Lithospermum erythrorhizon and Forsythia suspensa Prevent Collagen Degradation and Maintain Skin Hydration by Regulating MMPs and HAS2/HYAL1 Signaling
Abstract
1. Introduction
2. Results
2.1. Analysis of the LF Ethanolic Extract Compounds
2.2. Quantification of the Total Polyphenol and Flavonoid Content in LF: Data Are Expressed as the Mean ± SD
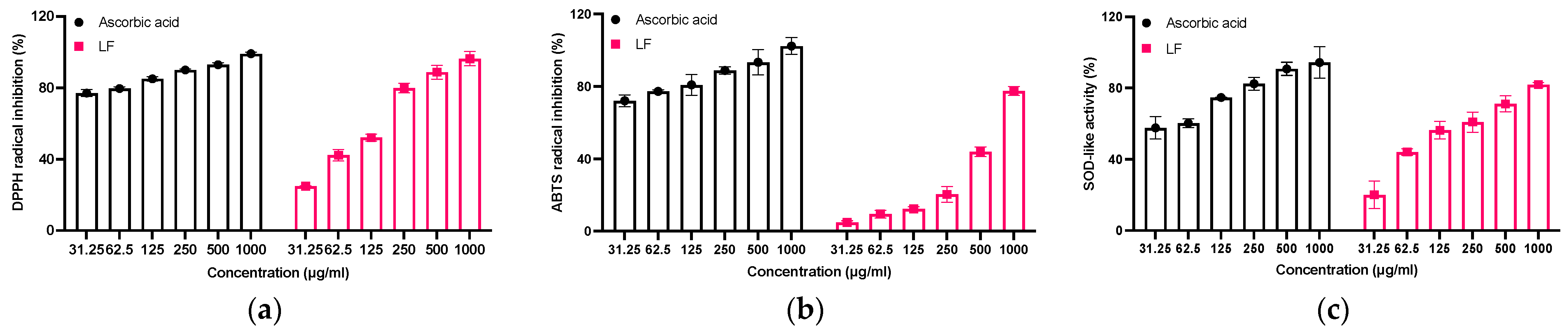
2.3. Evaluation of Antioxidant Activity of LF
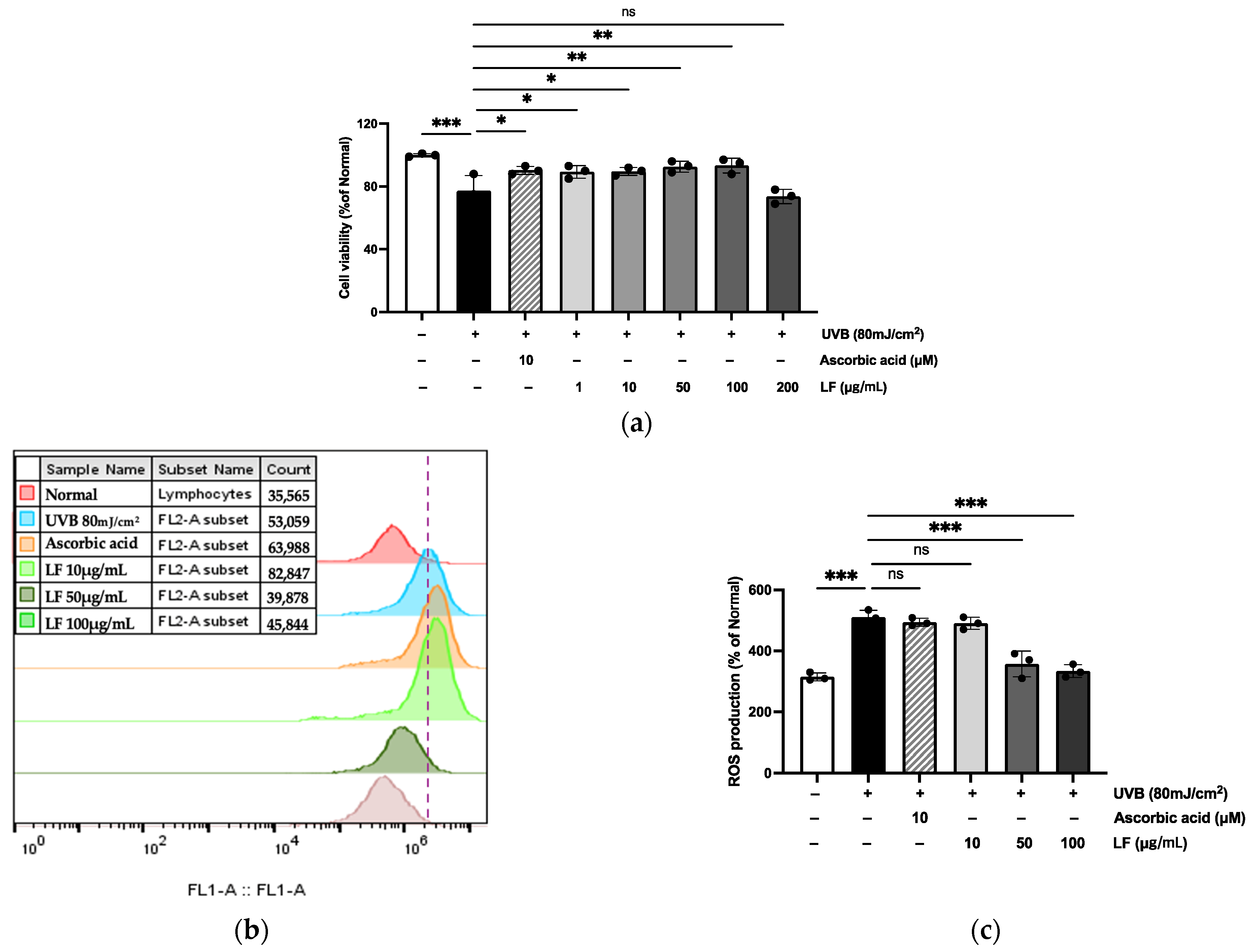
2.4. Toxicity and Cytoprotective Effects of LF on UVB-Irradiated HaCaT Cells
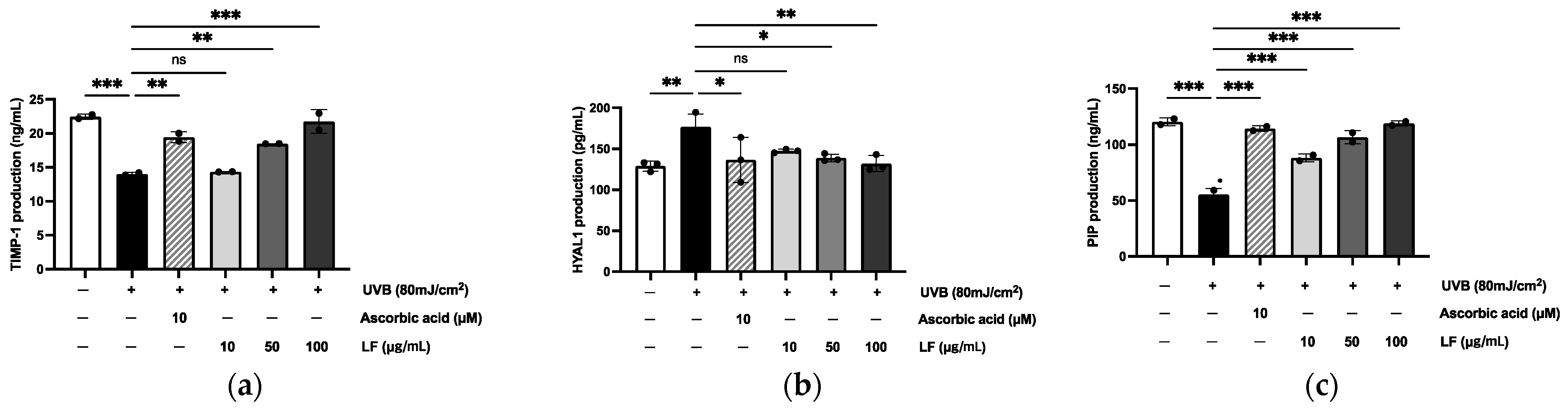
2.5. Regulation of TIMP-1, HYAL1, and PIP Expression in HaCaT Cells
2.6. MMP-1, MMP-2, MMP-3, MMP-9 Regulation Effect on UVB-Irradiated HaCaT Cells
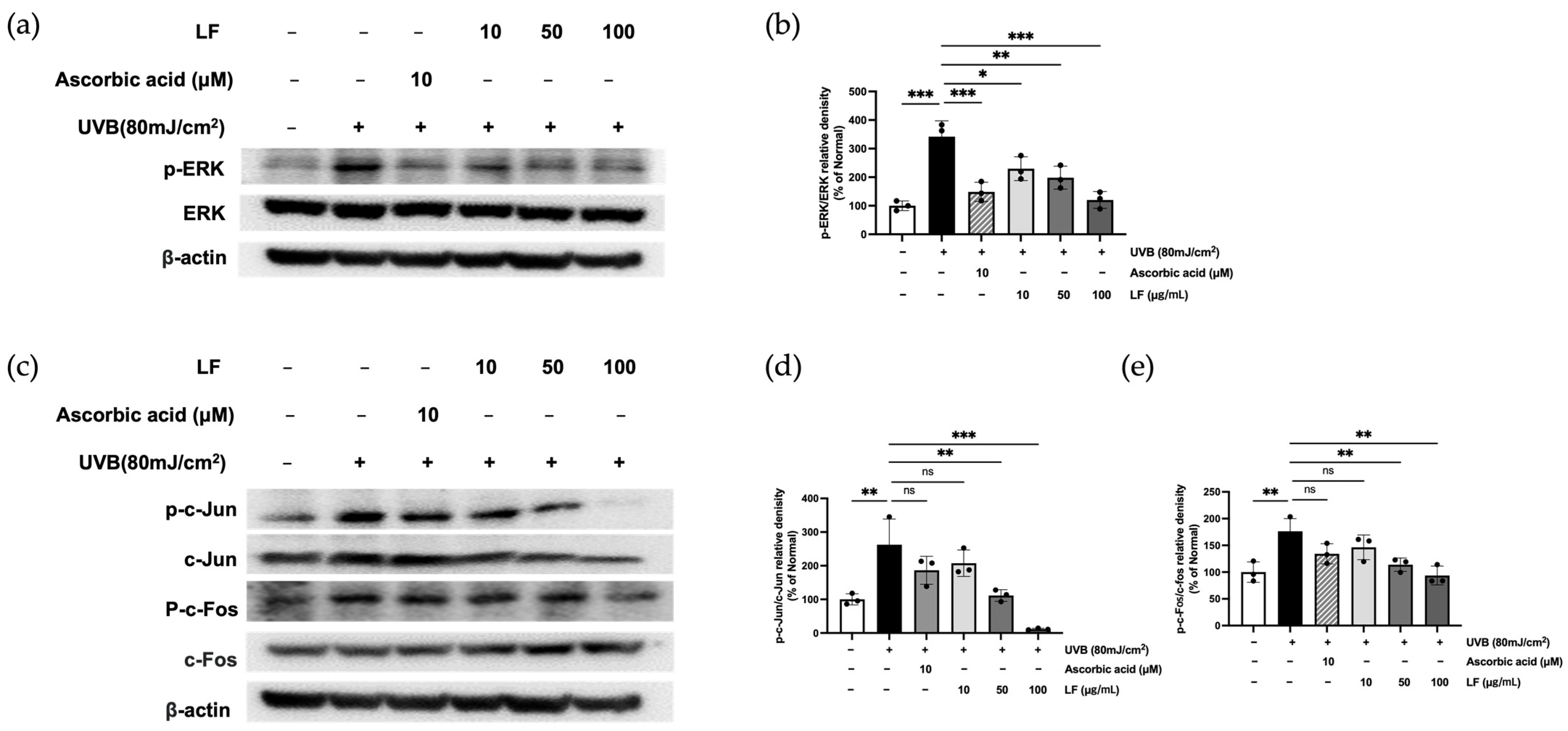
2.7. ERK Signaling and AP-1 Activation by LF Treatment
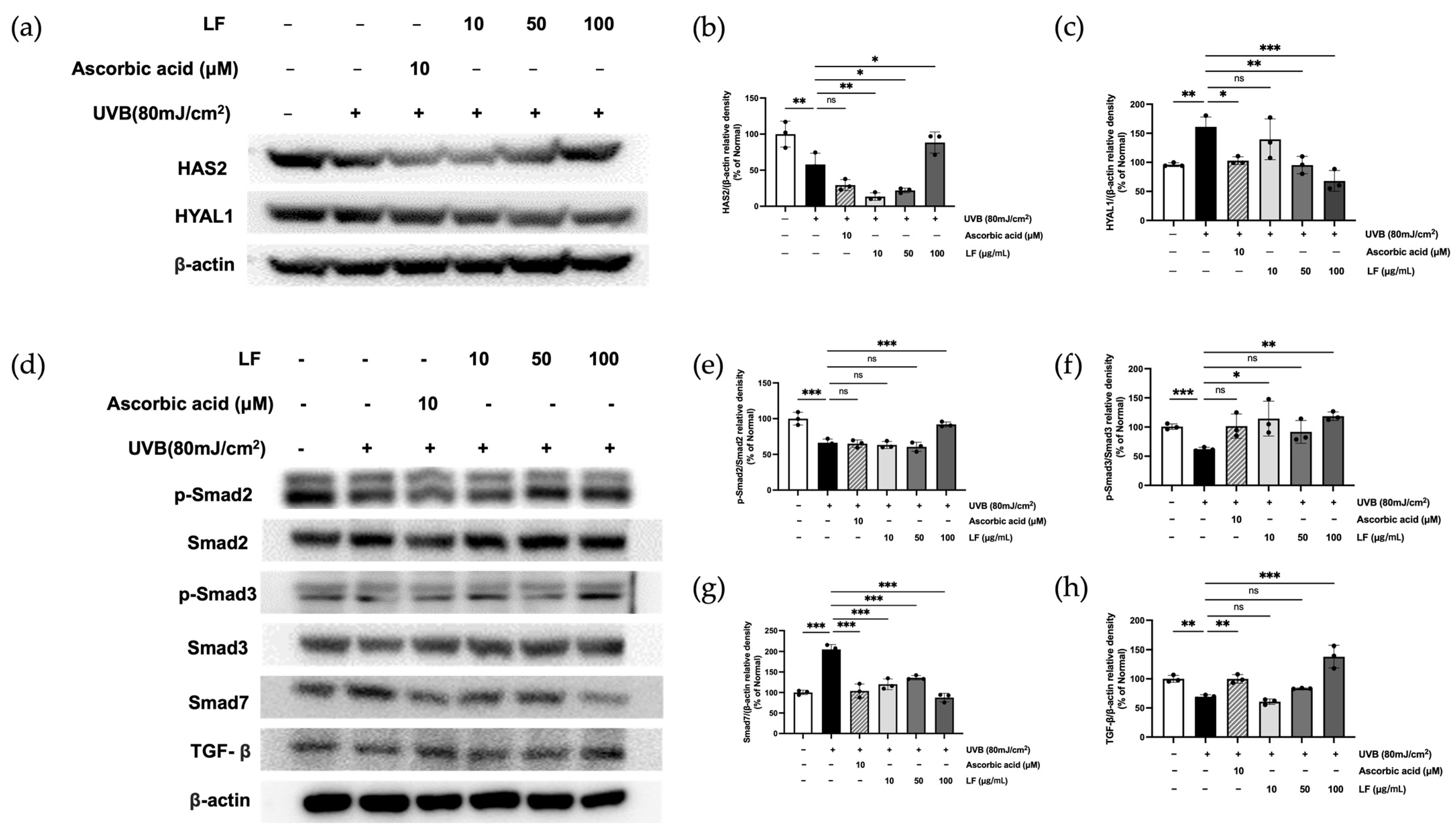
2.8. LF Treatment on TGF-β/Smad7 Signaling and HYAL and HAS Regulation Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. HPLC Analysis
4.4. Assessment of Total Phenolic and Flavonoid Contents
4.5. Evaluation of Antioxidant Activity
4.6. Culture and Treatment HaCaT Cells
4.7. Cell Viability
4.8. ROS Inhibition Assay
4.9. ELISA
4.10. RT-PCR
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2022, 71, 817–831. [Google Scholar] [CrossRef] [PubMed]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Caudri, D.; Savenije, O.E.; Smit, H.A.; Postma, D.S.; Koppelman, G.H.; Wijga, A.H.; Kerkhof, M.; Gehring, U.; Hoekstra, M.O.; Brunekreef, B.; et al. Perinatal risk factors for wheezing phenotypes in the first 8 years of life. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2013, 43, 1395–1405. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Morita, T.; Hayakawa, T. BNIP3 upregulation via stimulation of ERK and JNK activity is required for the protection of keratinocytes from UVB-induced apoptosis. Cell Death Dis. 2017, 8, e2576. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Lee, H.M.; Oh, S.; Zheng, S.; Bellere, A.D.; Kim, M.; Yu, D.; Yi, T.H. Rosa davurica inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 signaling in UVB-irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Winston, L.A.; Hunter, T. JAK2, Ras, and Raf Are Required for Activation of Extracellular Signal-regulated Kinase/Mitogen-activated Protein Kinase by Growth Hormone (∗). J. Biol. Chem. 1995, 270, 30837–30840. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Tanemura, S.; Ohata, S.; Shimizu, N.; Seo, J.; Nishitai, G.; Watanabe, T.; Nakagawa, K.; Kishimoto, H.; Wada, T.; et al. Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic function. J. Biol. Chem. 2002, 277, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S. Homeostasis of extracellular matrix by TGF-beta and lefty. Front. Biosci. A J. Virtual Libr. 2002, 7, d1231–d1246. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Okazaki, I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007, 56, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Amano, S. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp. Dermatol. 2016, 25 (Suppl. S3), 14–19. [Google Scholar] [CrossRef]
- Zhang, M.; Hwang, E.; Lin, P.; Gao, W.; Ngo, H.T.; Yi, T.H. Prunella vulgaris L. exerts a protective effect against extrinsic aging through NF-κB, MAPKs, AP-1, and TGF-β/Smad signaling pathways in UVB-aged normal human dermal fibroblasts. Rejuvenation Res. 2018, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Šínová, R.; Pavlík, V.; Ondrej, M.; Velebný, V.; Nešporová, K. Hyaluronan: A key player or just a bystander in skin photoaging? Exp. Dermatol. 2022, 31, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.S.; Jung, H.K.; Hong, J.H. Protective effect of mulberry and Lithospermum erythrorhizon extracts on anti-aging against photodamage. J. Korean Soc. Food Sci. Nutr. 2013, 42, 1744–1752. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Yoon, T.; Jang, S.; Kim, H.K. Forsythia suspensa Suppresses House Dust Mite Extract-Induced Atopic Dermatitis in NC/Nga Mice. PLoS ONE 2016, 11, e0167687. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Dou, J.; Zhang, S.; Yu, H.; Zhang, S. Shikonin potentiates skin wound healing in Sprague-Dawley rats by stimulating fibroblast and endothelial cell proliferation and angiogenesis. J. Gene Med. 2024, 26, e3633. [Google Scholar] [CrossRef]
- Yue, Y.; Fang, Y.; Jia, R.; Cao, K.; Chen, X.; Xia, H.; Cheng, Z. Study on the Antioxidant Effect of Shikonin-Loaded β-Cyclodextrin Forming Host–Guest Complexes That Prevent Skin from Photoaging. Int. J. Mol. Sci. 2023, 24, 15177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, W.; Wang, P.; Dong, L.; Du, H.; Li, N.; Liu, G.; Zhang, R.; Wang, L.; Sun, T. Investigating the active components and mechanistic effects of Forsythia suspensa Leaf against RSV via the PI3K/Akt-NLRP3 pathway. Heliyon 2024, 10, e38285. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Miličević, A. Flavonoid Oxidation Potentials and Antioxidant Activities-Theoretical Models Based on Oxidation Mechanisms and Related Changes in Electronic Structure. Int. J. Mol. Sci. 2024, 25, 5011. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B.; et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef]
- Liao, P.L.; Lin, C.H.; Li, C.H.; Tsai, C.H.; Ho, J.D.; Chiou, G.C.; Kang, J.J.; Cheng, Y.W. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy. Sci. Rep. 2017, 7, 44985. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Liu, Q.P.; Zhou, Y.X.; Chen, Y.Y.; An, P.; Xing, Y.Z.; Zhang, L.; Jia, M.; Zhang, H. Forsythiasides: A review of the pharmacological effects. Front. Cardiovasc. Med. 2022, 9, 971491. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Bigg, H.F.; Rowan, A.D.; Barker, M.D.; Cawston, T.E. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J. 2007, 274, 1246–1255. [Google Scholar] [CrossRef]
- Kähäri, V.M.; Saarialho-Kere, U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]



| TPC (mg GAE/g Extract) | ||
| Deuterium-depleted water | Dimethyl sulfoxide | |
| LF | 350.48 ± 4.59 | 1340 ± 2.19 |
| TFC (mg QE/g Extract) | ||
| Deuterium-depleted water | Dimethyl sulfoxide | |
| LF | 313.2 ± 7.09 | 144.2 ± 2.03 |
| NCBI Accession Code | Primera | Sequence (5-3) | |
|---|---|---|---|
| NM_001145938.2 | Human MMP-1 | Sense | TGC GCA CAA ATC CCT TCT AC |
| Antisense | TTC AAG CCC ATT TGG CAG TT | ||
| NM_004530.5 | Human MMP-2 | Sense | CCC GAG GTT GGA CCT ACA AG |
| Antisense | CTT CCC CGT CAC CTC CAA TC | ||
| NM_002422.5 | Human MMP-3 | Sense | CCC GAG GTT GGA CCT ACA AG |
| Antisense | CTT CCC CGT CAC CTC CAA TC | ||
| NM_004994.3 | Human MMP-9 | Sense | TGT ACC GCT ATG GTT ACA CT |
| Antisense | GGC AGG GAC AGT TGC TTC AG | ||
| XM_011526432.2 | Human GAPDH | Sense | ACC ACA GTC CAT GCC ATC AC |
| Antisense | CCA CCA CCC TGT TGC TGT AG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Zheng, Q.; Nguyen, T.T.M.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Lithospermum erythrorhizon and Forsythia suspensa Prevent Collagen Degradation and Maintain Skin Hydration by Regulating MMPs and HAS2/HYAL1 Signaling. Molecules 2025, 30, 1083. https://doi.org/10.3390/molecules30051083
Jin X, Zheng Q, Nguyen TTM, Park S-J, Yi G-S, Yang S-J, Yi T-H. Lithospermum erythrorhizon and Forsythia suspensa Prevent Collagen Degradation and Maintain Skin Hydration by Regulating MMPs and HAS2/HYAL1 Signaling. Molecules. 2025; 30(5):1083. https://doi.org/10.3390/molecules30051083
Chicago/Turabian StyleJin, Xiangji, Qiwen Zheng, Trang Thi Minh Nguyen, Se-Jig Park, Gyeong-Seon Yi, Su-Jin Yang, and Tae-Hoo Yi. 2025. "Lithospermum erythrorhizon and Forsythia suspensa Prevent Collagen Degradation and Maintain Skin Hydration by Regulating MMPs and HAS2/HYAL1 Signaling" Molecules 30, no. 5: 1083. https://doi.org/10.3390/molecules30051083
APA StyleJin, X., Zheng, Q., Nguyen, T. T. M., Park, S.-J., Yi, G.-S., Yang, S.-J., & Yi, T.-H. (2025). Lithospermum erythrorhizon and Forsythia suspensa Prevent Collagen Degradation and Maintain Skin Hydration by Regulating MMPs and HAS2/HYAL1 Signaling. Molecules, 30(5), 1083. https://doi.org/10.3390/molecules30051083









