One-Pot Synthesis and Immobilization of Gold Nanoparticles Using Peptidyl Microbeads
Abstract
1. Introduction
2. Results
2.1. Design and Synthesis of Au Ion-Reducing Peptide-Immobilized Beads
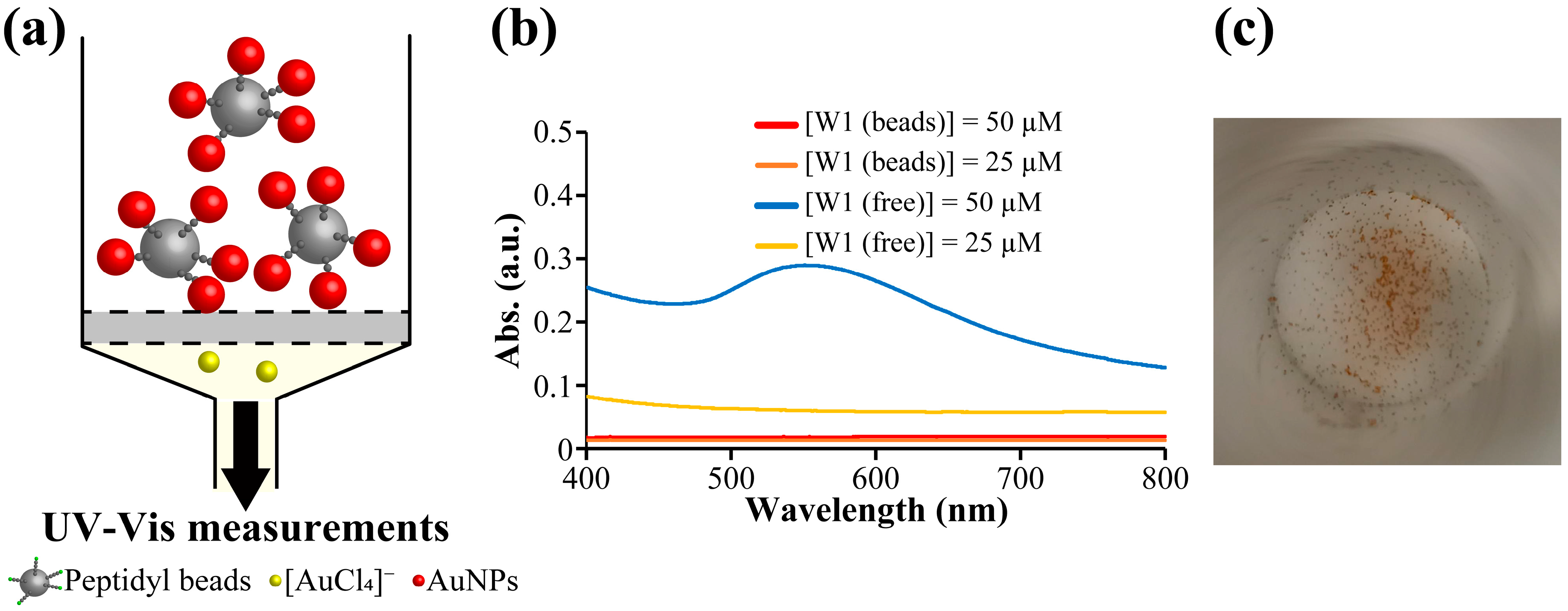
2.2. Preparation of Immobilized AuNPs Using W1-Beads
2.2.1. Preparation of Immobilized AuNPs
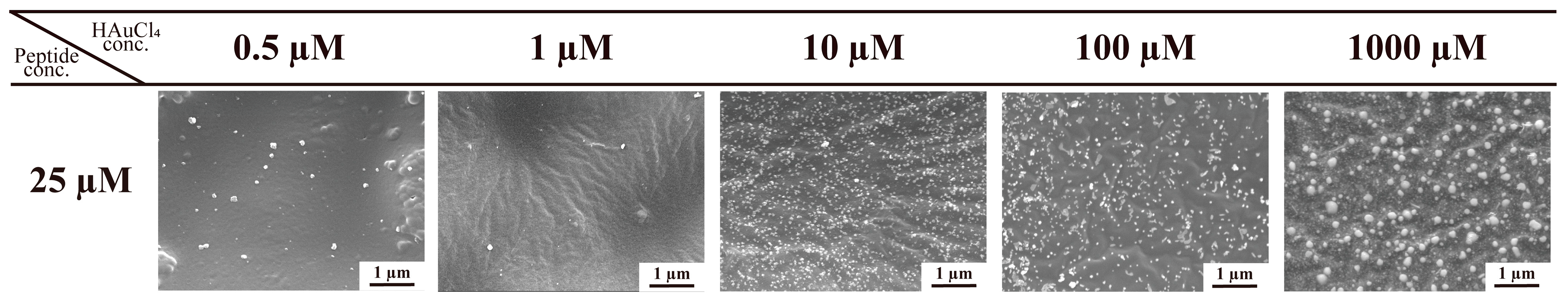
2.2.2. Immobilization of AuNPs at Various Concentrations of W1 and HAuCl4
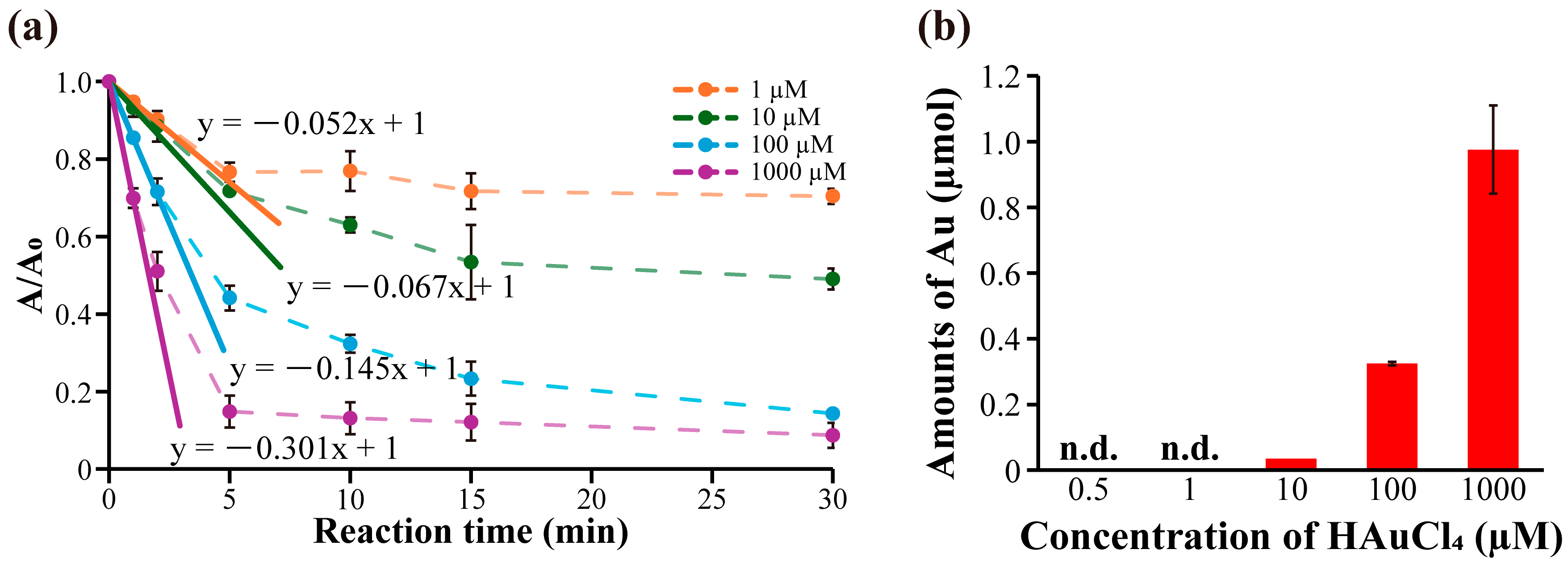
2.3. Evaluation of Initial Catalytic Reaction Rates of Immobilized AuNPs
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Peptidyl Beads and Peptide
3.3. Synthesis of Ac-Beads
3.4. Reduction in Au Ions and Immobilization of AuNPs Using Peptidyl Beads or Free Peptide
3.5. UV-Vis Measurement After Au Ion Reduction Reaction
3.6. Scanning Electron Microscopy (SEM), SEM-Energy Dispersive X-Ray Spectroscopy (SEM-EDX) Measurement, and Particle Size Analysis
3.7. Inductively Coupled Plasma-Atomic Emission Spectroscopy Measurement
3.8. Initial Catalytic Reaction Rates Evaluation of Immobilized AuNPs Using the Reduction of 4-Nitrophenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Mo, X.; Phillips, D.L.; Tse, E.C.M. Immobilization of a Molecular Copper Complex and a Carboxylate-Terminated Cocatalyst on a Metal Oxide Electrode for Enhanced Electrocatalytic Oxygen Reduction. ACS Catal. 2023, 13, 5599–5608. [Google Scholar] [CrossRef]
- Xu, H.; Sang, Y.; Xu, B.; Zhang, L.; Zhang, L.; Xu, H. Immobilization of Gold Nanoparticles on Poly(4-vinylpyridine)-Grafted Carbon Nanotubes as Heterogeneous Catalysts for Hydrogenation of 4-Nitrophenol. ACS Appl. Nano Mater. 2020, 3, 12169–12177. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kaneko, K.; Hara, M.; Matsui, M.; Morita, K.; Maruyama, T. Covalent immobilization of gold nanoparticles on a plastic substrate and subsequent immobilization of biomolecules. RSC Adv. 2021, 11, 23409–23417. [Google Scholar] [CrossRef]
- Hashmi, A.S.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Burtsev, V.; Miliutina, E.; Ulbrich, P.; Elashnikov, R.; Svorcik, V.; Orendac, M.; Lyutakov, O. Immobilization of Gold Nanoparticles in Localized Surface Plasmon Polariton-Coupled Hot Spots via Photolytic Dimerization of Aromatic Amine Groups for SERS Detection in a Microfluidic Regime. ACS Appl. Nano Mater. 2022, 5, 1836–1844. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.L. In situ formation and immobilization of gold nanoparticles on polydimethylsiloxane (PDMS) exhibiting catalase-mimetic activity. Chem. Commun. 2020, 56, 6416–6419. [Google Scholar] [CrossRef]
- Ross, M.B.; Mirkin, C.A.; Schatz, G.C. Optical Properties of One-, Two-, and Three-Dimensional Arrays of Plasmonic Nanostructures. J. Phys. Chem. C 2016, 120, 816–830. [Google Scholar] [CrossRef]
- Chandrasekharan, N.; Kamat, P.V. Assembling Gold Nanoparticles as Nanostructured Films Using an Electrophoretic Approach. Nano Lett. 2001, 1, 67–70. [Google Scholar] [CrossRef]
- Chen, C.L.; Rosi, N.L. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew. Chem. Int. Ed. 2010, 49, 1924–1942. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ozaki, N.; Nagasawa, H. Purification and Structural Determination of a Phosphorylated Peptide with Anti-calcification and Chitin-binding Activities in the Exoskeleton of the Crayfish, Procambarus clarkii. Biosci. Biotechnol. Biochem. 2001, 65, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.J.; Knecht, M.R. Solution Effects on Peptide-Mediated Reduction and Stabilization of Au Nanoparticles. Langmuir 2017, 33, 13757–13765. [Google Scholar] [CrossRef]
- Slocik, J.M.; Stone, M.O.; Naik, R.R. Synthesis of gold nanoparticles using multifunctional peptides. Small 2005, 1, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Pigliacelli, C.; Sanjeeva, K.B.; Nonappa; Pizzi, A.; Gori, A.; Bombelli, F.B.; Metrangolo, P. In Situ Generation of Chiroptically-Active Gold-Peptide Superstructures Promoted by Iodination. ACS Nano 2019, 13, 2158–2166. [Google Scholar] [CrossRef]
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chem. Eur. J. 2007, 13, 3160–3168. [Google Scholar] [CrossRef]
- Ozaki, M.; Yoshida, S.; Oura, M.; Tsuruoka, T.; Usui, K. Effect of tryptophan residues on gold mineralization by a gold reducing peptide. RSC Adv. 2020, 10, 40461–40466. [Google Scholar] [CrossRef]
- Yoshida, S.; Tomizaki, K.Y.; Usui, K. Shape control of Au nanostructures using peptides for biotechnological applications. Chem. Commun. 2023, 59, 13239–13244. [Google Scholar] [CrossRef]
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Miyazaki, H.; Hamada, Y.; Takaishi, H.; Minamino, Y.; Ikeda, H.; Mekata, H.; Takaishi, M.; Yamashita, K.; Usui, K. Development of a chromophore-solid phase peptide reaction assay (C-SPRA) for assessing skin sensitization in vitro. Analyst 2020, 145, 3211–3216. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.J.; Hughes, Z.E.; Walsh, T.R.; Knecht, M.R. Peptide Sequence Effects Control the Single Pot Reduction, Nucleation, and Growth of Au Nanoparticles. J. Phys. Chem. C 2016, 120, 18917–18924. [Google Scholar] [CrossRef]
- Chan, C.W.; White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-Dependent Catalytic Activity and Dynamics of Gold Nanoparticles at the Single-Molecule Level. J. Am. Chem. Soc. 2009, 132, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ, U. S. National Institutes of Health, Bethesda, ML, USA, 1997–2018. Available online: https://imagej.net/ij/ (accessed on 30 March 2025).
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172. [Google Scholar] [CrossRef]
- Arrigoni, F.; Prosdocimi, T.; Mollica, L.; De Gioia, L.; Zampella, G.; Bertini, L. Copper reduction and dioxygen activation in Cu-amyloid beta peptide complexes: Insight from molecular modelling. Metallomics 2018, 10, 1618–1630. [Google Scholar] [CrossRef]


| Concentration of HAuCl4 | ||||||
|---|---|---|---|---|---|---|
| 0.5 µM | 1 µM | 10 µM | 100 µM | 1000 µM | ||
| Concentration of peptide | 5 µM | n.d. | 70.6 ± 54.5 nm | 37.3 ± 13.7 nm | 85.7 ± 47.0 nm | 38.2 ± 32.1 nm |
| 10 µM | n.d. | 43.2 ± 15.8 nm | 47.8 ± 14.1 nm | 106.0 ± 27.0 nm | 61.9 ± 25.8 nm | |
| 25 µM | 26.9 ± 6.6 nm | 26.3 ± 13.9 nm | 37.7 ± 11.5 nm | 39.4 ± 12.6 nm | 46.0 ± 35.3 nm | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Yoshida, K.; Isozaki, T.; Oura, M.; Ozaki, M.; Tsuruoka, T.; Usui, K. One-Pot Synthesis and Immobilization of Gold Nanoparticles Using Peptidyl Microbeads. Molecules 2025, 30, 1689. https://doi.org/10.3390/molecules30081689
Yoshida S, Yoshida K, Isozaki T, Oura M, Ozaki M, Tsuruoka T, Usui K. One-Pot Synthesis and Immobilization of Gold Nanoparticles Using Peptidyl Microbeads. Molecules. 2025; 30(8):1689. https://doi.org/10.3390/molecules30081689
Chicago/Turabian StyleYoshida, Shuhei, Koki Yoshida, Taichi Isozaki, Maho Oura, Makoto Ozaki, Takaaki Tsuruoka, and Kenji Usui. 2025. "One-Pot Synthesis and Immobilization of Gold Nanoparticles Using Peptidyl Microbeads" Molecules 30, no. 8: 1689. https://doi.org/10.3390/molecules30081689
APA StyleYoshida, S., Yoshida, K., Isozaki, T., Oura, M., Ozaki, M., Tsuruoka, T., & Usui, K. (2025). One-Pot Synthesis and Immobilization of Gold Nanoparticles Using Peptidyl Microbeads. Molecules, 30(8), 1689. https://doi.org/10.3390/molecules30081689







