curves of unnotched and notched PCAIP7.5-0.3. (h) Digital image of stretching test. (i) Force–displacement curves of PCAIP7.5-0.3 in the puncture test.
In the application of ICE material, durability is one of the key parameters in addition to mechanical properties. Therefore, cyclic tensile tests were conducted on PCAIPX elastomers to investigate their fatigue resistance. Figure 5e presents the typical tensile loading–unloading curves of the PCAIP7.5-0.3 elastomer measured at different strains (100%, 200%, 300%, 400%, and 500%). The stress–strain curves exhibit pronounced hysteresis loops, indicating the reversible dissociation of dynamic hydrogen bonds within the elastomer, which dissipates a portion of the stretching energy. Additionally, continuous tensile loading–unloading tests were performed on PCAIP7.5-0.3 at a fixed strain of 100% to study its viscoelastic behavior under this condition, with results shown in Figure 5f. It can be observed that, although hysteresis loops are still evident, the stress loss does not significantly diminish after five cycles of stretching, suggesting that the elastomer possesses favorable recovery characteristics.
Tear resistance is another critical mechanical property of ICEs. High tear resistance enables elastomers to withstand significant loads without continuous degradation. To characterize its tear resistance, notched tensile tests were conducted to evaluate the ability of PCAIPX to resist tearing in the presence of defects and damage (Figure 5g,h). The tear energy was calculated using the Greensmith method [47,48]. The results indicate that, even with a notch of 1 mm, the PCAIP7.5-0.3 elastomer exhibits a tear elongation at break of 886% and a tear strength of 0.2 MPa. Additionally, its tear energy is as high as 4.47 kJ/m2. This is attributed to the dynamic dissociation and reformation of hydrogen bonds between the carboxyl groups of IA and PA. The molecular chains of the polymer undergo stick–slip motion under the influence of external forces, effectively transferring the stress at the crack tip to the entire polymer elastomer network and preventing lateral crack propagation. A puncture needle with a diameter of 1 mm was also used to compress the PCAIP7.5-0.3 at a rate of 200 mm/min until the sample with a thickness of 0.4 mm was completely penetrated. The PCAIP7.5-0.3 exhibits nice puncture resistance. As can be seen from the obtained puncture force–displacement curves in Figure 5i, the puncture force of PCAIP7.5-0.3 is determined to be 6.7 N, and the maximum displacement reaches 21 mm.
2.4. Adhesion Performance
The PCAIPX elastomer network is rich in -COOH, -OH, and zwitterionic groups, which endow the material with excellent adhesive properties. We evaluated the adhesion of the PCAIP7.5-0.3 elastomer to various substrates, including glass, copper, and paper. To quantify the adhesion strength between the elastomer and these three substrates, the PCAIP7.5-0.3 elastomer was assembled between the substrates and subjected to overlap shear tests using a universal tensile machine. The adhesion curves are shown in Figure 6a. The PCAIP7.5 elastomer exhibited the highest adhesion force to paper (15 N), followed by copper (8.5 N), and the lowest to glass (5.4 N). The adhesion between the PCAIP7.5-0.3 elastomer and the various substrates is attributed to the non-covalent interactions between the functional groups on the substrate surfaces and the abundant −OH, −COOH, and zwitterionic groups within the elastomer network. The ability of the PCAIP7.5-0.3 elastomer to readily adhere to a wide range of substrates facilitates its application in the field of sensors.
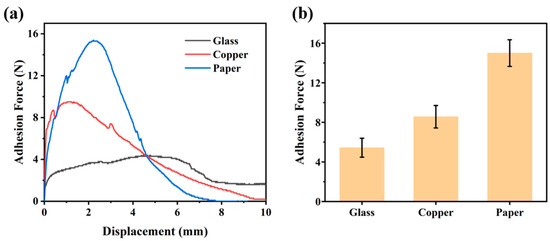
Figure 6.
(a) Adhesion strength curves and (b) adhesion strength values of PCAIP7.5-0.3 on different substrates.
2.5. Application of PCAIPX Elastomers as Strain Sensors
The PCAIPX elastomers exhibit adjustable ionic conductivity and elasticity, endowing them with strain-sensitive electrical response behavior. As shown in Figure 7a, with the increase in the content of PA, its ionic conductivity shows a distinct increasing trend. This is because the increase in the content of PA leads to an increase in the water content, which promotes the ionization of ChCl and further enhances the ionic conductivity. A simple strain sensor was fabricated by connecting the ends of the PCAIP7.5 elastomer to conductive copper wires using 3M insulating tape. As depicted in Figure 7b, the relative resistance of the sensor gradually increased with increasing tensile strain. The gauge factor (GF) was calculated to be 0.61 over the strain range of 0–100%, indicating the elastomer’s high sensitivity to strain. The PCAIP7.5 exhibited better performance than other ion-conducting elastomers in terms of both GF and ionic conductivity, which is shown in Figure 7c. Additionally, the simple strain sensor constructed from PCAIP7.5 was subjected to cyclic tensile sensing tests at various strains (10–100%). As shown in Figure 7d, the relative change in resistance increased with increasing strain. Moreover, stability is one of the critical criteria for evaluating sensor materials. The PCAIP7.5 elastomer was tested for 100 cycles at a strain of 50%. As illustrated in Figure 7e, the overall trend of the curve remained unchanged throughout the 100 cycles, demonstrating the elastomer’s favorable cyclic stability. The strain sensor based on this elastomer was employed to monitor human body movements in real time. The detection results shown in Figure 7f–i reveal that the sensor exhibited repeatable and stable resistance change signals in response to repetitive bending motions of the wrist, fingers, elbow, and knee. This indicates that the PCAIPX-based sensor has a high recognition capability for movements of different body parts. The above results suggest that the conductive elastomer based on PCAIPX is a promising material for the fabrication of intelligent sensors and holds significant potential for applications in human motion monitoring and related fields.
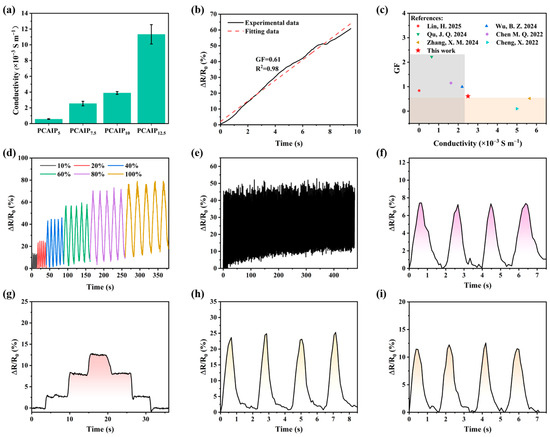
Figure 7.
(a) Ionic conductivity of PCAIPx elastomers with different PA contents. (b) Changes in the relative resistance of the sensor with variations in strains. (c) GF and ionic conductivity compared with values from the literature [51,52,53,54,55,56]. (d) Real-time monitoring of relative resistance at different strains. (e) Cyclic stability test of the sensor under 50% strain for 100 cycles. Real-time monitoring of repeated motions of (f) finger, (g) wrist, (h) elbow, and (i) knee by the PCAIP7.5-based sensor.
3. Materials and Methods
3.1. Materials
Choline chloride (ChCl, 99%, Adamas-Beta, Shanghai, China), acrylic acid (AA, 99% Aladdin, Shanghai, China), itaconic acid (IA, 99%, Aladdin), phytic acid (PA, 50% aqueous solution, Macklin, Shanghai, China), and 2-hydroxy-2-methylpropiophenone (photoinitiator 1173, 97%, Macklin, Shanghai, China) were used as received if not otherwise mentioned.
Software and version used: Origin 2024, Office 365, TH2832, Trios 5.1.1.
3.2. Synthesis of Phosphorus–Nitrogen-Containing PCAIPX Elastomers
3.2.1. Preparation of ChCl/AA/IA (CAI) and ChCl/AA/IA/PA (CAIPX) PDES
Before use, ChCl was recrystallized from ethanol, AA was dried over a 4 Å molecular sieve, and IA as the hydrogen bond donor was dried under vacuum at 50 °C for 2 h. ChCl, AA, and IA were added to a round-bottom flask at a molar ratio of 1:2:0.3, and then PA with different mass fractions was added. The obtained mixture was heated at 90 °C and stirred for 1 h, and a transparent and colorless PDES could be obtained. The obtained PDES was named CAIPx, where x represents the mass fraction of PA. The PDES prepared from ChCl, AA, and IA at a molar ratio of 1:2:0.3 using the same method as above was named CAI.
To verify the effects of different contents of IA in the polymer, while keeping the molar ratio of ChCl to AA at 1:2 and the mass fraction of PA at 7.5 wt%, PDESs with different IA contents were prepared using the same method. The obtained PDESs were named PCAI7.5-Y, where Y represents the molar ratio of IA to ChCl.
3.2.2. Preparation of Elastomers via Photopolymerization of CAI and CAIPX
The following typical procedure was used for preparing PCAI and PCAIPX elastomers: 0.1 mol% (relative to AA and IA monomers) photoinitiator 1173 was added to the above obtained PDES and stirred for 30 min at room temperature until a clear and transparent colorless solution was formed. The resulting solution was then poured into a polytetrafluoroethylene mold with dimensions of 4 cm × 2 cm × 0.1 cm (length × width × thickness) and irradiated under a UV lamp (B-100AP, UVP Analytik Jena, Jena, Germany) with a wavelength of 365 nm for 10 min. The light intensity was measured to be 10 mW cm−2 using a UV radiometer (UV-A, Beijing Shida Photoelectric Technology, Beijing, China). The obtained products were named PCAIPX, where x represents the mass fraction of PA. The product prepared by the photopolymerization of CAI without PA was named PCAI. The elastomers prepared by the photopolymerization of CAIP7.5-Y were named PCAIP7.5-Y, where Y represents the molar ratio of IA to ChCl. The detailed contents used for preparing the ICEs are listed in Table 3.

Table 3.
Detailed contents used for preparing ICEs.
3.3. Characterization
3.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
The structural characterization of the obtained products was carried out on an FT-IR instrument (Vertex70, Bruker, Billerica, MA, USA). The samples to be tested were directly placed on the diamond reflection crystal, and the test was carried out in the ATR total reflection mode. The scanning range was 600–4000 cm−1, the spectral resolution was 4 cm−1, and the number of scans was 32.
3.3.2. Thermogravimetry Analysis (TGA)
TGA was performed on an instrument (Discovery, TA, New Castle, DE, USA) with about 5 mg of the sample in the platinum plate. Beginning with equilibrating at 25 °C, the temperature was ramped from 25 to 700 °C at a heating rate of 10 °C/min under a nitrogen atmosphere.
3.3.3. Differential Scanning Calorimeter (DSC)
DSC curves were obtained using a DSC 2010 instrument (TA, New Castle, DE, USA) from −80 to 100 °C at a heating rate of 10 °C/min with a 5 min isothermal hold at the maximum and minimum temperatures. All glass transition temperatures (Tg) of the samples were reported according to the second heating scans.
3.3.4. Mechanical Tests
The mechanical tests were performed at room temperature on a tensile testing machine (Instron 5965, Missouri S&T, Rolla, MO, USA, 5 kN load cell).
The fracture energy test was conducted using the Greensmith method from a single-edge notched sample with a crack length of 1 mm at room temperature on a tensile testing machine (Instron 5965, 5 kN load cell). The tensile speed was set to 50 mm/min, and the size of samples with and without notches was 12 mm × 2.0 mm × 0.5 mm. The testing condition was at room temperature (25 °C). The fracture energy (GC) was calculated by the following equation:
where C represents the length of the notch (1 mm), λC represents the fracture elongation of the notched sample, and W represents the strain energy calculated by integrating the stress–strain curve of the unnotched sample until εC (εC = λC − 1).
3.3.5. Puncture Resistance Test
The film sample with a thickness of about 0.4 mm was fixed between two rectangular metal sheets. The film deformed freely during the compression process, carried by a metal needle with a diameter of 1 mm. The needle was positioned perpendicularly and moved down to the film in a compression machine equipped with a compression speed of 200 mm/min.
3.3.6. Electrochemical Impedance Spectroscopy (EIS)
EIS was applied using an electrochemical workstation (CS350, Corrtest, Wuhan, China) with a test frequency range of 1 Hz–100 kHz. An elastomer film and two symmetrical stainless-steel electrodes (SS, Φ = 14 mm) were assembled into a blocking cell (SS/elastomer/SS). The ionic conductivity (σ) of the elastomer was calculated according to the following equation:
where R is the bulk resistance, L is the thickness of the elastomer (0.1 cm), and S is the contact area of the sample and electrodes.
σ = L/(R × S)
3.3.7. Scanning Electron Microscope (SEM)
The morphologies of the of the samples and char residues were observed by an SEM instrument (Hitachi SU8010, Hitachi, Tokyo, Japan). Prior to observation, both the sample and char residue were subjected to gold sputtering under conditions of 10 mA current for 60 s. The SEM was operated at an accelerating voltage of 5 kV for imaging.
3.3.8. Flame-Retardant Test
The limiting oxygen index (LOI) values of the material were measured on an instrument (SHI5706A, Guangzhou Xinhe Electronic Equipment, Guangzhou, China) using a 10 cm × 1 cm × 0.4 cm standard sample fixed in the oxygen index tester according to the requirements of GB/T 2406.2 [57]. Cone calorimetric testing (CCT) of the elastomers with a square sheet of 10 cm × 10 cm × 0.3 cm was performed on an instrument (FTT iCons Classic, FTT, Derby, UK), and the combustion test was conducted in the cone calorimeter with a radiant power of 35 kW/m2. We conducted three tests and took the average value of the LOI.
3.3.9. Sensing Performance Test
Sensing performances were measured using an LCR meter (TH2830, Changzhou Tonghui Electronics, Changzhou, China) at a frequency of 1.0 kHz and a constant voltage of 1.0 V. Two copper wires were connected to the test apparatus, and the other ends of the two copper wires were connected to each end of the elastomer (4 cm × 2 cm × 0.1 cm). Cyclic tensile sensing tests were performed using a tensile machine (Instron 5965, 5 kN load cell) to record the change in relative resistance signal with strain in real time. The testing condition was at room temperature (25 °C).
3.3.10. X-Ray Photoelectron Spectroscopy (XPS) Test
XPS was performed on an EXCALAB 250 XI (Thermo Scientifc, Waltham, MA, USA). The energy resolution error was less than 0.5 eV, and the sensitivity was better than 400 KCPS.
4. Conclusions
This work designed and synthesized a series of flame-retardant ICEs (PCAIPx) with multiple hydrogen bonds with adjustable chemical structures and P content. The incorporation of PA effectively rendered PCAIPx with good fire safety attributes, leading to the attainment of a UL-94 V-0 rating of all PCAIP elastomers. PCAI exhibited a pHRR of 225.9 kW/m2, and, when PA was subjected to the PCAI, the value of pHRR was 199.9 kW/m2 for the PCAIP5. The incorporation of 5% PA proved effective in diminishing the pHRR of PCAI. SEM images of the char layer of the pure PCAI contained more holes, while, with the introduction of PA, the char layer became denser with fewer holes. PCAIP7.5-0.3 exhibited a tensile strength of 0.6 MPa and an elongation at break of 2180%, which were higher than those of PCAIP7.5-0 (0.1 MPa). The stress–strain curves exhibit pronounced hysteresis loops, indicating the reversible dissociation of dynamic hydrogen bonds within the elastomer, which dissipates a portion of the stretching energy. The PCAIP7.5 elastomer exhibited a tear elongation at break of 886% and a tear strength of 0.2 MPa and exhibited the highest adhesion force to paper (15.0 N), followed by copper (8.6 N), and the lowest to glass (5.4 kPa). The PCAIP7.5 elastomer exhibited favorable ionic conductivity and elasticity, which endow it with strain-sensitive electrical response behavior. The detection results revealed that the sensor exhibited repeatable and stable resistance change signals in response to repetitive bending motions of the wrist, fingers, elbow, and knee. In conclusion, these flame-retardant ICEs hold potential for applications in electronics, sports equipment, and aviation.
Author Contributions
Conceptualization, J.H., L.Z. and S.L.; Methodology, S.L., H.C., C.Z. and J.H.; formal analysis, S.L. and H.C.; investigation, S.L.; writing—original draft preparation, S.L.; writing—review and editing, J.H. and L.Z.; supervision, J.H. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Liu, B.-W.; Zhao, H.-B.; Wang, Y.-Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, e2107905. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.-Y.; Cao, C.-F.; Qu, Y.-X.; Zhang, G.-D.; Zhao, L.; Cao, K.; Song, P.; Tang, L.-C. Smart fire-warning materials and sensors: Design principle, performances, and applications. Mater. Sci. Eng. R Rep. 2022, 150, 100690. [Google Scholar] [CrossRef]
- Wang, Y.D.; Ma, L.; Yuan, J.; Zhu, Z.M.; Liu, X.M.; Li, D.S.; He, L.Q.; Xiao, F. Furfural-based P/N/S flame retardant towards high-performance epoxy resins with flame retardancy, toughness, low dielectric properties and UV resistance. Polym. Degrad. Stab. 2023, 212, 110343. [Google Scholar] [CrossRef]
- Rao, W.; Zhao, P.; Yu, C.; Zhao, H.-B.; Wang, Y.-Z. High strength, low flammability, and smoke suppression for epoxy thermoset enabled by a low-loading phosphorus-nitrogen-silicon compound. Compos. Part B 2021, 211, 108640. [Google Scholar] [CrossRef]
- Liang, J.; Yang, W.; Yuen, A.C.Y.; Long, H.; Qiu, S.; De Cachinho Cordeiro, I.M.; Wang, W.; Chen, T.B.Y.; Hu, Y.; Yeoh, G.H. Peanut Shell Derived Carbon Combined with Nano Cobalt: An Effective Flame Retardant for Epoxy Resin. Molecules 2021, 26, 6662. [Google Scholar] [CrossRef]
- Huo, S.; Sai, T.; Ran, S.Y.; Guo, Z.H.; Fang, Z.P.; Song, P.G.; Wang, H. A hyperbranched P/N/B-containing oligomer as multifunctional flame retardant for epoxy resins. Compos. Part B 2022, 234, 109701. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, M.; Chen, B.; Zhang, H.; Zheng, B. Understanding the curing kinetics of boron-based hyperbranched polysiloxane reinforced and toughened epoxy resin by rheology. Chem. Eng. J. 2023, 467, 143542. [Google Scholar] [CrossRef]
- Chi, Z.Y.; Guo, Z.W.; Xu, Z.; Zhang, M.J.; Li, M.; Shang, L.; Ao, Y.H. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- Wan, C.; Duan, H.J.; Zhang, C.H.; Cao, J.F.; Zou, J.H.; Zhang, J.J.; Ma, H.R. A P/N/S-containing compound toward enhanced fire safety epoxy resin with well-balanced performance. Polym. Degrad. Stab. 2021, 192, 109698. [Google Scholar] [CrossRef]
- Cui, X.Y.; Wu, Q.; Sun, J.; Gu, X.Y.; Li, H.F.; Zhang, S. Preparation of 4-formylphenylboronic modified chitosan and its effects on the flame retardancy of poly(lactic acid). Polym. Degrad. Stab. 2022, 202, 110037. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.Y.; Wang, S.P.; Hu, J.Y.; Liu, X.Q. Hyperbranched flame retardant for epoxy resin modification: Simultaneously improved flame retardancy, toughness and strength as well as glass transition temperature. Chem. Eng. J. 2022, 428, 131226. [Google Scholar] [CrossRef]
- Qian, Y.X.; Luo, Y.B.; Li, Y.; Xiong, T.S.; Wang, L.Y.; Zhang, W.G.; Gang, S.F.; Li, X.; Jiang, Q.H.; Yang, J.Y. Enhanced electromagnetic wave absorption, thermal conductivity and flame retardancy of BCN@LDH/EP for advanced electronic packing materials. Chem. Eng. J. 2023, 467, 143433. [Google Scholar] [CrossRef]
- Yang, Q.S.; Wang, J.; Chen, X.; Yang, S.; Huo, S.Q.; Chen, Q.F.; Guo, P.Z.; Wang, X.; Liu, F.; Chen, W.; et al. A phosphorus-containing tertiary amine hardener enabled flame retardant, heat resistant and mechanically strong yet tough epoxy resins. Chem. Eng. J. 2023, 468, 143811. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, Y.S.; Liu, P.J.; Xu, C.; Liu, Y.; Wang, Q. The preparation and application of a graphene-based hybrid flame retardant containing a long-chain phosphaphenanthrene. Sci. Rep. 2017, 7, 8759. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Qi, X.L.; Wang, D.Y. Recent Progress on Metal-Organic Framework and Its Derivatives as Novel Fire Retardants to Polymeric Materials. Nano-Micro Lett. 2020, 12, 173. [Google Scholar] [CrossRef]
- Huo, S.Q.; Guo, Y.; Yang, Q.S.; Wang, H.; Song, P. Two-dimensional nanomaterials for flame-retardant polymer composites: A mini review. Adv. Nanocompos. 2024, 1, 240–247. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.Q.; Shen, L.; Fang, Z.P.; Zhang, X.M.; Wang, J.J.; Zhang, B.Y. A phosphorus-, nitrogen- and carbon-containing polyelectrolyte complex: Preparation, characterization and its flame retardant performance on polypropylene. RSC Adv. 2014, 4, 48285–48292. [Google Scholar] [CrossRef]
- Hou, B.Y.; Shan, X.Y.; Li, B.J.; Zhang, Y.; Xu, B.; Chen, Z.D.; Cui, Q.Q.; Li, J.C. Phosphorus-nitrogen-containing flame-retardant plasticizers derived from L-lactic acid for poly (lactic acid) improved toughness, flame retardancy and crystallization. Chem. Eng. J. 2024, 500, 157143. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Zhao, Z.; Wu, M. Ecofriendly and durable flame-retardant cotton fabric based on alkyl/N/B/P modified meglumine with high efficiency. Prog. Org. Coat. 2023, 185, 107915. [Google Scholar] [CrossRef]
- Vahabi, H.; Wu, H.; Saeb, M.R.; Koo, J.H.; Ramakrishna, S. Electrospinning for developing flame retardant polymer materials: Current status and future perspectives. Polymer 2021, 217, 123466. [Google Scholar] [CrossRef]
- Qin, P.F.; Yi, D.Q.; Hao, J.W.; Ye, X.M.; Gao, M.; Song, T.L. Fabrication of melamine trimetaphosphate 2D supermolecule and its superior performance on flame retardancy, mechanical and dielectric properties of epoxy resin. Compos. Part B 2021, 225, 109269. [Google Scholar] [CrossRef]
- Sun, J.H.; Zhang, D.; Wang, B.T.; Xia, Y.; Zhang, Y.H.; Guo, Z.H.; Fang, Z.P.; Li, J.; Chen, P. Flame retardancy and toughness of epoxy resin induced by a star-shaped flame retardant containing P/Si/B. React. Funct. Polym. 2023, 190, 105649. [Google Scholar] [CrossRef]
- Qi, Y.Z.; Ye, X.L.; Huan, X.Y.; Xu, Q.; Ma, S.K.; Bao, D.M.; Zhou, G.Y.; Zhang, D.H.; Zhang, Y.P.; Du, H.J. P/N/S flame retardant based on DOPS-triazine groups for improving the flame retardancy, char formation properties and mechanical properties of epoxy resin. Eur. Polym. J. 2024, 202, 112634. [Google Scholar] [CrossRef]
- Huo, S.Q.; Song, P.; Yu, B.; Ran, S.Y.; Chevali, V.S.; Liu, L.; Fang, Z.P.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Qiu, S.; Ma, C.; Wang, X.; Zhou, X.; Feng, X.; Yuen, R.K.K.; Hu, Y. Melamine-containing polyphosphazene wrapped ammonium polyphosphate: A novel multifunctional organic-inorganic hybrid flame retardant. J. Hazard. Mater. 2018, 344, 839–848. [Google Scholar] [CrossRef]
- Mao, Y.W.; Wang, W.B.; Huang, W.Y.; Cai, H.P. Flame retardant, transparent, low dielectric and low smoke density EP composites implemented with reactive flame retardants containing P/N/B. Polym. Degrad. Stab. 2024, 230, 111078. [Google Scholar] [CrossRef]
- Wang, Y.D.; Liu, L.Y.; Ma, L.; Yuan, J.; Wang, L.X.; Wang, H.; Xiao, F.; Zhu, Z.M. Transparent, flame retardant, mechanically strengthened and low dielectric EP composites enabled by a reactive bio-based P/N flame retardant. Polym. Degrad. Stab. 2022, 204, 110106. [Google Scholar] [CrossRef]
- Wang, S.H.; Jiang, Y.C.; Tong, X.; Li, Y.J.; Sun, J.; Qian, L.J.; Li, H.F.; Gu, X.Y.; Zhang, S. The fabrication of a boron nitride/ammonium polyphosphate skeleton based on ice template method for thermal conductive and flame retardant epoxy. Polym. Degrad. Stab. 2024, 219, 110606. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zhang, K.T.; Wang, J.R.; Wang, Y.Z. Biopolymer-Based Flame Retardants and Flame-Retardant Materials. Adv. Mater. 2025, e2414880. [Google Scholar] [CrossRef]
- Ridard, H.; Duvigneau, J.; Mayer-Gall, T.; Ali, W.; Wurm, F.R. Biobased Flame-Retardant Polylactic Acid Foams through Lignin-Based Nanocarriers Encapsulating Deoxyribonucleic Acid. ACS Sustain. Chem. Eng. 2024, 12, 14866–14878. [Google Scholar] [CrossRef]
- Song, K.; Bi, X.; Yu, C.; Pan, Y.T.; Xiao, P.; Wang, J.; Song, J.I.; He, J.; Yang, R. Structure of Metal-Organic Frameworks Eco-Modulated by Acid-Base Balance toward Biobased Flame Retardant in Polyurea Composites. ACS Appl. Mater. Interfaces 2024, 16, 15227–15241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Yan, X.X.; Zeng, F.L.; Zhang, H.; Li, P.Y.; Zhang, H.Y.; Li, N.; Guan, Q.B.; You, Z.W. Low-Cost Intrinsic Flame-Retardant Bio-Based High Performance Polyurethane and its Application in Triboelectric Nanogenerators. Adv. Sci. 2025, 12, e2412258. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhu, Y.L.; Niu, W.W.; Yang, X.; Jiang, Z.Y.; Lu, Z.Y.; Liu, X.K.; Sun, J.Q. Healable and Recyclable Elastomers with Record-High Mechanical Robustness, Unprecedented Crack Tolerance, and Superhigh Elastic Restorability. Adv. Mater. 2021, 33, e2101498. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, B.; Xu, Z.Y.; Yang, J.H.; Liu, W.G. An unparalleled H-bonding and ion-bonding crosslinked waterborne polyurethane with super toughness and unprecedented fracture energy. Mater. Horiz. 2021, 8, 2742–2749. [Google Scholar] [CrossRef]
- Ou, F.Y.; Xie, T.; Li, X.Z.; Zhang, Z.C.; Ning, C.; Tuo, L.; Pan, W.Y.; Wang, C.S.; Duan, X.Y.; Liang, Q.H.; et al. Liquid-free ionic conductive elastomers with high mechanical properties and ionic conductivity for multifunctional sensors and triboelectric nanogenerators. Mater. Horiz. 2024, 11, 2191–2205. [Google Scholar] [CrossRef]
- Hao, X.-Y.; Yu, B.; Li, L.; Ju, H.; Tian, M.; Cao, P.-F. Semi-Interpenetrating Polyurethane Network with Fatigue Elimination and Upcycled Mechanical Performance. Macromolecules 2024, 57, 5063–5072. [Google Scholar] [CrossRef]
- Luo, Y.L.; Chen, J.L.; Situ, G.H.; Li, C.C.; Zhang, C.R.; Li, F.Z.; Li, C.-H.; Luo, Z.Y.; Zhang, X. Aromatic disulfide-induced self-reinforcing polyurethane elastomer with self-healability. Chem. Eng. J. 2023, 469, 143958. [Google Scholar] [CrossRef]
- Shang, X.; Jin, Y.; Du, W.N.; Bai, L.; Zhou, R.; Zeng, W.H.; Lin, K.Y. Flame-Retardant and Self-Healing Waterborne Polyurethane Based on Organic Selenium. ACS Appl. Mater. Interfaces 2023, 15, 16118–16131. [Google Scholar] [CrossRef]
- Deng, H.H.; Xie, F.; Shi, H.B.; Li, Y.F.; Liu, S.Y.; Zhang, C.Q. UV resistance, anticorrosion and high toughness bio-based waterborne polyurethane enabled by a Sorbitan monooleate. Chem. Eng. J. 2022, 446, 137124. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Spiering, G.A.; Moore, R.B.; Long, T.E. Quadruple Hydrogen Bond-Containing A-AB-A Triblock Copolymers: Probing the Influence of Hydrogen Bonding in the Central Block. Molecules 2021, 26, 4705. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.Y.; Liu, Y.Y.; Miao, H.Y.; Huang, G.; Han, L.; Tong, L.L.; Fu, X.B.; Zheng, C.Y.; Huang, X.J.; Zhang, X.; et al. Stretchable and high sensitive ionic conductive hydrogel for the direction recognizable motion detection sensor. Giant 2023, 16, 100199. [Google Scholar] [CrossRef]
- Chang, K.Q.; Zhang, C.; Liu, T.X. A Comprehensive Review on Fabrication and Structural Design of Polymer Composites for Wearable Pressure Sensors. Polym. Sci. Technol. 2025, 1, 3–24. [Google Scholar] [CrossRef]
- Gong, S.; Lu, Y.; Yin, J.L.; Levin, A.; Cheng, W.L. Materials-Driven Soft Wearable Bioelectronics for Connected Healthcare. Chem. Rev. 2024, 124, 455–553. [Google Scholar] [CrossRef]
- Luo, C.; Huang, Z.K.; Guo, Z.H.; Yue, K. Recent Progresses in Liquid-Free Soft Ionic Conductive Elastomers. Chin. J. Chem. 2023, 41, 835–860. [Google Scholar] [CrossRef]
- Yan, C.C.; Li, W.Z.; Liu, Z.Y.; Zheng, S.J.; Hu, Y.; Zhou, Y.J.; Guo, J.N.; Ou, X.; Li, Q.N.; Yu, J.T.; et al. Ionogels: Preparation, Properties and Applications. Adv. Funct. Mater. 2024, 34, 2314408. [Google Scholar] [CrossRef]
- Jin, Y.T.; Li, J.T.; Zhang, M.Z.; He, J.L.; Ni, P.H. Unexpected mechanically robust ionic conductive elastomer constructed from an itaconic acid-involved polymerizable DES. Chem. Commun. 2023, 59, 12998–13001. [Google Scholar] [CrossRef]
- Qian, Z.Y.; Pan, C.L.; Chen, H.; Zhang, M.Z.; He, J.L.; Ni, P.H. Development of an Ionic Conductive Elastomer from the Photocopolymerization of a Ternary Polymerizable Deep Eutectic Solvent for Human Motions Sensing. Macromol. Rapid Commun. 2025, 46, e2400798. [Google Scholar] [CrossRef]
- Li, R.A.; Fan, T.; Chen, G.X.; Xie, H.J.; Su, B.; He, M.H. Highly Transparent, Self-Healing Conductive Elastomers Enabled by Synergistic Hydrogen Bonding Interactions. Chem. Eng. J. 2020, 393, 124685. [Google Scholar] [CrossRef]
- Zhang, K.L.; Li, R.A.; Chen, G.X.; Yang, J.M.; Tian, J.F.; He, M.H. Polymerizable Deep Eutectic Solvent-Based Mechanically Strong and Ultra-Stretchable Conductive Elastomers for Detecting Human Motions. J. Mater. Chem. A 2021, 9, 4890–4897. [Google Scholar] [CrossRef]
- Ding, X.-P.; Mao, Y.-J.; Huang, J.-F.; Lin, H. Fabrication of a Flexible and Transparent All-Solid-State Ionic Conductive Elastomer and Its Sensing Properties. Ind. Eng. Chem. Res. 2025, 64, 5720–5728. [Google Scholar] [CrossRef]
- Xu, W.K.; Shen, T.; Ding, Y.T.; Ye, H.J.; Wu, B.Z.; Chen, F. Wearable and Recyclable Water-Toleration Sensor Derived from Lipoic Acid. Small 2024, 20, e2310072. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Lu, W.N.; Qu, J.Q. Rapid Preparation of High Strength, High Stretchability, Transparent, Self-Healing Conductive Elastomers for Strain Sensors by Photo-Initiated Copolymerization of Two Novel Polymerizable Deep Eutectic Solvents. Eur. Polym. J. 2024, 210, 112999. [Google Scholar] [CrossRef]
- Du, D.Y.; Zhou, J.H.; Shi, D.J.; Dong, W.F.; Chen, M.Q. Cross-Linked, Transient Ionic Conductive Elastomer with Extreme Stretchability, Healability, and Degradability for Detecting Human Motions. ACS Appl. Polym. Mater. 2022, 4, 4972–4979. [Google Scholar] [CrossRef]
- Aierken, Y.; Xu, Y.S.; Xiang, S.F.; Peng, W.J.; Zhang, X.M.; Hakkarainen, M.; Ma, P.M. Reprocessable, Highly Transparent Ionic Conductive Elastomers Based on Beta-Amino Ester Chemistry for Sensing Devices. ACS Appl. Mater. Interfaces 2024, 16, 25374–25384. [Google Scholar] [CrossRef]
- Wen, X.; Xu, J.H.; Wang, H.B.; Du, Z.L.; Wang, S.; Cheng, X. High Strength, Self-Healing, and Anti-Freezing Polyurethane Ionogel Based on Multiple Hydrogen Bonding for Wearable Strain Sensor. Polym. Eng. Sci. 2022, 62, 3132–3143. [Google Scholar] [CrossRef]
- GB/T 2406.2-2009; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. National Standards of People’s Republic of China: Beijing, China, 2009.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).