Enhancing Methane Production in Anaerobic Digestion of Food Waste Using Co-Pyrolysis Biochar Derived from Digestate and Rice Straw
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of the Characteristics of Materials
2.2. Regulation of DB, RB, and DRB on Anaerobic Digestion of Food Waste
2.2.1. Impact on Yields of Biogas and Methane
2.2.2. Variations in pH and Volatile Fatty Acids
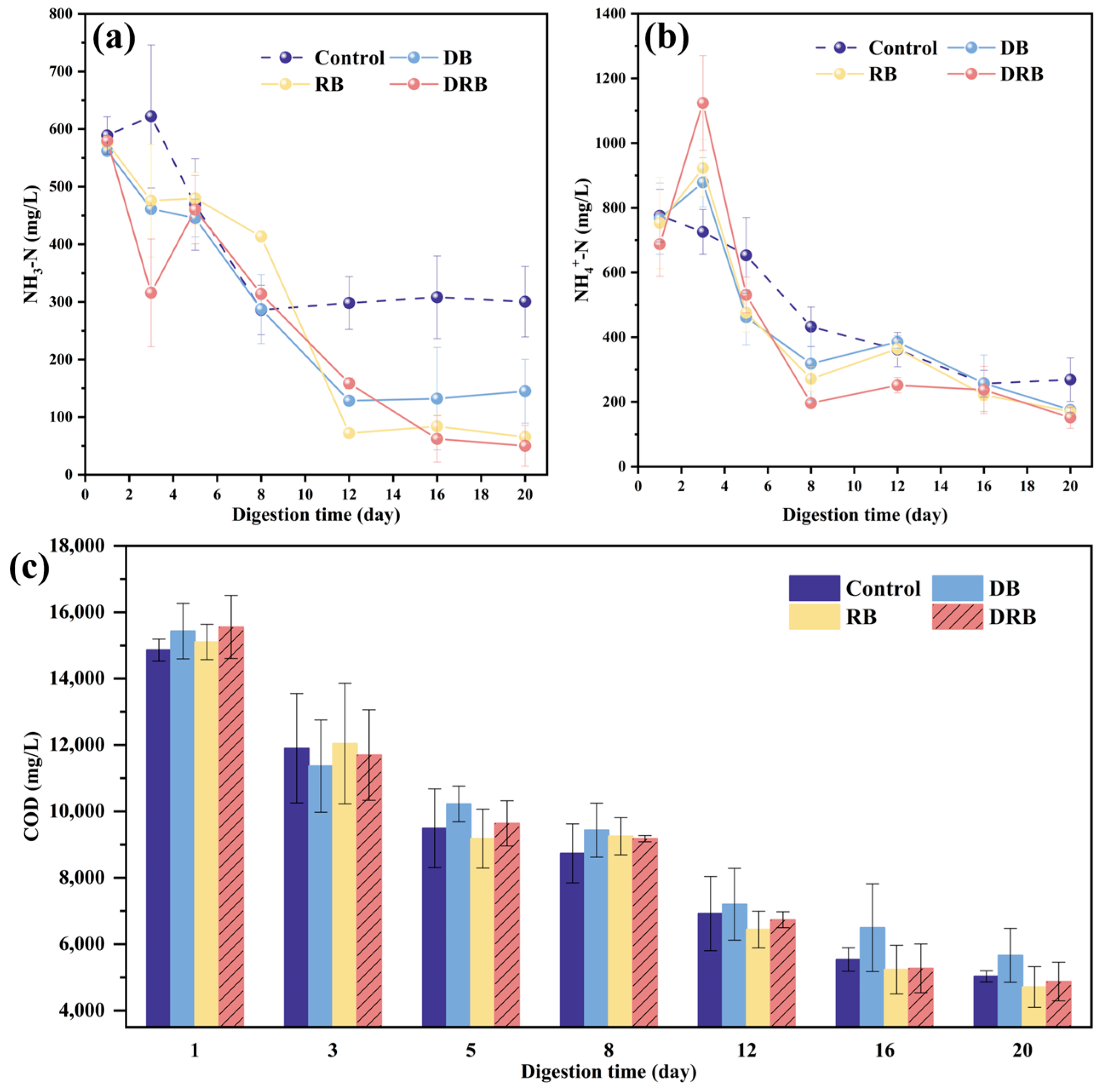
2.2.3. Ammonia Nitrogen and COD
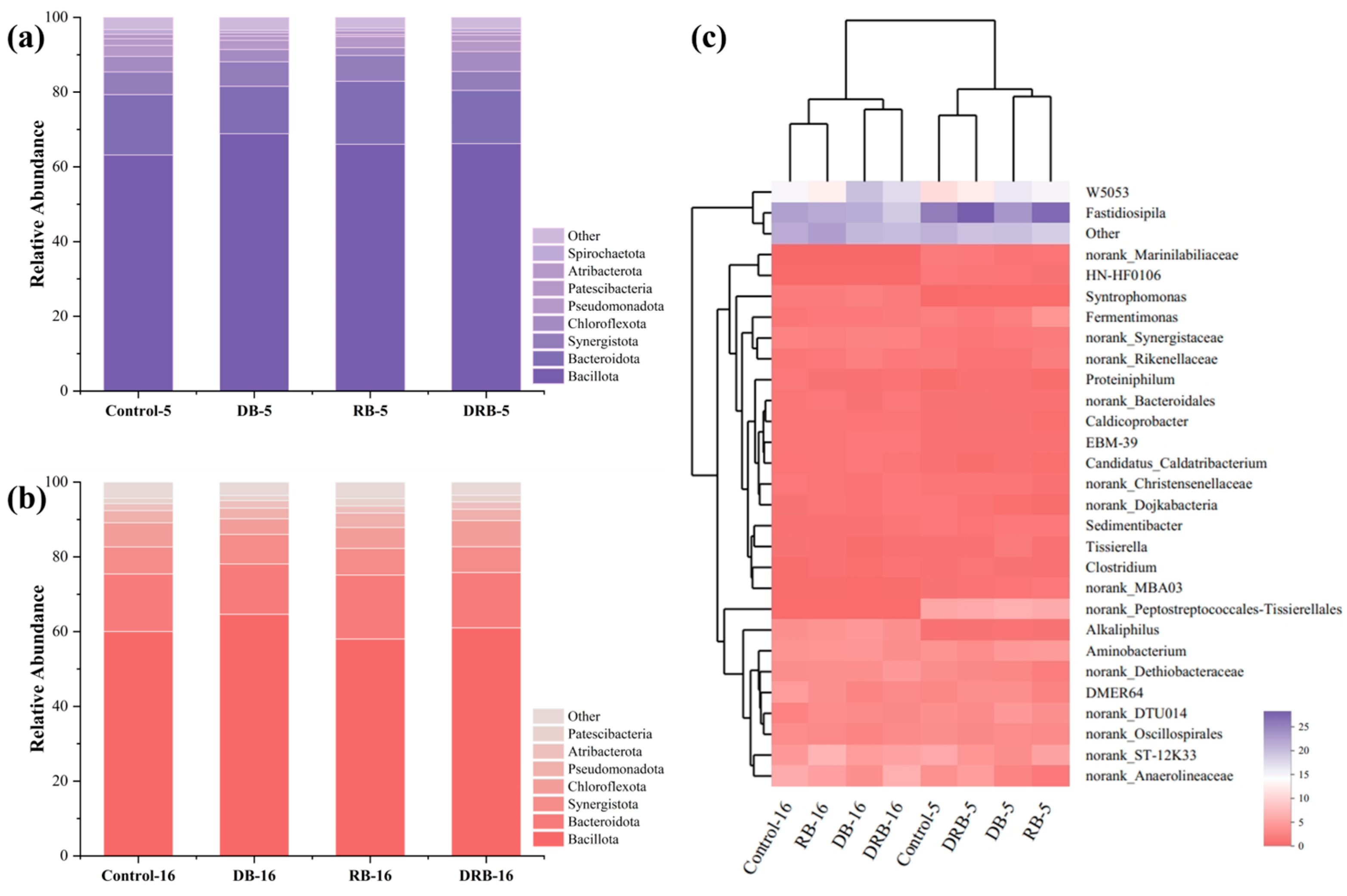
2.3. Microbial Diversity Analysis
2.3.1. Bacterial Community Analysis
2.3.2. Archaeal Community Analysis
3. Materials and Methods
3.1. Materials
3.2. Biochar Preparation
3.3. Anaerobic Digestion Experimental Design and Set-Up
3.4. Analytical Methods
3.5. Analysis of Microbial Community
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| FW | food waste |
| DRB | digestate and rice straw |
| DIET | direct interspecies electron transfer |
| VFAs | volatile fatty acids |
| TVFAs | total volatile fatty acids |
| BC | biochar |
| TS | total solids |
| VS | volatile solids |
| DB | digestate biochar |
| RB | rice-straw biochar |
| AS | anaerobic sludge |
| DG | digestate |
| SBET | specific surface area |
| SEM | scanning electron microscope |
| FTIR | Fourier-transform infrared spectroscopy |
| BET | Brunauer–Emmett–Teller |
| COD | chemical oxygen demand |
| NH3-N | ammonia nitrogen |
References
- Hoy, Z.X.; Woon, K.S.; Chin, W.C.; Van Fan, Y.; Yoo, S.J. Curbing Global Solid Waste Emissions toward Net-Zero Warming Futures. Science 2023, 382, 797–800. [Google Scholar] [CrossRef]
- Xu, S.; Bi, G.; Zou, J.; Li, H.; Chen, M.; Tang, Z.; Yu, Q.; Xie, J.; Chen, Y. Effect of Hydrochar from Biogas Slurry Co-Hydrothermal Carbonization with Biomass on Anaerobic Digestion Performance of Food Waste. Ind. Crops Prod. 2024, 221, 119361. [Google Scholar] [CrossRef]
- Anglou, F.Z.; Sanders, R.E.; Stamatopoulos, I. Of the First Five US States with Food Waste Bans, Massachusetts Alone Has Reduced Landfill Waste. Science 2024, 385, 1236–1240. [Google Scholar] [CrossRef]
- Jin, C.; Sun, S.; Yang, D.; Sheng, W.; Ma, Y.; He, W.; Li, G. Anaerobic Digestion: An Alternative Resource Treatment Option for Food Waste in China. Sci. Total Environ. 2021, 779, 146397. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A Critical Review on Biochar for Enhancing Biogas Production from Anaerobic Digestion of Food Waste and Sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- De Clercq, D.; Wen, Z.; Fan, F.; Caicedo, L. Biomethane Production Potential from Restaurant Food Waste in Megacities and Project Level-Bottlenecks: A Case Study in Beijing. Renew. Sustain. Energy Rev. 2016, 59, 1676–1685. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Koch, K.; Bolzonella, D.; Drewes, J.E. Full Scale Co-Digestion of Wastewater Sludge and Food Waste: Bottlenecks and Possibilities. Renew. Sustain. Energy Rev. 2017, 72, 354–362. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance Evaluation of Anaerobic Digestion Technology for Energy Recovery from Organic Fraction of Municipal Solid Waste: A Review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y.; Sun, C.; Wang, Z.; Ye, S.; Yu, Q.; Zhang, Y. New Insights into Inhibition of High Fe(III) Content on Anaerobic Digestion of Waste-Activated Sludge. Sci. Total Environ. 2024, 916, 170147. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Q.; Zhao, X.; Cai, Y.; Ma, X.; Fu, J.; Wang, P.; Wang, Y.; Liu, W.; Ren, L. A Review of the Technologies Used for Preserving Anaerobic Digestion Inoculum. Renew. Sustain. Energy Rev. 2023, 188, 113804. [Google Scholar] [CrossRef]
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. Methane Production and Conductive Materials: A Critical Review. Environ. Sci. Technol. 2018, 52, 10241–10253. Available online: https://pubs.acs.org/doi/10.1021/acs.est.8b01913#_i20 (accessed on 24 March 2025). [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H. Current Status of Food Waste Generation and Management in China. Bioresour. Technol. 2019, 273, 654–665. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, C. Thermophilic-Mesophilic Temperature Phase Anaerobic Co-Digestion Compared with Single Phase Co-Digestion of Sewage Sludge and Food Waste. Sci. Rep. 2024, 14, 11967. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.Y.; Cho, J.; Kim, J.Y. Releasing Characteristics and Fate of Heavy Metals from Phytoremediation Crop Residues during Anaerobic Digestion. Chemosphere 2018, 191, 520–526. [Google Scholar] [CrossRef]
- Devi, P.; Eskicioglu, C. Effects of Biochar on Anaerobic Digestion: A Review. Environ. Chem. Lett. 2024, 22, 2845–2886. [Google Scholar] [CrossRef]
- Xin, L.; Guo, Z.; Xiao, X.; Xu, W.; Geng, R.; Wang, W. Feasibility of Anaerobic Digestion for Contaminated Rice Straw Inoculated with Waste Activated Sludge. Bioresour. Technol. 2018, 266, 45–50. [Google Scholar] [CrossRef]
- Lu, J.-S.; Chang, J.-S.; Lee, D.-J. Adding Carbon-Based Materials on Anaerobic Digestion Performance: A Mini-Review. Bioresour. Technol. 2020, 300, 122696. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wang, X. Effects of Alkalinity Sources on the Stability of Anaerobic Digestion from Food Waste. Waste Manag. Res. 2015, 33, 1033–1040. [Google Scholar] [CrossRef]
- Dai, X.-F.; Bai, Y.; Lian, S.-J.; Qi, X.-J.; Feng, K.; Fu, S.-F.; Guo, R.-B. Distinct Mechanisms between Free Iron Species and Magnetite Addition in Anaerobic Digestion on Alleviating Ammonia Inhibition. ACS EST Eng. 2024, 4, 1990–2001. [Google Scholar] [CrossRef]
- Liu, B.; Tian, G.; Zhou, K.; Yang, Y.; Yang, Z.; Zhang, T.; Bian, B. Option of Treatment Scenario for Food Waste Anaerobic Digestion By-Products Based on Comprehensive Evaluation and Scenario Promotion Benefits Analysis. J. Clean. Prod. 2022, 374, 133861. [Google Scholar] [CrossRef]
- Zhu, X.; Yellezuome, D.; Wang, Z.; Liu, X.; Liu, R. Comprehensive Insights into the Effects of Acidogenic Off-Gas Utilization on Successive Biogas Production, Microbial Community Structure and Metabolite Distribution during Two-Stage Anaerobic Digestion. Water Res. 2024, 258, 121740. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of Different Types of Biochar on the Anaerobic Digestion of Chicken Manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef]
- Yuan, T.; Shi, X.; Sun, R.; Ko, J.H.; Xu, Q. Simultaneous Addition of Biochar and Zero-Valent Iron to Improve Food Waste Anaerobic Digestion. J. Clean. Prod. 2021, 278, 123627. [Google Scholar] [CrossRef]
- Li, X.; Shimizu, N. Biochar-Promoted Methane Production and Mitigation of Acidification during Thermophilic Anaerobic Co-Digestion of Food Waste with Crude Glycerol: Comparison with Re-Inoculation. Sustain. Environ. Res. 2023, 33, 4. [Google Scholar] [CrossRef]
- Valença, R.B.; Santos, L.A.D.; Firmo, A.L.B.; Silva, L.C.S.D.; Lucena, T.V.D.; Santos, A.F.D.M.S.; Jucá, J.F.T. Influence of Sodium Bicarbonate (NaHCO3) on the Methane Generation Potential of Organic Food Waste. J. Clean. Prod. 2021, 317, 128390. [Google Scholar] [CrossRef]
- Wei, X.; Fan, L.; Li, Y.; Wang, W.; Zhu, Z.; Zhran, M.; Shen, J.; Kim, P.J.; Wu, J.; Ge, T.; et al. Subsurface Methane Dynamics of a Paddy Field under Long-Term Fertilization: 13C-Evidence from in-Situ Belowground Labeling. J. Clean. Prod. 2021, 325, 129285. [Google Scholar] [CrossRef]
- Charoensuk, P.; Chaiwong, S.; Suwunwong, T.; Halley, P.J.; Suwantong, O. Preparation and Utilization of Biochar from Agricultural Wastes as Ethylene Absorber for ‘Gros Michel’ Banana Ripening. Ind. Crops Prod. 2024, 222, 119860. [Google Scholar] [CrossRef]
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of Methylene Blue from Aqueous Solution by Cattle Manure-Derived Low Temperature Biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, L.; Chen, C.; Xu, F.; Peng, H.; Song, Y.; Chen, G. Preparation of Iron Oxide-Modified Digestate Biochar and Effect on Anaerobic Digestion of Kitchen Waste. Bioresour. Technol. 2024, 398, 130515. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Fang, Y.; Lai, W.; Xu, S.; Lichtfouse, E. Enhancing Thermophilic Anaerobic Co-Digestion of Sewage Sludge and Food Waste with Biogas Residue Biochar. Renew. Energy 2022, 188, 465–475. [Google Scholar] [CrossRef]
- Yue, Y.; Lin, Q.; Li, G.; Zhao, X.; Chen, H. Biochar Amends Saline Soil and Enhances Maize Growth: Three-Year Field Experiment Findings. Agronomy 2023, 13, 1111. [Google Scholar] [CrossRef]
- Sheng, H.; Yin, Y.; Xiang, L.; Wang, Z.; Harindintwali, J.D.; Cheng, J.; Ge, J.; Zhang, L.; Jiang, X.; Yu, X.; et al. Sorption of N-Acyl Homoserine Lactones on Maize Straw Derived Biochars: Characterization, Kinetics and Isotherm Analysis. Chemosphere 2022, 299, 134446. [Google Scholar] [CrossRef]
- Faisal, S.; Ebaid, R.; Xiong, M.; Huang, J.; Wang, Q.; El-Hefnawy, M.; Abomohra, A. Maximizing the Energy Recovery from Rice Straw through Two-Step Conversion Using Eggshell-Catalytic Pyrolysis Followed by Enhanced Anaerobic Digestion Using Calcium-Rich Biochar. Sci. Total Environ. 2023, 858, 159984. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, J.; Guan, D.; Wang, Y.; Xie, J.; Zhang, H.; Sun, Y.; Zhu, J.; Guo, L. A Comprehensive Review on the Preparation of Biochar from Digestate Sources and Its Application in Environmental Pollution Remediation. Sci. Total Environ. 2024, 912, 168822. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Insight into the Co-Pyrolysis of Different Blended Feedstocks to Biochar for the Adsorption of Organic and Inorganic Pollutants: A Review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Srivastava, R.; Singh, A.C. Catalytic Co-Pyrolysis of Paper Biomass and Plastic Mixtures (HDPE (High Density Polyethylene), PP (Polypropylene) and PET (Polyethylene Terephthalate)) and Product Analysis. Energy 2016, 103, 513–521. [Google Scholar] [CrossRef]
- Fan, S.; Li, H.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Cadmium Removal from Aqueous Solution by Biochar Obtained by Co-Pyrolysis of Sewage Sludge with Tea Waste. Res. Chem. Intermed. 2018, 44, 135–154. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A Review on Co-Pyrolysis of Biomass: An Optional Technique to Obtain a High-Grade Pyrolysis Oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Brebu, M.; Ucar, S.; Vasile, C.; Yanik, J. Co-Pyrolysis of Pine Cone with Synthetic Polymers. Fuel 2010, 89, 1911–1918. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y.; Zhu, A.; Fu, J.; Xia, Y.; Lan, G.; Fu, C.; Ma, Z.; Xue, J.; Tao, L.; et al. Effect of Different Digestate Biochars as Promoters via Sludge Anaerobic Digestion on Subsequent Pyrolysis Products: Focusing on the Nitrogen, Sulfur, and Chlorine Releasing Characteristics. Renew. Energy 2024, 226, 120366. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Sheng, L.; Xing, Y.; Liu, G.; Yao, G.; Ngo, H.H.; Li, Q.; Wang, X.C.; Li, Y.-Y.; et al. A Review on Facilitating Bio-Wastes Degradation and Energy Recovery Efficiencies in Anaerobic Digestion Systems with Biochar Amendment. Bioresour. Technol. 2020, 314, 123777. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct Interspecies Electron Transfer via Conductive Materials: A Perspective for Anaerobic Digestion Applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, M.; Liu, Y.; Gou, J.; Wei, Q.; Shen, B. Physico-Chemical Characteristics and the Adsorption of Ammonium of Biochar Pyrolyzed from Distilled Spirit Lees, Tobacco Fine and Chinese Medicine Residues. J. Anal. Appl. Pyrolysis 2021, 156, 105148. [Google Scholar] [CrossRef]
- Perego, C.; König, R.; Cuomo, M.; Pianta, E.; Maye, S.; Di Maggio, L.; Moser, M.; Fischer, F.; Principi, P. Shewanella Oneidensis and Methanosarcina Barkerii Augmentation and Conductive Material Effects on Long-Term Anaerobic Digestion Performance. Biotechnol. Biofuels Bioprod. 2025, 18, 10. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.-E.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon Cloth Stimulates Direct Interspecies Electron Transfer in Syntrophic Co-Cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Zhan, M.; Li, X.; Yan, J. Activation of Persulfate by CO2-Activated Biochar for Improved Phenolic Pollutant Degradation: Performance and Mechanism. Chem. Eng. J. 2020, 380, 122519. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, J.; Huang, C.; Wang, L.; Wang, X. Investigating the Potential of Mineral Precipitation in Co-Pyrolysis Biochar: Development of a Novel Cd (II) Adsorption Material Utilizing Dual Solid Waste. Bioresour. Technol. 2024, 402, 130762. [Google Scholar] [CrossRef]
- Singh, B.; Fang, Y.; Cowie, B.C.C.; Thomsen, L. NEXAFS and XPS Characterisation of Carbon Functional Groups of Fresh and Aged Biochars. Org. Geochem. 2014, 77, 1–10. [Google Scholar] [CrossRef]
- Bae, S.; Kim, D.; Lee, W. Degradation of Diclofenac by Pyrite Catalyzed Fenton Oxidation. Appl. Catal. B 2013, 134–135, 93–102. [Google Scholar] [CrossRef]
- Sun, X.; Dawson, S.R.; Parmentier, T.E.; Malta, G.; Davies, T.E.; He, Q.; Lu, L.; Morgan, D.J.; Carthey, N.; Johnston, P.; et al. Facile Synthesis of Precious-Metal Single-Site Catalysts Using Organic Solvents. Nat. Chem. 2020, 12, 560–567. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, J.; Liu, Q.; He, J.; Frost, R.L. Thermogravimetric Analysis–Mass Spectrometry (TG–MS) of Selected Chinese Kaolinites. Thermochim. Acta 2010, 507–508, 106–114. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Liu, L.; Yu, H. Application of Co-Pyrolysis Biochar for the Adsorption and Immobilization of Heavy Metals in Contaminated Environmental Substrates. J. Hazard. Mater. 2021, 420, 126655. [Google Scholar] [CrossRef]
- Biagini, E.; Tognotti, L. Comparison of Devolatilization/Char Oxidation and Direct Oxidation of Solid Fuels at Low Heating Rate. Energy Fuels 2006, 20, 986–992. Available online: https://pubs.acs.org/doi/full/10.1021/ef0503156 (accessed on 22 March 2025). [CrossRef]
- Harvey, O.R.; Kuo, L.-J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars). Environ. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef]
- Kong, X.; Yu, S.; Xu, S.; Fang, W.; Liu, J.; Li, H. Effect of Fe0 Addition on Volatile Fatty Acids Evolution on Anaerobic Digestion at High Organic Loading Rates. Waste Manag. 2018, 71, 719–727. [Google Scholar] [CrossRef]
- Yan, W.; Xu, H.; Lu, D.; Zhou, Y. Effects of Sludge Thermal Hydrolysis Pretreatment on Anaerobic Digestion and Downstream Processes: Mechanism, Challenges and Solutions. Bioresour. Technol. 2022, 344, 126248. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Chen, J.; Cui, L.; Li, W.; Gao, X.; Liu, Z. Water Quality Criteria of Total Ammonia Nitrogen (TAN) and Un-Ionized Ammonia (NH3-N) and Their Ecological Risk in the Liao River, China. Chemosphere 2020, 243, 125328. [Google Scholar] [CrossRef]
- Constable, M.; Charlton, M.; Jensen, F.; McDonald, K.; Craig, G.; Taylor, K.W. An Ecological Risk Assessment of Ammonia in the Aquatic Environment. Hum. Ecol. Risk Assess. Int. J. 2003, 9, 527–548. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of Slow Pyrolyzed Wood and Rice Husks Biochar for Adsorption of Ammonium Nitrogen from Piggery Manure Anaerobic Digestate Slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Kui, D. Sorption Kinetic Studies of Ammonium from Aqueous Solution on Different Inorganic and Organic Media. Acta Chim. Slov. 2013, 60, 103. [Google Scholar]
- Halim, A.A.; Latif, M.T.; Ithnin, A. Ammonia Removal from Aqueous Solution Using Organic Acid Modified Activated Carbon. World Appl. Sci. 2013, 24, 1–6. [Google Scholar]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A Review of Biochar Properties and Their Roles in Mitigating Challenges with Anaerobic Digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Ke, T.; Yun, S.; Wang, K.; An, J.; Liu, L.; Liu, J. Enhanced Anaerobic Co-Digestion Performance by Using Surface-Annealed Titanium Spheres at Different Atmospheres. Bioresour. Technol. 2022, 347, 126341. [Google Scholar] [CrossRef]
- Zhang, L.; Tsui, T.-H.; Wah Tong, Y.; Sharon, S.; Shoseyov, O.; Liu, R. Biochar Applications in Microbial Fermentation Processes for Producing Non-Methane Products: Current Status and Future Prospects. Bioresour. Technol. 2023, 386, 129478. [Google Scholar] [CrossRef]
- Bao, R.; Wei, Y.; Guan, R.; Li, X.; Lu, X.; Rong, S.; Zuo, X.; Yuan, H. High-Solids Anaerobic Co-Digestion Performances and Microbial Community Dynamics in Co-Digestion of Different Mixing Ratios with Food Waste and Highland Barley Straw. Energy 2023, 262, 125529. [Google Scholar] [CrossRef]
- Schnürer, A.; Singh, A.; Bi, S.; Qiao, W.; Westerholm, M. Miniphocaeibacter Halophilus Sp. Nov., an Ammonium-Tolerant Acetate-Producing Bacterium Isolated from a Biogas System. Int. J. Syst. Evol. Microbiol. 2022, 72, 005328. [Google Scholar] [CrossRef]
- Lin, H.-C.; Pan, S.-Y.; Lin, Y.-I.; Cao, D.-T.N. Enhanced Biomethane Production with a Low Carbon Footprint via Anaerobic Co-Digestion of Swine Wastewater with Rice Husk. Sci. Total Environ. 2023, 891, 164612. [Google Scholar] [CrossRef]
- Besteman, M.S.; Doloman, A.; Sousa, D.Z. Transcriptomic Evidence for an Energetically Advantageous Relationship between Syntrophomonas Wolfei and Methanothrix Soehngenii. Environ. Microbiol. Rep. 2024, 16, e13276. [Google Scholar] [CrossRef]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an Enhancer in Methanogenic Degradation of Volatile Fatty Acids under Ammonia-Stressed Condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Chen, Z.; Ye, Z.; Lyu, R. Multi-Omics Analysis Unravels Effects of Salt and Oil on Substance Transformation, Microbial Community, and Transcriptional Activity in Food Waste Anaerobic Digestion. Bioresour. Technol. 2023, 387, 129684. [Google Scholar] [CrossRef]
- Le, T.-S.; Bui, X.-T.; Nguyen, P.-D.; Hao Ngo, H.; Dang, B.-T.; Le Quang, D.-T.; Thi Pham, T.; Visvanathan, C.; Diels, L. Bacterial Community Composition in a Two-Stage Anaerobic Membrane Bioreactor for Co-Digestion of Food Waste and Food Court Wastewater. Bioresour. Technol. 2024, 391, 129925. [Google Scholar] [CrossRef]
- Hu, Y.; Tong, K.; Guo, Q.; Zhang, B.; Jiao, L.; Li, M.; Zhang, Z. Effect of Controlled Temperature and Biomass Addition on the Formed Environmental Persistent Free Radicals (EPFRs) in Sewage Sludge-Based Biochar from Pyrolysis Treatment. J. Anal. Appl. Pyrolysis 2022, 162, 105460. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Xie, D.; Guan, W.; Yang, M.; Zhao, P.; Gao, M.; Wang, Q.; Wu, C. Adding Activated Carbon to the System with Added Zero-Valent Iron Further Improves Anaerobic Digestion Performance by Alleviating Ammonia Inhibition and Promoting DIET. J. Environ. Chem. Eng. 2021, 9, 106616. [Google Scholar] [CrossRef]
- Chen, S.; Tao, Z.; Yao, F.; Wu, B.; He, L.; Hou, K.; Pi, Z.; Fu, J.; Yin, H.; Huang, Q.; et al. Enhanced Anaerobic Co-Digestion of Waste Activated Sludge and Food Waste by Sulfidated Microscale Zerovalent Iron: Insights in Direct Interspecies Electron Transfer Mechanism. Bioresour. Technol. 2020, 316, 123901. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Niu, Y.; Zuo, Z.; Zhang, J.; Wei, Y. Effect of Zero-Valent Iron Combined with Carbon-Based Materials on the Mitigation of Ammonia Inhibition during Anaerobic Digestion. Bioresour. Technol. 2020, 311, 123503. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of Trace Element Supplementation on the Performance of Anaerobic Digestion Process: A Critical Review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y. Effects of Fe-Mn-Modified Biochar Addition on Anaerobic Digestion of Sewage Sludge: Biomethane Production, Heavy Metal Speciation and Performance Stability. Bioresour. Technol. 2020, 313, 123695. [Google Scholar] [CrossRef]
- Kalogiannis, A.; Vasiliadou, I.A.; Tsiamis, A.; Galiatsatos, I.; Stathopoulou, P.; Tsiamis, G.; Stamatelatou, K. Enhancement of Biodegradability of Chicken Manure via the Addition of Zeolite in a Two-Stage Dry Anaerobic Digestion Configuration. Molecules 2024, 29, 2568. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Di, L.; Zhang, Q.; Wang, F.; Wang, H.; Liu, H.; Yi, W.; Zhang, Z.; Zhang, D. Effect of Nano-Fe3O4 Biochar on Anaerobic Digestion of Chicken Manure under High Ammonia Nitrogen Concentration. J. Clean. Prod. 2022, 375, 134107. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, C.; Wu, P.; Ding, P.; Zhang, Y.; Cui, M.; Liu, H. Algae Biochar Enhanced Methanogenesis by Enriching Specific Methanogens at Low Inoculation Ratio during Sludge Anaerobic Digestion. Bioresour. Technol. 2021, 338, 125493. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, H.; Zhang, Y.; Cui, M.; Fu, B.; Liu, H. Insight into Sludge Anaerobic Digestion with Granular Activated Carbon Addition: Methanogenic Acceleration and Methane Reduction Relief. Bioresour. Technol. 2021, 319, 124131. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. Available online: https://www.nature.com/articles/nmeth.2604 (accessed on 24 March 2025). [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. Available online: https://www.nature.com/articles/s41587-020-0548-6 (accessed on 24 March 2025). [CrossRef]




| C (%) | N (%) | H (%) | S (%) | TS (%) | VS (%) | SBET (m2/g) | Fe (mg/g) | Ni (mg/g) | Co (mg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| FW | 0.7 ± 0.04 | 0.84 ± 0.06 | 12.34 ± 0.15 | <0.1 | 23.70 ± 0.01 | 21.15 ± 0.26 | ||||
| AS | 9.98 ± 0.98 | 1.22 ± 0.09 | 5.73 ± 0.08 | 0.16 ± 0.01 | 2.29 ± 0.11 | 1.90 ± 0.33 | ||||
| RS | 34.68 ± 0.08 | 0.46 ± 0.01 | 4.93 ± 0.09 | 0.14 ± 0.01 | 92.56 ± 0.03 | 85.67 ± 0.26 | ||||
| DG | 20.80 ± 0.92 | 3.78 ± 0.09 | 3.27 ± 0.32 | 0.51 ± 0.02 | 95.62 ± 0.31 | 90.26 ± 0.55 | ||||
| DB | 13.87 ± 0.01 | 0.84 ± 0.04 | 0.95 ± 0.01 | 0.93 ± 0.01 | 21.91 | 3.57 | 0.008 | 0.754 | ||
| RB | 40.36 ± 0.03 | 0.55 ± 0.04 | 1.51 ± 0.02 | 0.25 ± 0.01 | 205.10 | 3.12 | 0.008 | 0.752 | ||
| DRB | 25.81 ± 0.20 | 1.19 ± 0.04 | 1.18 ± 0.03 | 0.36 ± 0.02 | 126.04 | 3.89 | 0.009 | 0.713 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Liu, H.; Liu, L.; Yan, Z.; Chui, C.; Yang, N.; Wang, C.; Shen, G.; Chen, Q. Enhancing Methane Production in Anaerobic Digestion of Food Waste Using Co-Pyrolysis Biochar Derived from Digestate and Rice Straw. Molecules 2025, 30, 1766. https://doi.org/10.3390/molecules30081766
Yang Q, Liu H, Liu L, Yan Z, Chui C, Yang N, Wang C, Shen G, Chen Q. Enhancing Methane Production in Anaerobic Digestion of Food Waste Using Co-Pyrolysis Biochar Derived from Digestate and Rice Straw. Molecules. 2025; 30(8):1766. https://doi.org/10.3390/molecules30081766
Chicago/Turabian StyleYang, Qinyan, Huanran Liu, Li Liu, Zhen Yan, Chunmeng Chui, Niannian Yang, Chen Wang, Guoqing Shen, and Qincheng Chen. 2025. "Enhancing Methane Production in Anaerobic Digestion of Food Waste Using Co-Pyrolysis Biochar Derived from Digestate and Rice Straw" Molecules 30, no. 8: 1766. https://doi.org/10.3390/molecules30081766
APA StyleYang, Q., Liu, H., Liu, L., Yan, Z., Chui, C., Yang, N., Wang, C., Shen, G., & Chen, Q. (2025). Enhancing Methane Production in Anaerobic Digestion of Food Waste Using Co-Pyrolysis Biochar Derived from Digestate and Rice Straw. Molecules, 30(8), 1766. https://doi.org/10.3390/molecules30081766







