Direct Synthesis of Allylic Sulfones via Hydrosulfonylation of 1,3-Dienes with Sulfinic Acids
Abstract
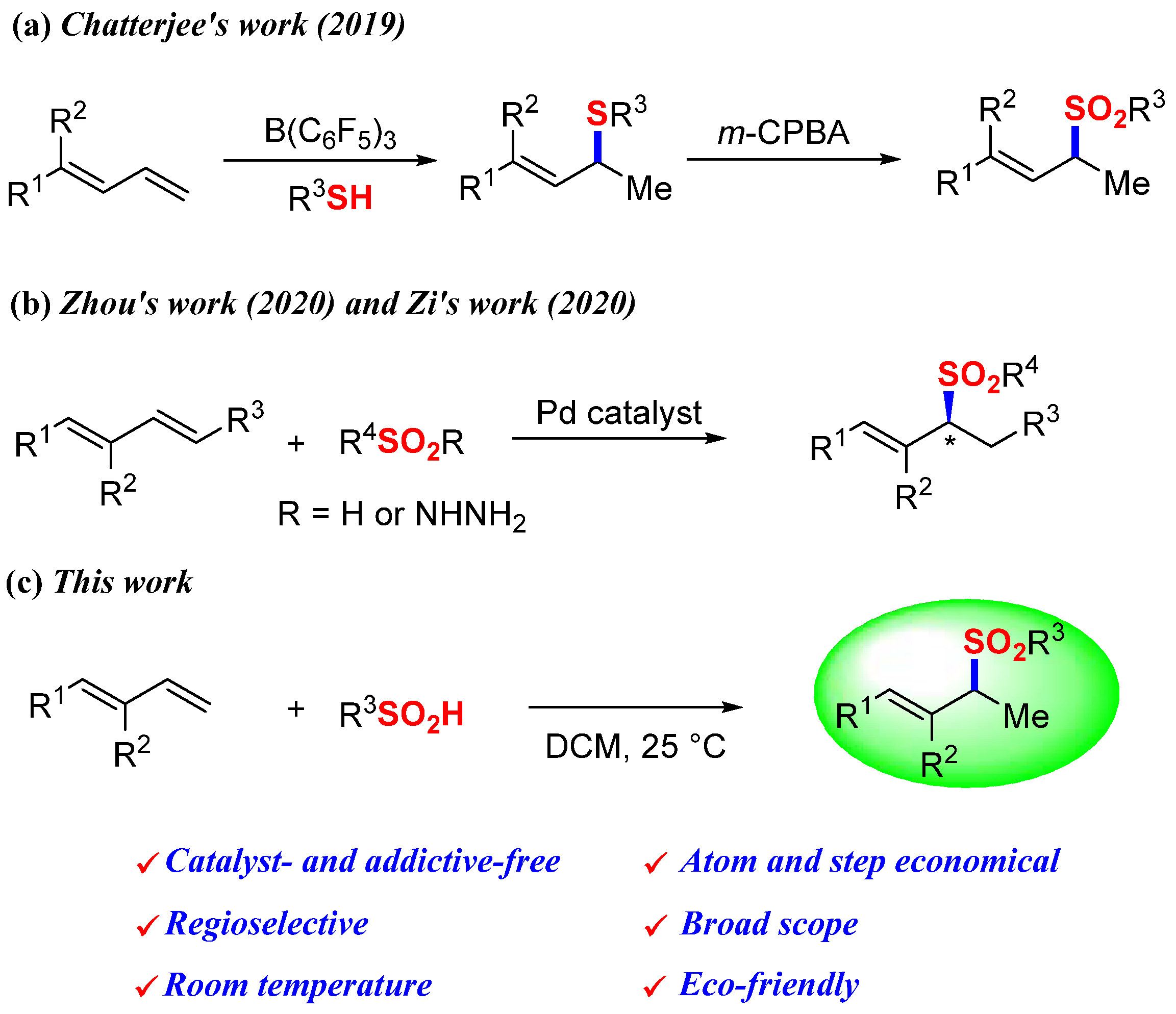
1. Introduction
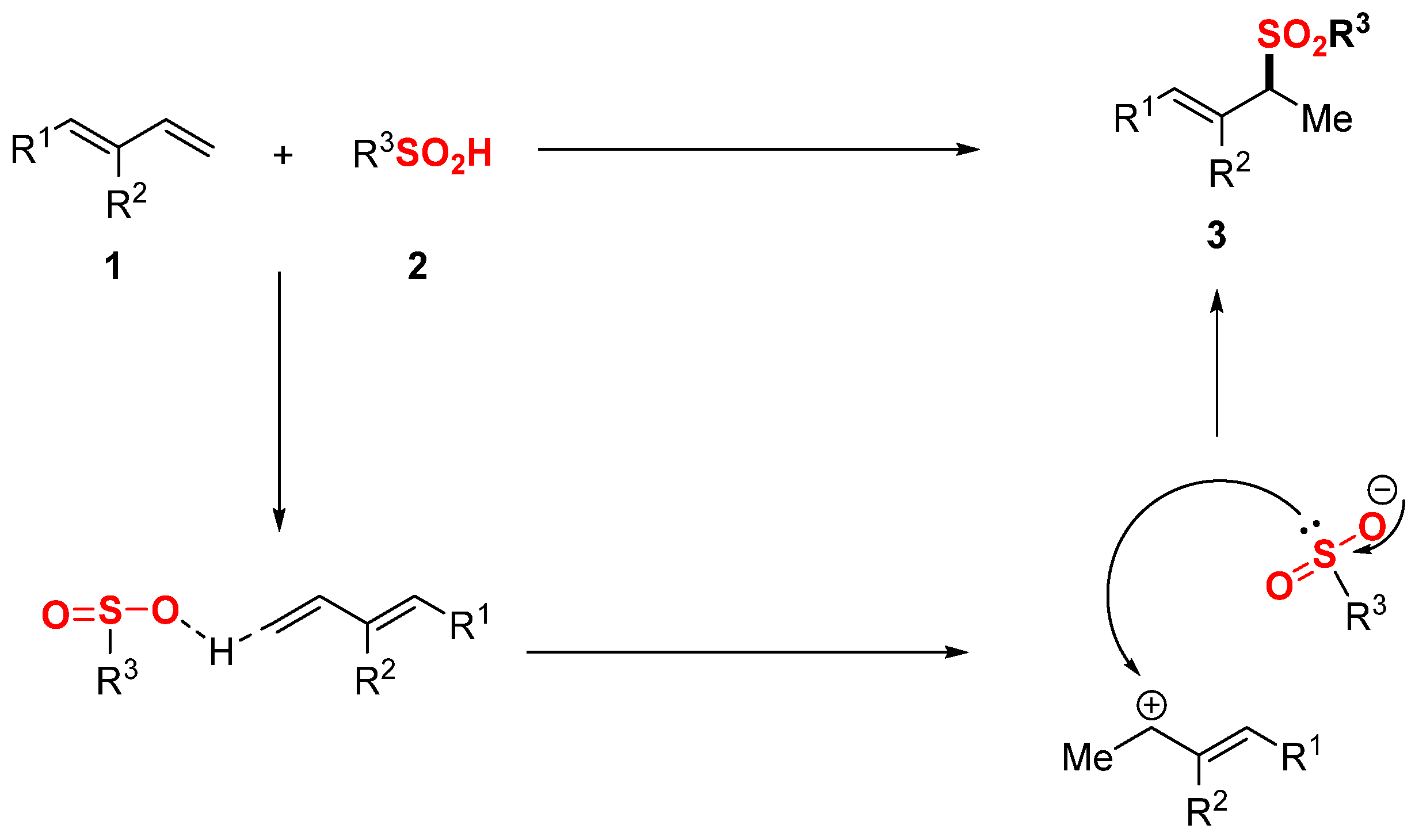
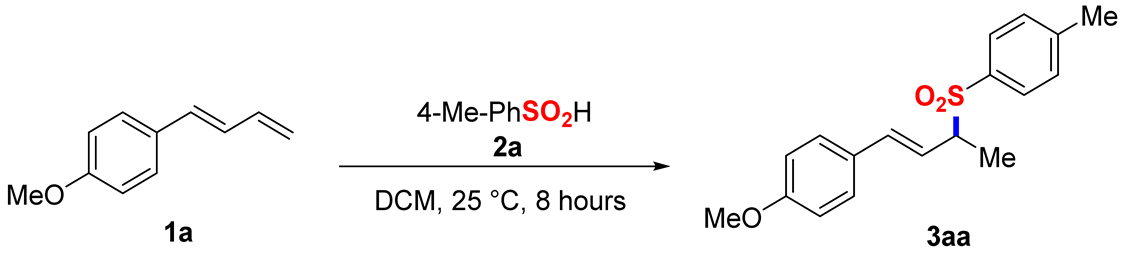
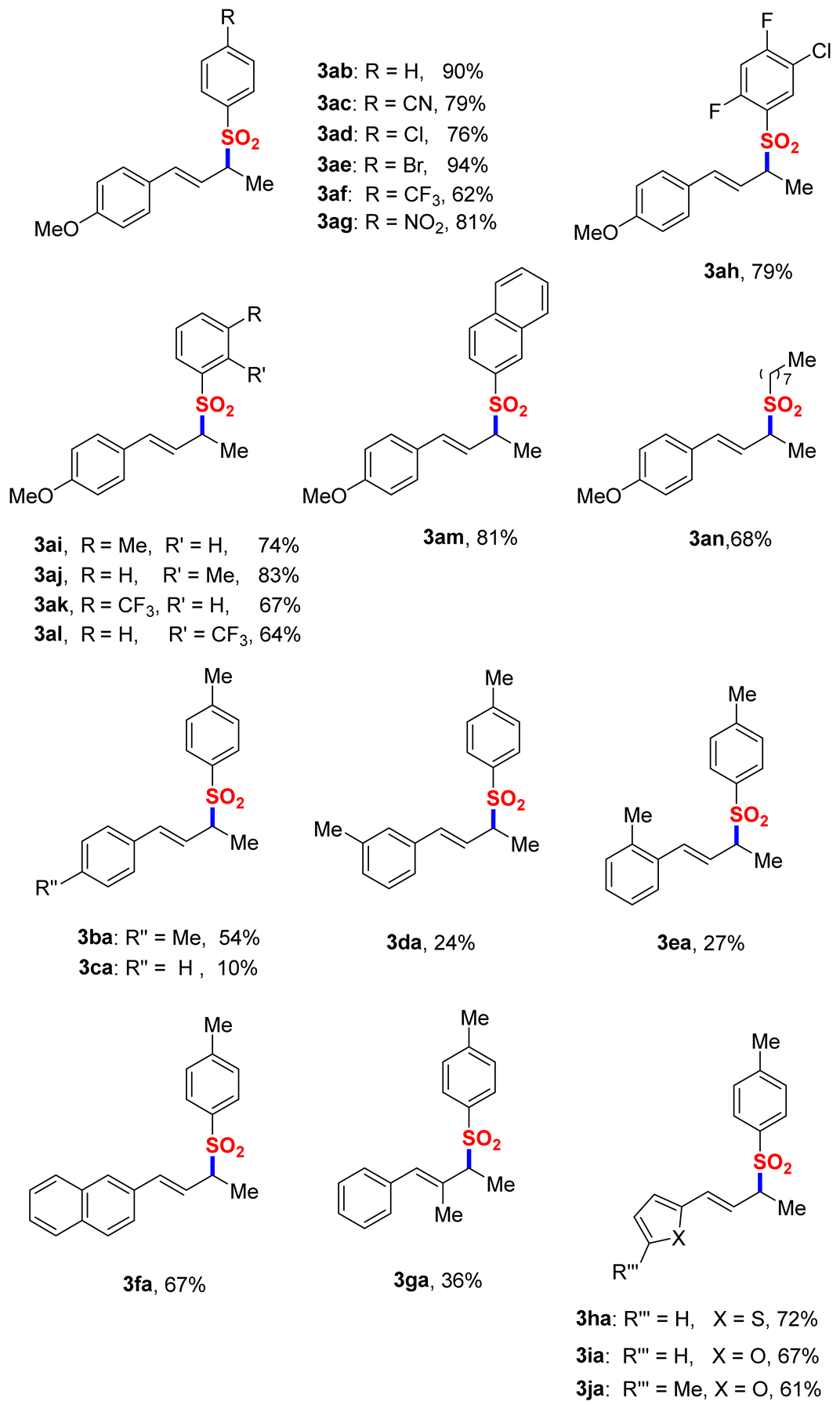
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azizi, N.; Dezfooli, S. Catalyst-free synthesis of imidazo [1,2-a] pyridines via Groebke multicomponent reaction. Environ. Chem. Lett. 2015, 14, 201–206. [Google Scholar] [CrossRef]
- Paprocki, D.; Wilk, M.; Madej, A.; Walde, P.; Ostaszewski, R. Catalyst-free synthesis of α-acyloxycarboxamides in aqueous media. Environ. Chem. Lett. 2018, 17, 1011–1016. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Z.; Zhang, X.; Tan, L.; Zhang, M.; Li, Y. Catalyst-free visible-light induced synthesis of nitrogen- and oxygen-containing heterocycles from 1,3-diketones. Environ. Chem. Lett. 2021, 19, 1831–1837. [Google Scholar] [CrossRef]
- Saikia, B.S.; Deb, M.L.; Baruah, P.K. Green synthesis of 1,3-oxazines by visible light-promoted catalyst-free C–H activation/cyclization of tertiary amines. Environ. Chem. Lett. 2021, 20, 109–118. [Google Scholar] [CrossRef]
- Dalu, F.; Scorciapino, M.A.; Cara, C.; Luridiana, A.; Musinu, A.; Casu, M.; Secci, F.; Cannas, C. A catalyst-free, waste-less ethanol-based solvothermal synthesis of amides. Green Chem. 2018, 20, 375–381. [Google Scholar] [CrossRef]
- Jin, C. Catalyst-free organic synthesis. Green Process. Synth. 2018, 7, 180. [Google Scholar] [CrossRef]
- Roshandel, S.; Suri, S.C.; Marcischak, J.C.; Rasul, G.; Surya Prakash, G.K. Catalyst and solvent free microwave-assisted synthesis of substituted 1,2,3-triazoles. Green Chem. 2018, 20, 3700–3704. [Google Scholar] [CrossRef]
- Mohan, V.; Dutta, B.; Ripani, R.; Jain, P.K. Room-temperature catalyst-free methane chlorination. Cell Rep. Phys. Sci. 2021, 2, 100545. [Google Scholar] [CrossRef]
- Chen, X.; Chang, X.; Zhang, S.; Lu, S.; Yang, L.; Sun, P. Air-triggered, catalyst-free decarboxylative oxysulfonylation of arylpropiolic acids with sodium sulfinates. Environ. Chem. Lett. 2022, 20, 2773–2779. [Google Scholar] [CrossRef]
- Trost, B.M.; Crawley, M.L. Asymmetric transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem. Rev. 2003, 103, 2921–2944. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, S. Metallkatalysierte enantioselektive Allylierungen in der asymmetrischen Synthese. Angew. Chem. 2008, 120, 264–303. [Google Scholar] [CrossRef]
- Corpas, J.; Kim-Lee, S.-H.; Mauleón, P.; Arrayás, R.G.; Carretero, J.C. Beyond classical sulfone chemistry: Metal-and photocatalytic approaches for C–S bond functionalization of sulfones. Chem. Soc. Rev. 2022, 51, 6774–6823. [Google Scholar] [CrossRef] [PubMed]
- Nambo, M.; Maekawa, Y.; Crudden, C.M. Desulfonylative transformations of sulfones by transition-metal catalysis, photocatalysis, and organocatalysis. ACS Catal. 2022, 12, 3013–3032. [Google Scholar] [CrossRef]
- Khan, A.; Zhao, H.; Zhang, M.; Khan, S.; Zhao, D. Regio-and Enantioselective Synthesis of Sulfone-Bearing Quaternary Carbon Stereocenters by Pd-Catalyzed Allylic Substitution. Angew. Chem. Int. Ed. 2020, 59, 1340–1345. [Google Scholar] [CrossRef]
- Pagès, L.; Lemouzy, S.; Taillefer, M.; Monnier, F. Easy Access to Allylic Sulfones Through Transition-Metal-Free Hydrosulfonylation of Allenes. J. Org. Chem. 2021, 86, 15695–15701. [Google Scholar] [CrossRef]
- Andrews, J.A.; Willis, M.C. DABSO–a reagent to revolutionize organosulfur chemistry. Synthesis 2022, 54, 1695–1707. [Google Scholar]
- Kalari, S.; Karale, U.B.; Rode, H.B. Selectfluor-mediated synthesis of β-acyl allyl sulfones/β-acyl allyl benzotriazoles from ketones/acetylenes, aryl sulfinates/benzotriazole, and DMSO as a dual-carbon synthon. J. Org. Chem. 2022, 87, 2435–2445. [Google Scholar] [CrossRef]
- Trost, B.M.; Organ, M.G.; O’Doherty, G.A. Asymmetric synthesis of allylic sulfones useful as asymmetric building blocks. J. Am. Chem. Soc. 1995, 117, 9662–9670. [Google Scholar] [CrossRef]
- Pritzius, A.B.; Breit, B. Z-Selective Hydrothiolation of Racemic 1, 3-Disubstituted Allenes: An Atom-Economic Rhodium-Catalyzed Dynamic Kinetic Resolution. Angew. Chem. Int. Ed. 2015, 54, 15818–15822. [Google Scholar] [CrossRef]
- Pritzius, A.B.; Breit, B. Asymmetric Rhodium-Catalyzed Addition of Thiols to Allenes: Synthesis of Branched Allylic Thioethers and Sulfones. Angew. Chem. Int. Ed. 2015, 54, 3121–3125. [Google Scholar] [CrossRef]
- Caldwell, D.J.; Mertens, B.; Kappler, K.; Senac, T.; Journel, R.; Wilson, P.; Meyerhoff, R.D.; Parke, N.J.; Mastrocco, F.; Mattson, B.; et al. A risk-based approach to managing active pharmaceutical ingredients in manufacturing effluent. Environ. Toxicol. Chem. 2016, 35, 813–822. [Google Scholar] [CrossRef]
- Long, J.; Shi, L.; Li, X.; Lv, H.; Zhang, X. Rhodium-Catalyzed Highly Regio-and Enantioselective Hydrogenation of Tetrasubstituted Allenyl Sulfones: An Efficient Access to Chiral Allylic Sulfones. Angew. Chem. 2018, 130, 13432–13435. [Google Scholar] [CrossRef]
- Yang, X.-H.; Davison, R.T.; Nie, S.-Z.; Cruz, F.A.; McGinnis, T.M.; Dong, V.M. Catalytic hydrothiolation: Counterion-controlled regioselectivity. J. Am. Chem. Soc. 2019, 141, 3006–3013. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Ecotoxicological assessment of pharmaceuticals and personal care products using predictive toxicology approaches. Green Chem. 2020, 22, 1458–1516. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, D.; Zi, W. Palladium-catalyzed regio-and enantioselective hydrosulfonylation of 1, 3-dienes with sulfinic acids: Scope, mechanism, and origin of selectivity. J. Am. Chem. Soc. 2020, 142, 15860–15869. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 2021, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Cheng, L.; Xiao, L.J.; Xie, J.H.; Zhou, Q.L. Palladium-Catalyzed Asymmetric Hydrosulfonylation of 1, 3-Dienes with Sulfonyl Hydrazides. Angew. Chem. Int. Ed. 2021, 60, 2948–2951. [Google Scholar] [CrossRef]
- Adamson, N.J.; Malcolmson, S.J. Catalytic enantio-and regioselective addition of nucleophiles in the intermolecular hydrofunctionalization of 1, 3-dienes. ACS Catal. 2019, 10, 1060–1076. [Google Scholar] [CrossRef]
- Kumar, G.; Qu, Z.-W.; Ghosh, S.; Grimme, S.; Chatterjee, I. Boron lewis acid-catalyzed regioselective hydrothiolation of conjugated dienes with thiols. ACS Catal. 2019, 9, 11627–11633. [Google Scholar] [CrossRef]
- Wang, G.; Gao, L.; Chen, H.; Liu, X.; Cao, J.; Chen, S.; Cheng, X.; Li, S. Chemoselective Borane-Catalyzed Hydroarylation of 1, 3-Dienes with Phenols. Angew. Chem. Int. Ed. 2019, 58, 1694–1699. [Google Scholar] [CrossRef]
- Wu, X.; Gong, L.-Z. Palladium (0)-catalyzed difunctionalization of 1, 3-dienes: From racemic to enantioselective. Synthesis 2019, 51, 122–134. [Google Scholar] [CrossRef]
- Lu, F.-D.; Lu, L.-Q.; He, G.-F.; Bai, J.-C.; Xiao, W.-J. Enantioselective radical carbocyanation of 1, 3-dienes via photocatalytic generation of allylcopper complexes. J. Am. Chem. Soc. 2021, 143, 4168–4173. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-Z.; Xiao, W.-J.; Chen, J.-R. Recent advances in radical-mediated transformations of 1, 3-dienes. Chin. J. Catal. 2022, 43, 548–557. [Google Scholar] [CrossRef]
- Chang, X.; Chen, X.; Zhang, S.; Lu, S.; Zhao, Y.; Zhang, D.; Yang, L.; Ma, Y.; Sun, P. Synthesis of branched allylic sulfones by regioselective boron-catalysed hydrosulfonylation. Environ. Chem. Lett. 2023, 21, 681–687. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, K.; Zhang, D.; Sun, P. Solvent Controlled Transformation between Sulfonyl Hydrazides and Alkynes: Divergent Synthesis of Benzo[b]thiophene-1,1-dioxides and (E)-β-iodo Vinylsulfones. Adv. Synth. Catal. 2019, 361, 597–602. [Google Scholar] [CrossRef]
- Lu, S.; Chen, X.; Chang, X.; Zhang, S.; Zhang, D.; Zhao, Y.; Yang, L.; Ma, Y.; Sun, P. Boron-catalysed transition-metal-free arylation and alkenylation of allylic alcohols with boronic acids. RSC Adv. 2023, 13, 3329–3332. [Google Scholar] [CrossRef]
- Andraos, J. Unification of reaction metrics for green chemistry II: Evaluation of named organic reactions and application to reaction discovery. Org. Process Res. Dev. 2005, 9, 404–431. [Google Scholar] [CrossRef]
- Vilotijevic, I.; Jamison, T.F. Epoxide-opening cascades in the synthesis of polycyclic polyether natural products. Angew. Chem. Int. Ed. 2009, 48, 5250–5281. [Google Scholar] [CrossRef]
- Meninno, S.; Roselli, A.; Capobianco, A.; Overgaard, J.; Lattanzi, A. Diastereodivergent and enantioselective access to spiroepoxides via organocatalytic epoxidation of unsaturated pyrazolones. Org. Lett. 2017, 19, 5030–5033. [Google Scholar] [CrossRef]
- Petsi, M.; Orfanidou, M.; Zografos, A.L. Organocatalytic epoxidation and allylic oxidation of alkenes by molecular oxygen. Green Chem. 2021, 23, 9172–9178. [Google Scholar] [CrossRef]
- Sartori, S.K.; Miranda, I.L.; Diaz, M.A.; Diaz-Muñoz, G. Sharpless asymmetric epoxidation: Applications in the synthesis of bioactive natural products. Mini-Rev. Org. Chem. 2021, 18, 606–620. [Google Scholar] [CrossRef]
- Ariki, Z.T.; Maekawa, Y.; Nambo, M.; Crudden, C.M. Preparation of quaternary centers via Nickel-catalyzed Suzuki–Miyaura cross-coupling of tertiary sulfones. J. Am. Chem. Soc. 2018, 140, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Lu, K.; Huang, J.; Li, Z.-H.; Ding, T.-M.; Chen, Z.-M. Brønsted acid-catalyzed solvent-controlled regioselective hydrothiolation and diastereoselective cascade cyclization of dienes. Chem. Commun. 2021, 57, 12639–12642. [Google Scholar] [CrossRef]
- Flaget, A.; Zhang, C.; Mazet, C. Ni-Catalyzed Enantioselective Hydrofunctionalizations of 1,3-Dienes. ACS Catal. 2022, 12, 15638–15647. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.J.P.; Jia, T.; Procter, D.J. Copper-Catalyzed Functionalization of 1,3-Dienes: Hydrofunctionalization, Borofunctionalization, and Difunctionalization. ACS Catal. 2020, 10, 1485–1499. [Google Scholar] [CrossRef]
- Puenner, F.; Schmidt, A.; Hilt, G. Up the Hill: Selective Double-Bond Isomerization of Terminal 1, 3-Dienes towards Z-1, 3-Dienes or 2Z, 4E-Dienes. Angew. Chem. Int. Ed. 2012, 5, 1270–1273. [Google Scholar] [CrossRef]
- Preuß, T.; Saak, W.; Doye, S. Titanium-catalyzed intermolecular hydroaminoalkylation of conjugated dienes. Chem.-A Eur. J. 2013, 19, 3833. [Google Scholar] [CrossRef]
- Qian, P.; Deng, Y.; Mei, H.; Han, J.; Zhou, J.; Pan, Y. Visible-light photoredox catalyzed oxidative/reductive cyclization reaction of N-cyanamide alkenes for the synthesis of sulfonated quinazolinones. Org. Lett. 2017, 19, 4798–4801. [Google Scholar] [CrossRef]

 | ||
| Entry | Variation from the Standard Conditions a | Yield b |
| 1 | None | 94% (93%) c |
| 2 | Added 10 mol% BF3·OEt2 | 35% |
| 3 | With petroleum ether instead of DCM | 32% |
| 4 | With CYH instead of DCM | 25% |
| 5 | With toluene instead of DCM | 30% |
| 6 | With THF instead of DCM | 0 |
| 7 | With EA instead of DCM | 0 |
| 8 | With acetone instead of DCM | 0 |
| 9 | With MeOH instead of DCM | 0 |
| 10 | With MeCN instead of DCM | 0 |
| 11 | With MTBE instead of DCM | 0 |
| 12 | 40 °C | 69% |
| 13 | 70 °C | 56% |
| 14 | 1a/2a = 1.2 | 67% |
| 15 | 1a/2a = 1.5 | 83% |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Zhang, S.; Zhang, J.; Ren, Y.; Chang, X.; Sun, P. Direct Synthesis of Allylic Sulfones via Hydrosulfonylation of 1,3-Dienes with Sulfinic Acids. Molecules 2025, 30, 1785. https://doi.org/10.3390/molecules30081785
Guo K, Zhang S, Zhang J, Ren Y, Chang X, Sun P. Direct Synthesis of Allylic Sulfones via Hydrosulfonylation of 1,3-Dienes with Sulfinic Acids. Molecules. 2025; 30(8):1785. https://doi.org/10.3390/molecules30081785
Chicago/Turabian StyleGuo, Ke, Shuaichen Zhang, Jing Zhang, Yu Ren, Xiaoqiang Chang, and Peng Sun. 2025. "Direct Synthesis of Allylic Sulfones via Hydrosulfonylation of 1,3-Dienes with Sulfinic Acids" Molecules 30, no. 8: 1785. https://doi.org/10.3390/molecules30081785
APA StyleGuo, K., Zhang, S., Zhang, J., Ren, Y., Chang, X., & Sun, P. (2025). Direct Synthesis of Allylic Sulfones via Hydrosulfonylation of 1,3-Dienes with Sulfinic Acids. Molecules, 30(8), 1785. https://doi.org/10.3390/molecules30081785






