Selenium in Action: Exploring the Biological Wonders of Hydroselenite Salts
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry and Characterization
2.2. Water Solubility Studies
2.3. Biological Evaluation
2.3.1. Antibacterial Activity of the Reported Compounds
2.3.2. Evaluation of the Antiproliferative Activities of the Antibiotics and Their Corresponding Salts
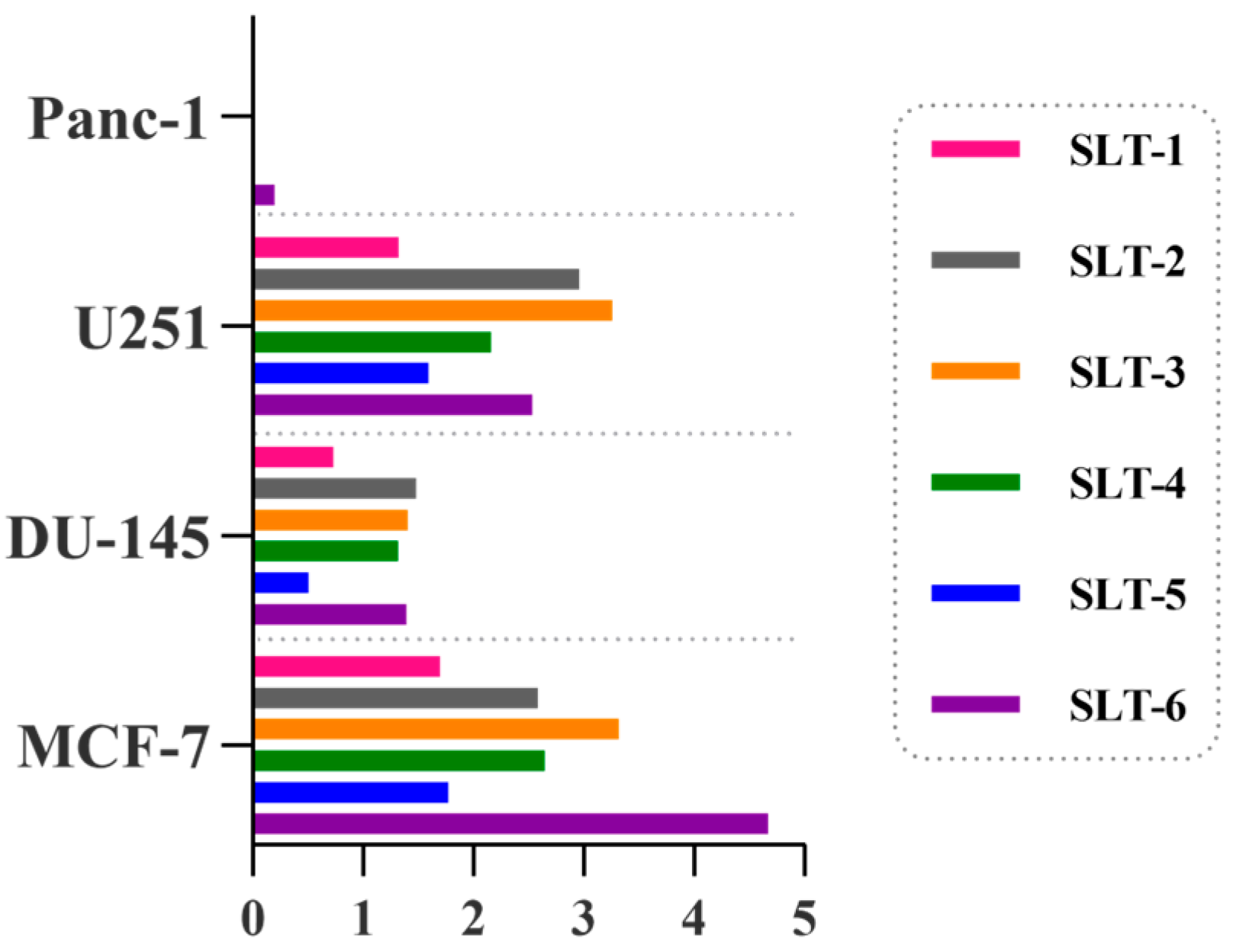
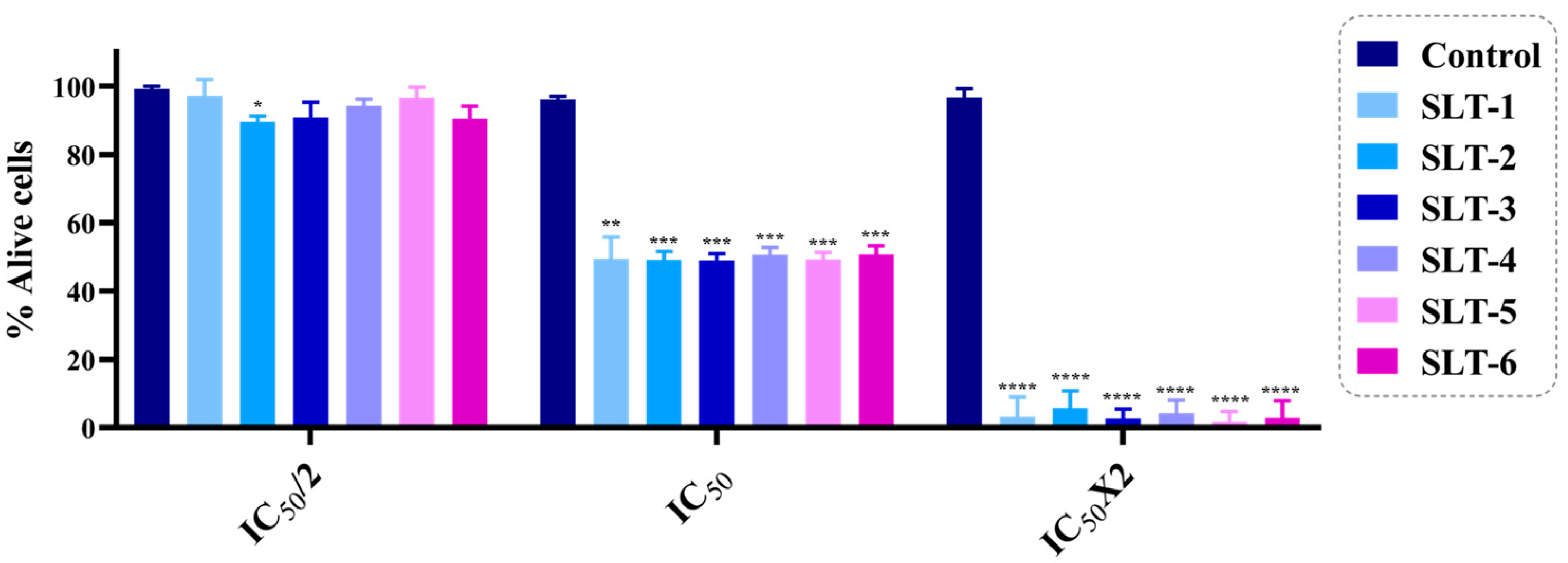
2.3.3. The Hydroselenite Salts Inhibited the Cell Viability of U251 Cancer Cells
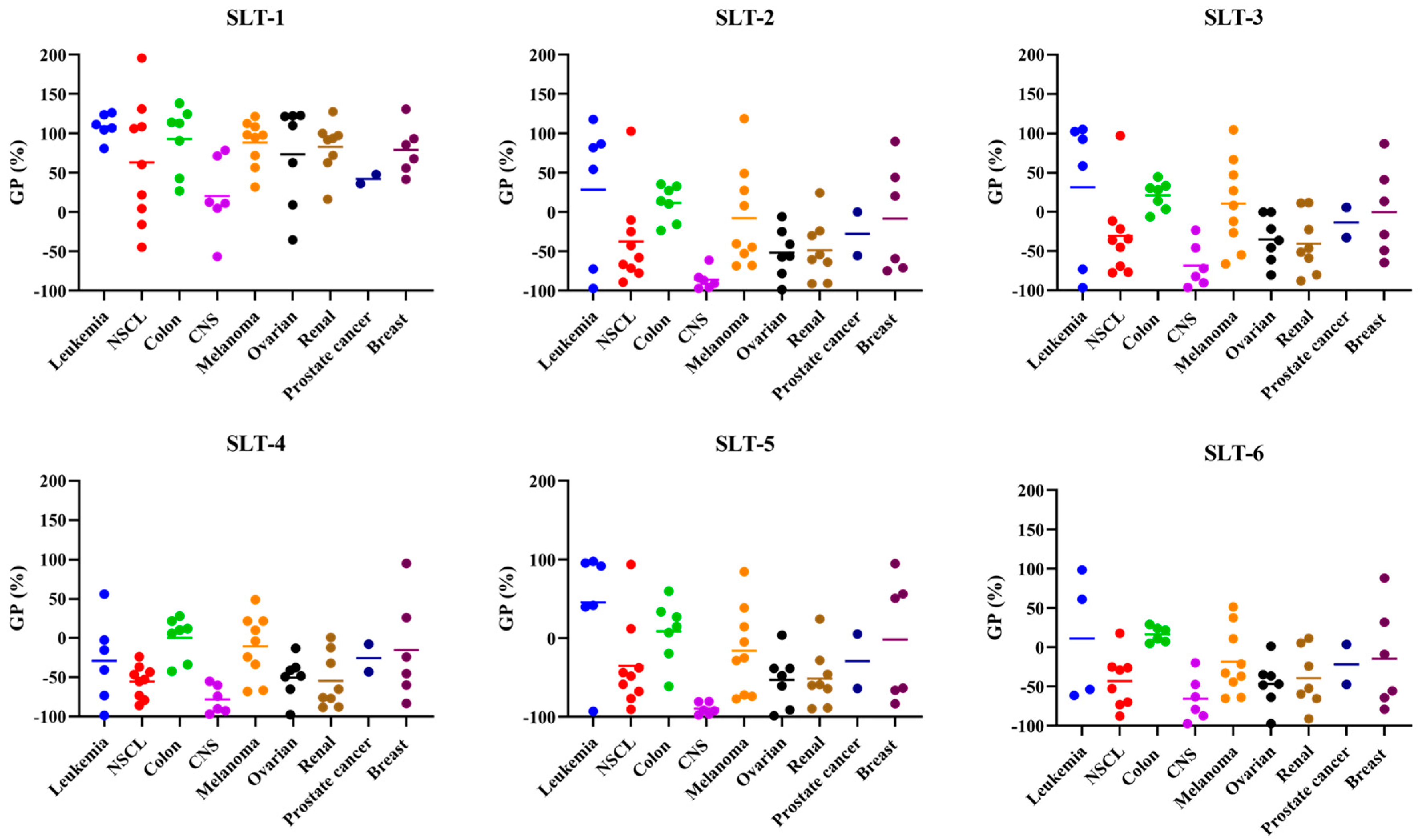
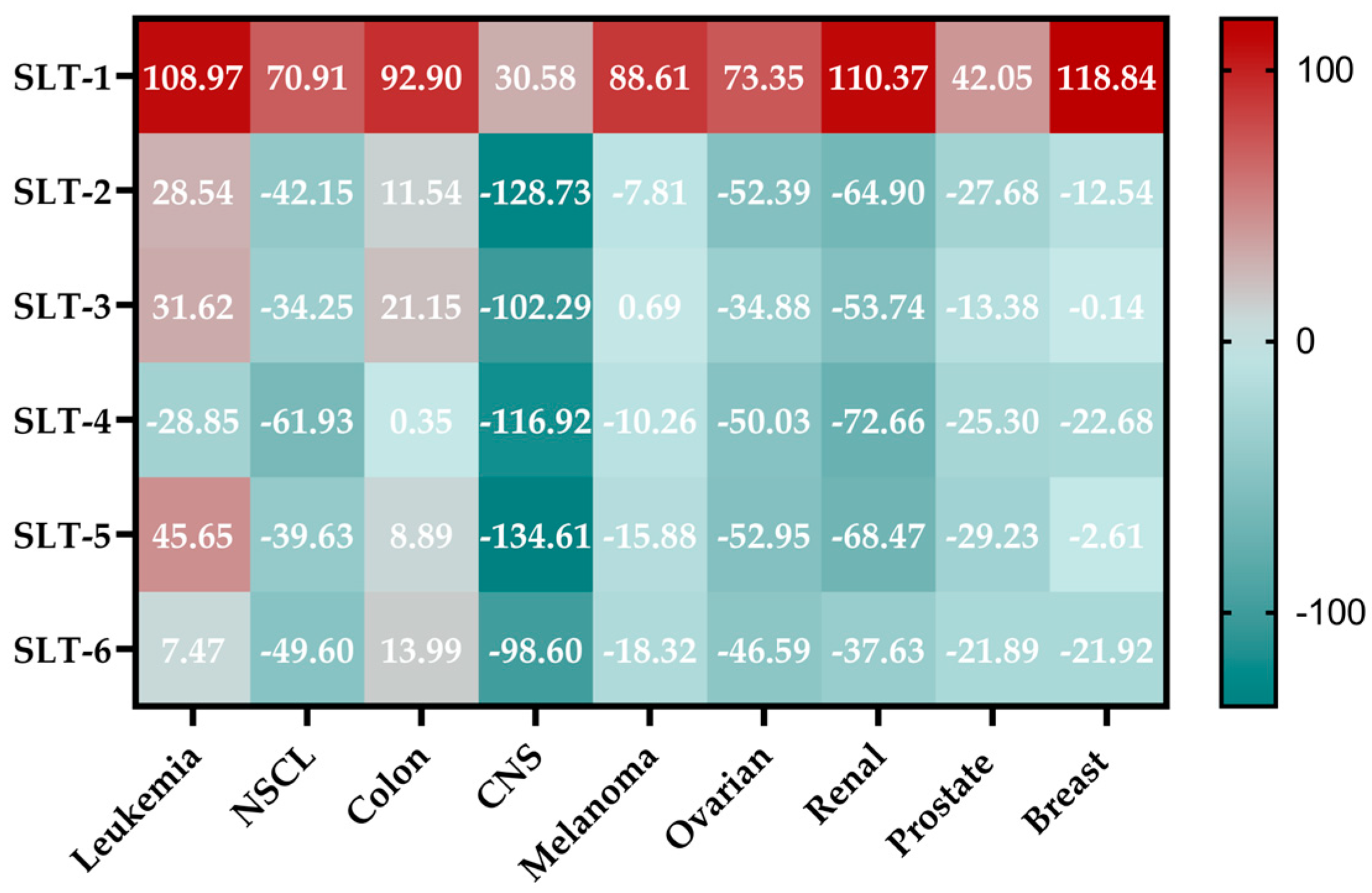
2.3.4. NCI-60 Analysis of the Hydroselenite Salts
2.3.5. Five-Dose Assay
2.3.6. SLT-2 and SLT-6 Induced Apoptosis in U251 Cancer Cells
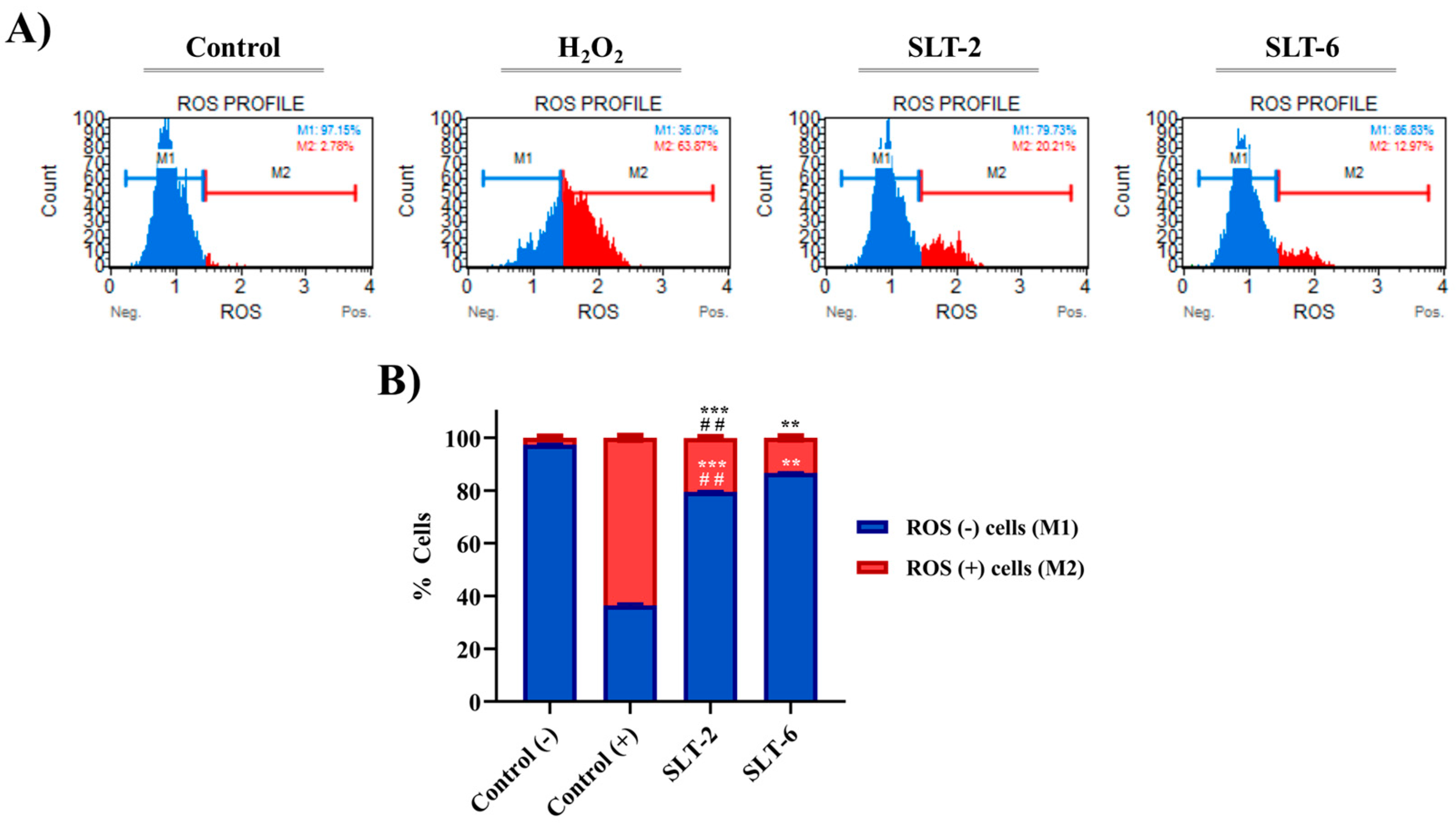
2.3.7. Influence of SLT-2 and SLT-6 on ROS Levels
3. Materials and Methods
3.1. Chemistry
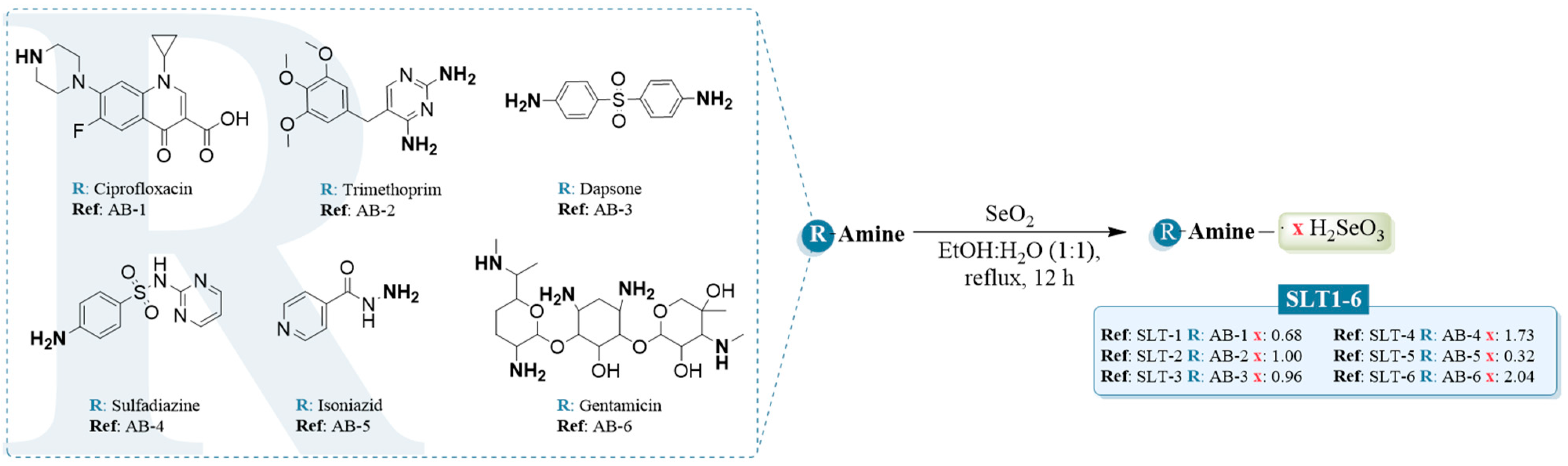
3.1.1. General Procedure for the Synthesis of the Hydroselenite Salts (SLT-1-6)
- 1-Cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid · 0.68 hydroselenite (SLT-1). The compound was obtained from AB-1 as a white solid according to the general procedure described above. Yield: 95%, m.p: 189.7 °C. 1H NMR (400 MHz, D2O) δ 8.58 (s, 1H, NH), 7.47 (s, 1H, Aryl), 7.45 (s, 1H, Aryl), 3.65–3.62 (m, 1H, Aliph), 3.57–3.55 (m, 4H, Aliph), 3.46–3.43 (m, 4H, Aliph), 1.35 (m, 2H, Aliph), 1.13 (m, 2H, Aliph). 13C NMR (101 MHz, D2O) δ 176.1 (C=O), 169.2 (C=O), 152.2 (d, JC-F = 249.3 Hz), 148.4, 144.6 (d, JC-F = 10.2 Hz), 139.0, 119.0, 110.8 (d, JC-F = 23.5 Hz), 106.7, 106.1, 46.3 (CH2, piperazine), 43.2 (CH2, piperazine), 36.1 (CH, cyclopropyl), 7.4 (CH2, cyclopropyl). 77Se NMR (76 MHz, D2O) δ (ppm): 1318. Anal. Calcd for C17H18FN3O3 ·0.68 H2SeO3 (%): C, 48.72; H, 4.62; N, 10.03. Found: C, 48.65; H, 4.88; N, 9.89.
- 5-(3,4,5-Trimethoxybenzyl)pyrimidine-2,4-diamine · 1.0 hydroselenite (SLT-2). The compound was obtained from AB-2 as a pale orange solid according to the general procedure described above. Yield: 93%, m.p: 61.6°C. 1H NMR (400 MHz, D2O) δ 7.18 (s, 1H, Aryl), 6.42 (s, 2H, Aryl), 3.65 (s, 6H, Aliph), 3.59 (s, 3H, Aliph), 3.50 (s, 2H, Aliph). 13C NMR (101 MHz, D2O) δ 164.4, 154.3, 152.6, 139.6, 135.5, 133.1, 109.0, 106.0, 60.8 (-OCH3), 55.9 (-OCH3), 32.4 (-CH2-). 77Se NMR (76 MHz, D2O) δ (ppm): 1309. Anal. Calcd for C14H18N4O3 · 1.0 H2SeO3 (%): C, C, 46.78; H, 4.77; N, 13.36. Found: C, 46.92; H, 4.62; N, 13.50.
- 4,4′-Sulfonyldianiline · 0.96 hydroselenite (SLT-3). The compound was obtained from AB-3 as a purple solid according to the general procedure described above. Yield: 95%, m.p: 106.7 °C. 1H NMR (400 MHz, DMSO) δ 7.44 (d, J = 8.8 Hz, 4H, Aryl), 6.58 (d, J = 8.9 Hz, 4H, Aryl). 13C NMR (101 MHz, DMSO) δ 153.2 (-C-NH2), 129.0, 128.6 (-C-SO2), 113.3. 77Se NMR (76 MHz, DMSO) δ (ppm): 1314. Anal. Calcd for C12H12N2O2S · 0.96 H2SeO3 (%): C, 38.73; H, 3.74; N, 7.53. Found: C, 38.49; H, 3.96; N, 7.39.
- 4-Amino-N-(pyrimidin-2-yl)benzenesulfonamide · 1.73 hydroselenite (SLT-4). The compound was obtained from AB-4 as a dark pink gel according to the general procedure described above. Yield: 84%. 1H NMR (400 MHz, D2O) δ 8.36 (d, J = 5.3 Hz, 2H, Aryl), 7.94 (d, J = 8.8 Hz, 2H, Aryl), 7.37 (d, J = 8.8 Hz, 2H, Aryl), 6.94 (t, J = 5.3 Hz, 1H, Aryl). 13C NMR (101 MHz, D2O) δ 159.2, 157.6, 155.6, 139.3, 128.8, 127.9, 123.3, 122.8, 112.6. 77Se NMR (76 MHz, D2O) δ (ppm): 1301. Anal. Calcd for C10H10N4O2S · 1.73 H2SeO3 (%): C, 25.36; H, 2.84; N, 11.83. Found: C, 25.01; H, 2.65; N, 12.01.
- Isonicotinohydrazide · 0.32 hydroselenite (SLT-5). The compound was obtained from AB-5 as a white solid according to the general procedure described above. Yield: 96%, m.p: 195.7 °C. 1H NMR (400 MHz, DMSO) δ 8.80 + 8.78 (d, J = 5.2 Hz, 2H, Aryl, signal split by hydrogen bond), 7.82 (d, J = 4.6 Hz, 2H, Aryl). 13C NMR (101 MHz, DMSO) δ 166.7 + 164.8 (C=O, signal split by hydrogen bond), 151.0, 139.8+ 138.6 (signal split by hydrogen bond), 123.2 + 121.8 (signal split by hydrogen bond). 77Se NMR (76 MHz, DMSO) δ 1315. Anal. Calcd for C6H7N3O · 0.32 H2SeO3 (%): C, 40.39; H, 4.28; N, 23.55. Found: C, 40.19; H, 4.17; N, 23.33.
- 2-((4,6-Diamino-3-((3-amino-6-(1-(methylamino)ethyl)tetrahydro-2H-pyran-2-yl)oxy)-2-hydroxycyclohexyl)oxy)-5-methyl-4-(methylamino)tetrahydro-2H-pyran-3,5-diol · 2.04 hydroselenite (SLT-6). The compound was obtained from AB-6 as an orange gel according to the general procedure described above. Yield: 94%. 1H NMR (400 MHz, D2O) δ 5.78 (ddd, J = 17.8, 9.6, 3.6 Hz, 1H), 5.01 (d, J = 3.7 Hz, 1H), 4.10 (dd, J = 10.9, 3.7 Hz, 1H), 4.06–3.93 (m, 1H), 3.89 (d, J = 12.8 Hz, 1H), 3.77–3.69 (m, 2H), 3.55–3.43 (m, 3H), 3.41 (d, J = 12.8 Hz, 1H), 3.37 (d, J = 10.9 Hz, 1H), 3.28–2.91 (m, 1H), 2.79 (s, 3H), 2.62 (s, 1H), 2.43 (dd, J = 13.2, 4.0 Hz, 1H), 2.03–1.70 (m, 4H), 1.47 (td, J = 13.4, 5.3 Hz, 1H), 1.22 (s, 3H), 1.17 (dt, J = 6.9, 3.1 Hz, 2H). 13C NMR (101 MHz, D2O) δ 101.1, 94.7, 83.6, 76.2, 76.1, 75.7, 74.5, 74.4, 70.1, 69.9, 69.1, 68.8, 67.8, 66.2, 66.0, 63.2, 57.4, 51.0, 49.7, 49.4, 48.5, 42.6, 34.5, 31.1, 27.6, 25.4, 22.9, 20.9, 20.5, 14.1, 12.3, 9.5. 77Se NMR (76 MHz, DMSO) δ 1302. Anal. Calcd for C21H43N5O7 · 2.04 H2SeO3 (%): C, 34.05; H, 6.36; N, 9.46. Found: C, 34.28; H, 6.71; N, 9.17.
3.1.2. Atomic Absorption Spectroscopy (AAS)
3.1.3. Water Solubility Assay by 1H-NMR
3.2. Biology
3.2.1. MIC and MBC Assays
3.2.2. Cell Culture Conditions
3.2.3. Cell Viability Assay
3.2.4. Trypan Blue Staining Assay
3.2.5. NCI-60 Analysis
3.2.6. Apoptosis Assays
3.2.7. ROS Measurement
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Wijdeven, R.H.; Pang, B.; Assaraf, Y.G.; Neefjes, J. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resist. Update 2016, 28, 65–81. [Google Scholar] [CrossRef]
- Ajulo, S.; Awosile, B. Global antimicrobial resistance and use surveillance system (GLASS 2022): Investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS ONE 2024, 19, e0297921. [Google Scholar] [CrossRef] [PubMed]
- Morán-Serradilla, C.; Angulo-Elizari, E.; Henriquez-Figuereo, A.; Sanmartín, C.; Sharma, A.K.; Plano, D. Seleno-Metabolites and Their Precursors: A New Dawn for Several Illnesses? Metabolites 2022, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, M.C.; Park, J.M.; Chung, A.S. Antitumor Effects of Selenium. Int. J. Mol. Sci. 2021, 22, 11844. [Google Scholar] [CrossRef]
- Rataan, A.O.; Geary, S.M.; Zakharia, Y.; Rustum, Y.M.; Salem, A.K. Potential Role of Selenium in the Treatment of Cancer and Viral Infections. Int. J. Mol. Sci. 2022, 23, 2215. [Google Scholar] [CrossRef]
- Morán-Serradilla, C.; Plano, D.; Sanmartín, C.; Sharma, A.K. Selenization of Small Molecule Drugs: A New Player on the Board. J. Med. Chem. 2024, 67, 7759–7787. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Ge, S.-W.; Wang, W.; Zheng, Q.; Zuo, Y.-W.; Zhong, C.-J.; Sun, B.-W. Novel perchlorate and phosphate salts of vinpocetine: Characterization, relative solid-state stability evaluation and Hirshfeld surface analysis. J. Mol. Struct. 2016, 1105, 1–10. [Google Scholar] [CrossRef]
- Palopa, J.A.; Planoa, D.; Morenoa, E.; Sanmartín, C. Novel quinazoline and pyrido [2,3-d]pyrimidine derivatives and their hydroselenite salts as antitumoral agents. ARKIVOC 2013, 2014, 187–206. [Google Scholar] [CrossRef]
- Elder, D.P.; Snodin, D.J. Drug substances presented as sulfonic acid salts: Overview of utility, safety and regulation. J. Pharm. Pharmacol. 2009, 61, 269–278. [Google Scholar] [CrossRef]
- Eom, T.; Khan, A. Polyselenonium salts: Synthesis through sequential selenium-epoxy ‘click’ chemistry and Se-alkylation. Chem. Commun. 2020, 56, 14271–14274. [Google Scholar] [CrossRef] [PubMed]
- Dai, A.; Wang, W.; Heng, X.; Shu, W.; Lu, S.; Xu, Y.; Wang, D.; Pan, X.; Li, N.; Chen, G.; et al. High-Efficiency Bactericidal and Biofilm Elimination Ability of the Biodegradable Alternating Sequence Main-Chain Polyselenium Salt. ACS Appl. Polym. Mater. 2024, 6, 4975–4984. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Zhang, J.; Pan, X.; Li, N.; Chen, G.; Zhu, J. Degradable Selenium-Containing Polymers for Low Cytotoxic Antibacterial Materials. ACS Macro Lett. 2022, 11, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Xing, D.; Pan, X.-Q.; Zhu, J. Synthesis and Antibacterial Activity of Selenium-functionalized Poly(ε-caprolactone). CJPS 2022, 40, 67–74. [Google Scholar] [CrossRef]
- Murai, T.; Nonoyama, T. Generation and characterization of aliphatic selenothioic acid salts. Tetrahedron 2012, 68, 10489–10495. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Dimitrijevs, P.; Domracheva, I.; Jaudzems, K.; Dambrova, M.; Arsenyan, P. Fused isoselenazolium salts suppress breast cancer cell growth by dramatic increase in pyruvate-dependent mitochondrial ROS production. Sci. Rep. 2020, 10, 21595. [Google Scholar] [CrossRef]
- Witek, K.; Nasim, M.J.; Bischoff, M.; Gaupp, R.; Arsenyan, P.; Vasiljeva, J.; Marć, M.A.; Olejarz, A.; Latacz, G.; Kieć-Kononowicz, K.; et al. Selenazolinium Salts as “Small Molecule Catalysts” with High Potency against ESKAPE Bacterial Pathogens. Molecules 2017, 22, 2174. [Google Scholar] [CrossRef]
- Arsenyan, P.; Rubina, K.; Shestakova, I.; Abele, E.; Abele, R.; Domracheva, I.; Nesterova, A.; Popelis, J.; Lukevics, E. Synthesis and cytotoxicity of silylalkylthio-substituted N-heterocycles and their hydroselenites. Appl. Organomet. Chem. 2003, 17, 825–830. [Google Scholar] [CrossRef]
- Lukevics, E.; Arsenyan, P.; Rubina, K.; Shestakova, I.; Domracheva, I.; Nesterova, A.; Popelis, J.; Pudova, O. Amino-acid hydroselenites: Synthesis and cytotoxicity. Appl. Organomet. Chem. 2002, 16, 235–238. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Henriquez-Figuereo, A.; Moreno, E.; Berzosa, M.; Encío, I.; Plano, D.; Sanmartín, C. Unveiling a New Selenocyanate as a Multitarget Candidate with Anticancer, Antileishmanial and Antibacterial Potential. Molecules 2022, 27, 7477. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Aliaga, C.; Encío, I.; Raza, A.; Sharma, A.K.; Aydillo, C.; Martínez-Sáez, N.; Sanmartín, C.; Plano, D. First Generation of Antioxidant Precursors for Bioisosteric Se-NSAIDs: Design, Synthesis, and In Vitro and In Vivo Anticancer Evaluation. Antioxidants 2023, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, J.; Du, Y.; Sun, M.; Xiang, Y.; Sheng, Y.; Ren, X.; Shao, J. Selenite induced breast cancer MCF7 cells apoptosis through endoplasmic reticulum stress and oxidative stress pathway. Chem.-Biol. Interact. 2021, 349, 109651. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Alves, V.; Sarmento-Ribeiro, A.B.; Mota-Pinto, A. Combined effect of sodium selenite and docetaxel on PC3 metastatic prostate cancer cell line. Biochem. Biophys. Res. Commun. 2011, 408, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Sohn, S.; Kwon, H.J.; Kim, S.U.; Kim, M.-J.; Lee, S.-J.; Choi, K.S. Sodium Selenite Induces Superoxide-Mediated Mitochondrial Damage and Subsequent Autophagic Cell Death in Malignant Glioma Cells. Cancer Res. 2007, 67, 6314–6324. [Google Scholar] [CrossRef]
- Doello, K.; Mesas, C.; Quiñonero, F.; Perazzoli, G.; Cabeza, L.; Prados, J.; Melguizo, C.; Ortiz, R. The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study. Cancers 2021, 13, 3169. [Google Scholar] [CrossRef]
- Fan, T.W.; El-Amouri, S.S.; Macedo, J.K.A.; Wang, Q.J.; Song, H.; Cassel, T.; Lane, A.N. Stable Isotope-Resolved Metabolomics Shows Metabolic Resistance to Anti-Cancer Selenite in 3D Spheroids versus 2D Cell Cultures. Metabolites 2018, 8, 40. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Jacobs, V.L.; Valdes, P.A.; Hickey, W.F.; De Leo, J.A. Current Review of in Vivo GBM Rodent Models: Emphasis on the CNS-1 Tumour Model. ASN Neuro 2011, 3, AN20110014. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Encío, I.; Raza, A.; Sharma, A.K.; Sanmartín, C.; Plano, D. Design, synthesis and anticancer evaluation of novel Se-NSAID hybrid molecules: Identification of a Se-indomethacin analog as a potential therapeutic for breast cancer. Eur. J. Med. Chem. 2022, 244, 114839. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]

| Ref. | Water Solubility (g/mL) |
|---|---|
| AB-1 | <1.62 × 10−4 |
| SLT-1 | 5.91 × 10−3 |
| AB-2 | 2.84 × 10−4 |
| SLT-2 | 4.92 × 10−3 |
| AB-3 | 1.52 × 10−4 |
| SLT-3 | 3.34 × 10−4 |
| AB-4 | 1.12 × 10−4 |
| SLT-4 | 3.08 × 10−3 |
| AB-5 | 5.35 × 10−3 |
| SLT-5 | 5.93 × 10−2 |
| Ref. | Gram-Positive Bacteria | Gram-Negative Bacteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | B. sphaericus | E. coli | K. pneumoniae | P. aeruginosa | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| AB-1 | 0.39 | 0.78 | 0.2 | 0.2 | 6.25 | 6.25 | 0.03 | 3.13 | 0.1 | 0.2 | 0.1 | 0.39 |
| SLT-1 | 0.78 | 0.78 | 0.78 | 1.56 | 6.25 | 6.25 | 0.2 | 0.78 | 0.05 | 0.05 | 0.2 | 1.56 |
| AB-2 | 1.56 | 3.13 | >200 | >200 | 1.56 | 6.25 | 1.56 | 6.25 | 3.13 | 12.5 | 50 | >200 |
| SLT-2 | 1.56 | 3.13 | >200 | >200 | 3.13 | 12.50 | 1.56 | 6.25 | 6.25 | 12.5 | 50 | >200 |
| AB-3 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| SLT-3 | 100 | 200 | 50 | 50 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| AB-4 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 50 | >200 |
| SLT-4 | 100 | >200 | 200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| AB-5 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| SLT-5 | >200 | >200 | 100 | 100 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| AB-6 | 25 | 50 | 0.39 | 0.39 | 0.2 | 0.78 | 25 | 25 | 1.56 | 3.13 | 6.56 | 12.5 |
| SLT-6 | 12.5 | 12.5 | 0.39 | 1.56 | 0.39 | 0.39 | 25 | 25 | 3.13 | 12.5 | 6.56 | 12.5 |
| H2SeO3 | 100 | 200 | 100 | 200 | 100 | 200 | 200 | >200 | 100 | 200 | 200 | >200 |
| MCF-7 | DU-145 | U251 | Panc-1 | NHDF | |
|---|---|---|---|---|---|
| AB-1 | 23.38 ± 7.61 | >100 | >100 | >100 | >100 |
| SLT-1 | 3.40 ± 0.47 | 7.95 ± 1.18 | 4.37 ± 1.21 | >100 | 5.77 ± 1.50 |
| AB-2 | >100 | >100 | >100 | >100 | 6.47 ± 1.79 |
| SLT-2 | 2.23 ± 0.52 | 3.91 ± 0.77 | 1.95 ± 0.42 | >100 | 5.41 ± 0.76 |
| AB-3 | >100 | >100 | >100 | >100 | >100 |
| SLT-3 | 1.74 ± 0.35 | 4.11 ± 0.61 | 1.77 ± 0.19 | >100 | 14.69 ± 6.27 |
| AB-4 | >100 | >100 | >100 | >100 | >100 |
| SLT-4 | 1.11 ± 0.14 | 2.23 ± 0.52 | 1.36 ± 0.27 | >100 | 2.94 ± 0.88 |
| AB-5 | >100 | 99.92 ± 0.20 | >100 | >100 | 41.23 ± 12.12 |
| SLT-5 | 5.18 ± 0.37 | 18.33 ± 3.03 | 5.77 ± 0.75 | >100 | 9.57 ± 2.36 |
| AB-6 | >100 | >100 | >100 | >100 | >100 |
| SLT-6 | 0.58 ± 0.14 | 1.95 ± 0.28 | 1.07 ± 0.12 | 13.85 ± 4.62 | 2.71 ± 0.29 |
| Subpanel | Compound (Mean GI50) | ||||
|---|---|---|---|---|---|
| SLT-2 | SLT-3 | SLT-4 | SLT-5 | SLT-6 | |
| Leukemia | 25.37 | 25.91 | 2.38 | 16.39 | 21.76 |
| NSCL | 10.90 | 9.54 | 1.24 | 11.38 | 4.05 |
| Colon | 17.42 | 12.59 | 4.54 | 15.60 | 6.93 |
| CNS | 1.55 | 1.42 | 0.97 | 1.80 | 1.09 |
| Melanoma | 15.04 | 15.30 | 15.75 | 17.33 | 18.67 |
| Ovarian | 6.36 | 5.63 | 4.35 | 6.77 | 4.67 |
| Renal | 3.68 | 3.75 | 2.61 | 3.88 | 2.92 |
| Prostate | 2.34 | 2.40 | 2.17 | 2.49 | 2.51 |
| Breast | 33.96 | 24.01 | 10.42 | 45.28 | 13.36 |
| Subpanel | Compound (Mean TGI) | ||||
|---|---|---|---|---|---|
| SLT-2 | SLT-3 | SLT-4 | SLT-5 | SLT-6 | |
| Leukemia | 16.90 | 29.30 | 20.39 | 36.23 | 25.50 |
| NSCL | 24.78 | 22.91 | 3.02 | 24.81 | 13.18 |
| Colon | 73.03 | 74.06 | 51.11 | 61.82 | 63.09 |
| CNS | 3.06 | 3.25 | 2.20 | 3.65 | 2.78 |
| Melanoma | 53.77 | 59.11 | 59.17 | 42.68 | 64.96 |
| Ovarian | 33.10 | 36.70 | 15.85 | 16.94 | 22.58 |
| Renal | 43.11 | 43.15 | 42.23 | 26.83 | 42.92 |
| Prostate | 4.67 | 4.86 | 3.73 | 5.57 | 4.61 |
| Breast | 61.69 | 61.56 | 48.84 | 62.07 | 53.47 |
| Subpanel | Compound (Mean LC50) | ||||
|---|---|---|---|---|---|
| SLT-2 | SLT-3 | SLT-4 | SLT-5 | SLT-6 | |
| Leukemia | 46.08 | 41.19 | 25.54 | 50.24 | 32.27 |
| NSCL | 64.29 | 68.94 | 59.10 | 46.25 | 64.44 |
| Colon | 100.00 | 100.00 | 89.16 | 78.47 | 100.00 |
| CNS | 5.69 | 24.71 | 4.43 | 7.48 | 28.78 |
| Melanoma | 96.30 | 84.11 | 87.90 | 62.92 | 86.62 |
| Ovarian | 68.26 | 68.50 | 53.48 | 44.75 | 69.95 |
| Renal | 68.91 | 81.26 | 62.48 | 60.96 | 68.98 |
| Prostate | 9.32 | - | - | 16.60 | - |
| Breast | 74.01 | 63.70 | 62.46 | 67.53 | 67.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morán-Serradilla, C.; Plano, D.; Pastor, Y.; Navarro-Blasco, I.; Raza, A.; Sharma, A.K.; Sanmartín, C. Selenium in Action: Exploring the Biological Wonders of Hydroselenite Salts. Molecules 2025, 30, 1714. https://doi.org/10.3390/molecules30081714
Morán-Serradilla C, Plano D, Pastor Y, Navarro-Blasco I, Raza A, Sharma AK, Sanmartín C. Selenium in Action: Exploring the Biological Wonders of Hydroselenite Salts. Molecules. 2025; 30(8):1714. https://doi.org/10.3390/molecules30081714
Chicago/Turabian StyleMorán-Serradilla, Cristina, Daniel Plano, Yadira Pastor, Iñigo Navarro-Blasco, Asif Raza, Arun K. Sharma, and Carmen Sanmartín. 2025. "Selenium in Action: Exploring the Biological Wonders of Hydroselenite Salts" Molecules 30, no. 8: 1714. https://doi.org/10.3390/molecules30081714
APA StyleMorán-Serradilla, C., Plano, D., Pastor, Y., Navarro-Blasco, I., Raza, A., Sharma, A. K., & Sanmartín, C. (2025). Selenium in Action: Exploring the Biological Wonders of Hydroselenite Salts. Molecules, 30(8), 1714. https://doi.org/10.3390/molecules30081714








