The Incorporation of Nanoconfined Poly(ionic liquid)s with Two-Dimensional Covalent Organic Frameworks to Enhance Proton Conduction
Abstract
1. Introduction
2. Results and Discussion
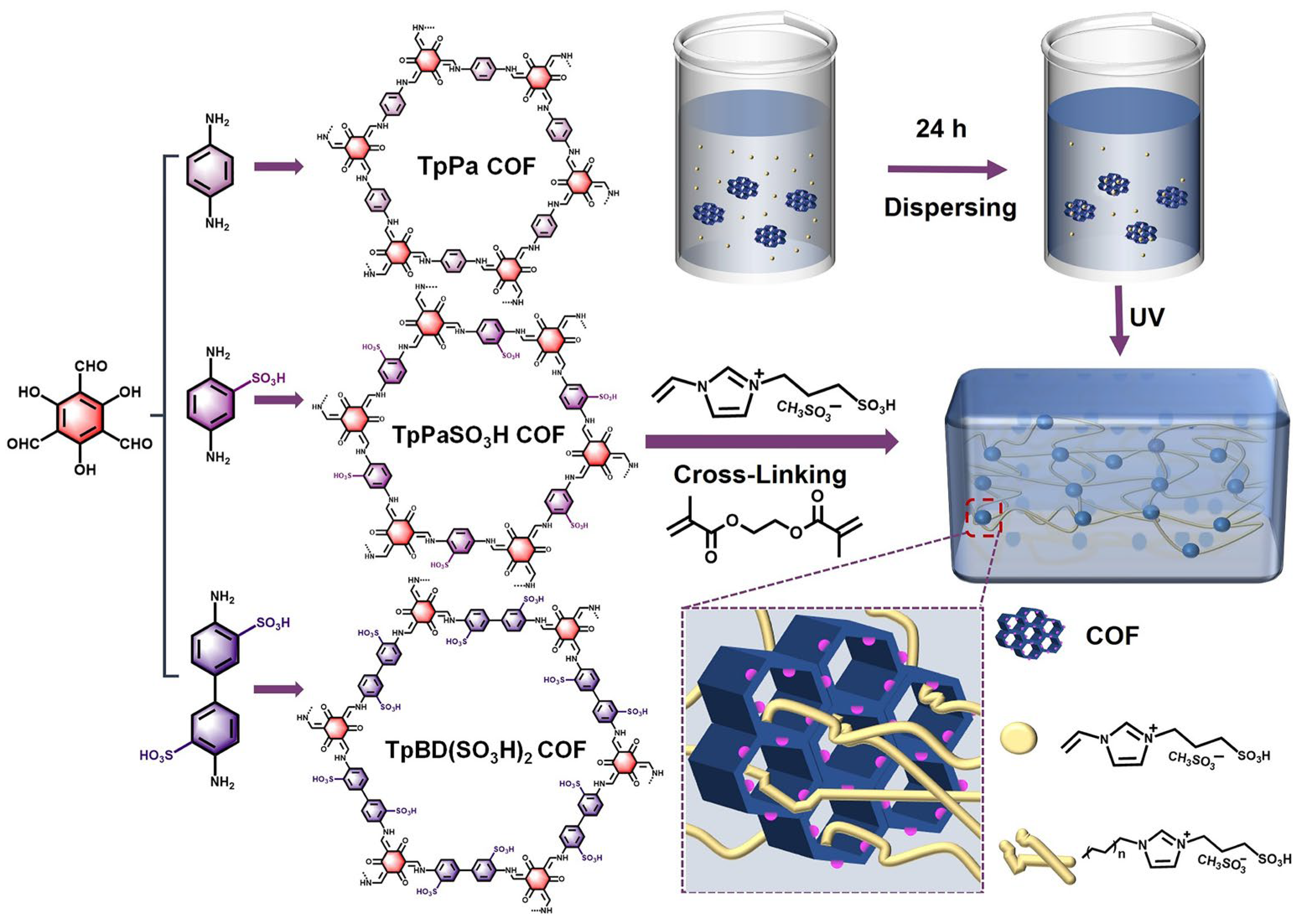
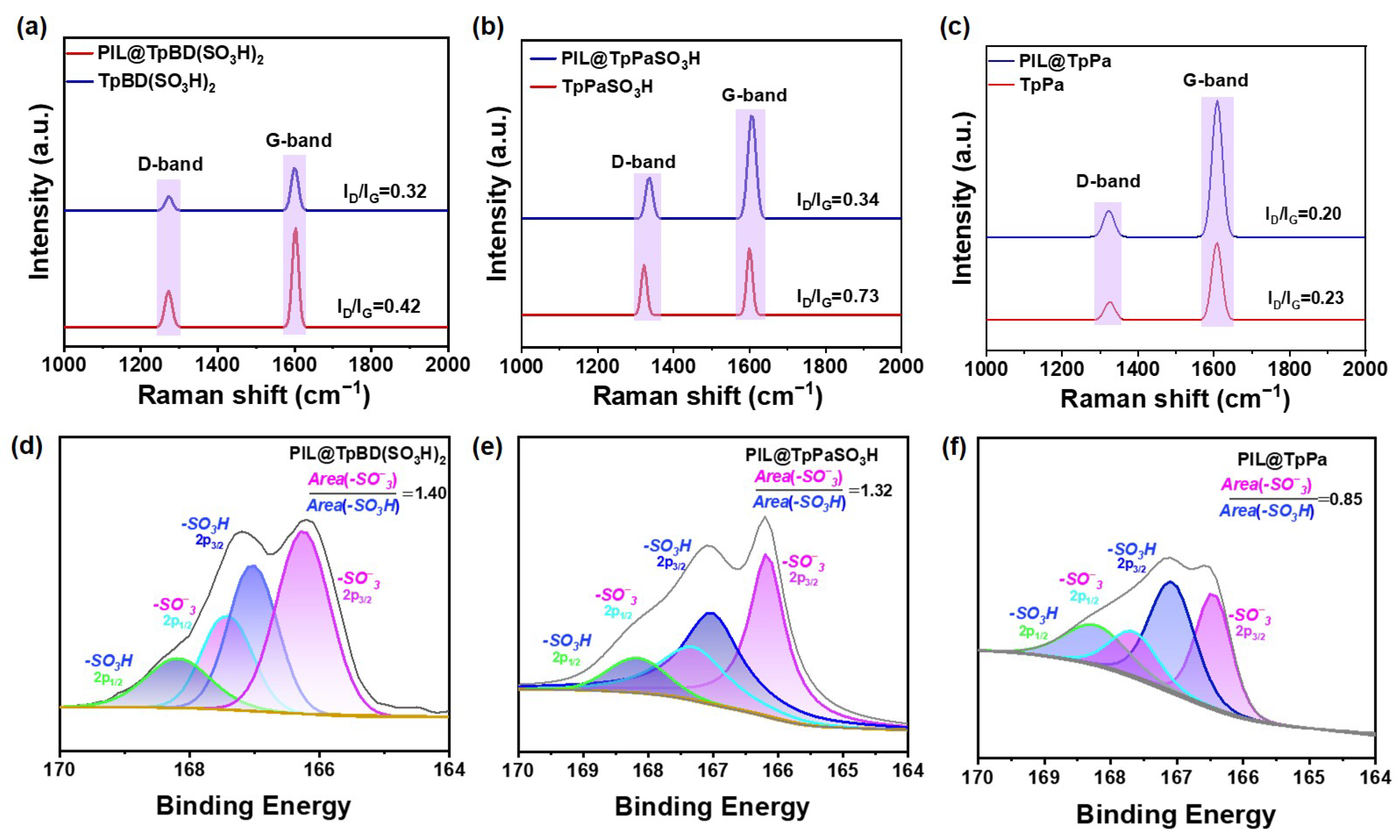
2.1. Preparation and Characterization of PIL@COF Composite Membranes
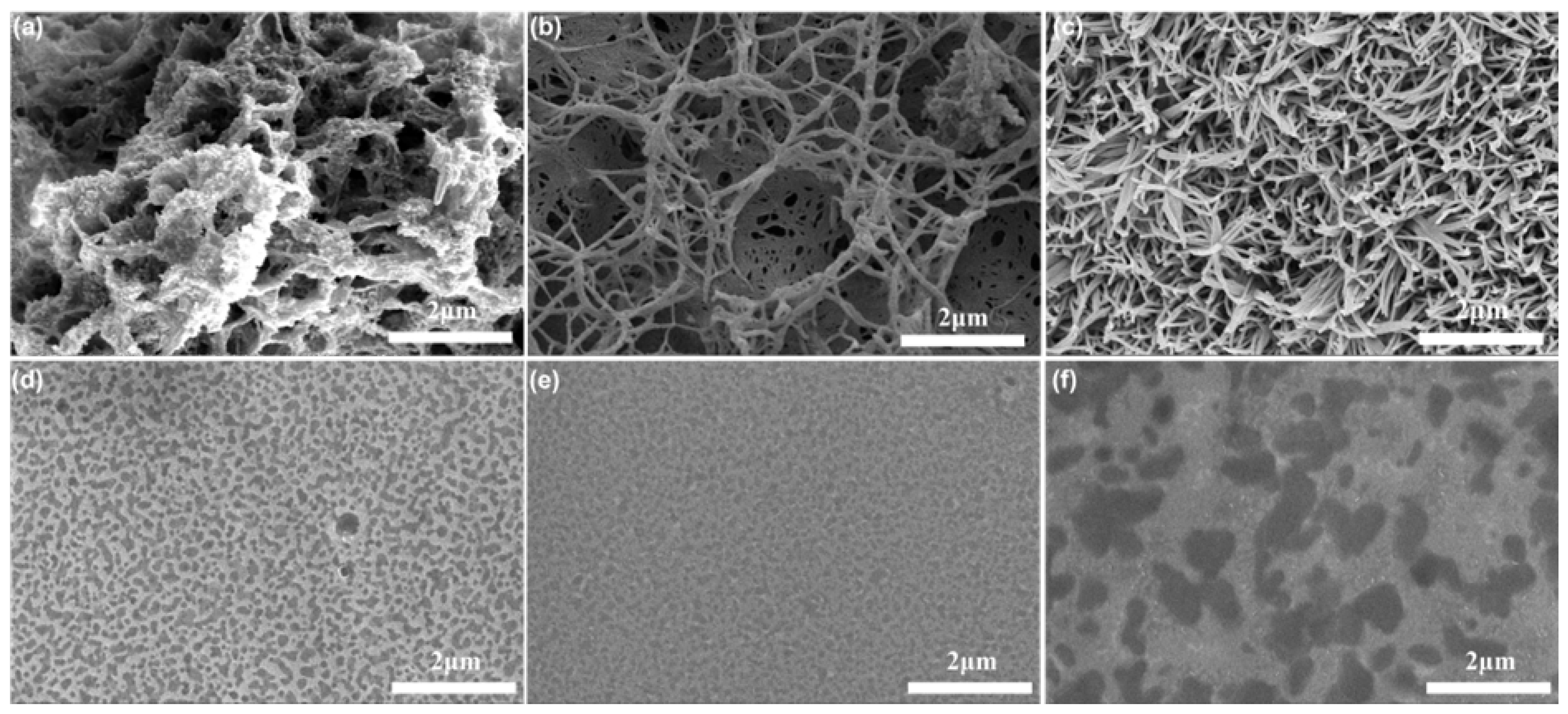
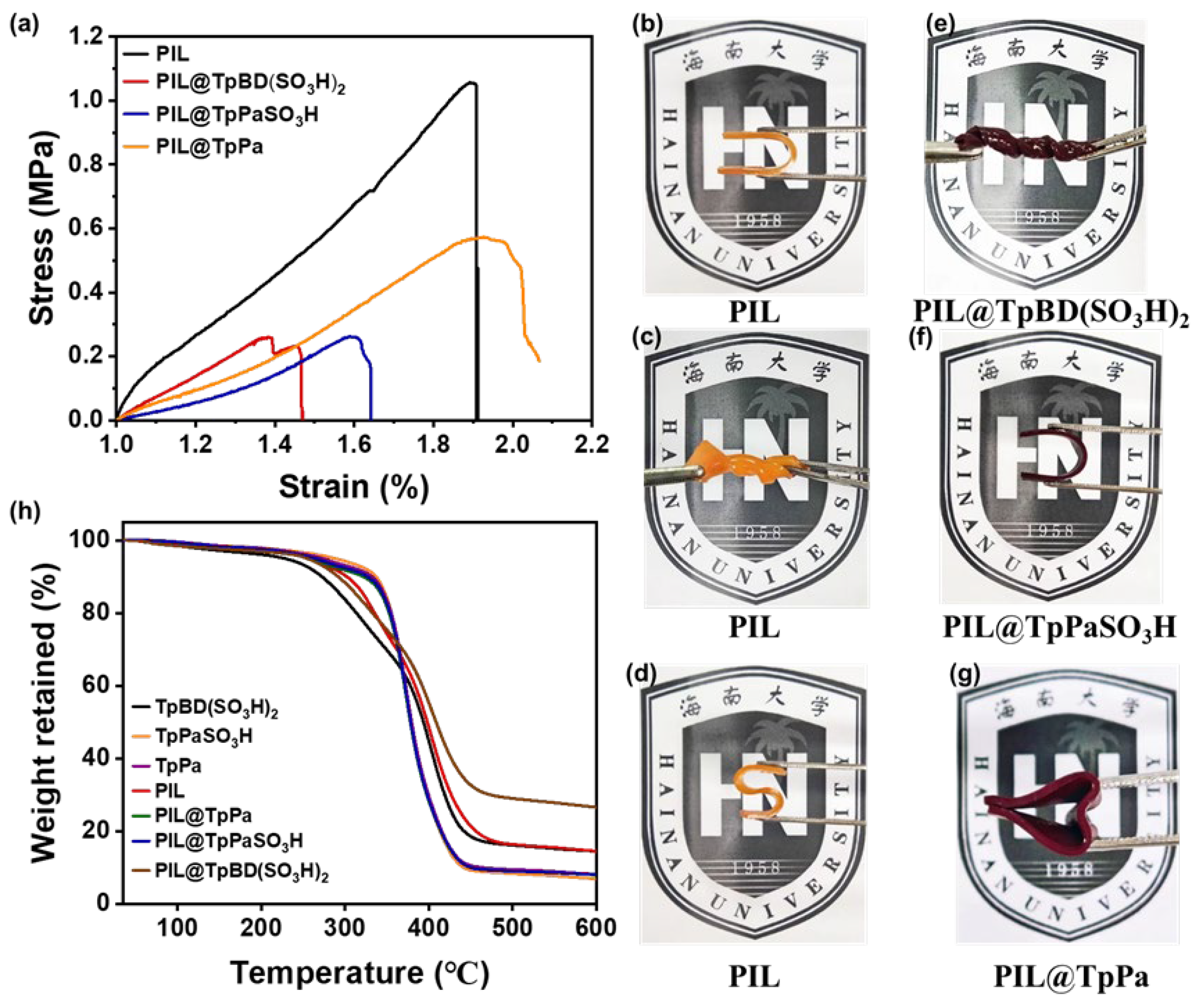
2.2. Morphology, Mechanical Properties, and Thermal Stability of PIL@COF Composite Membranes
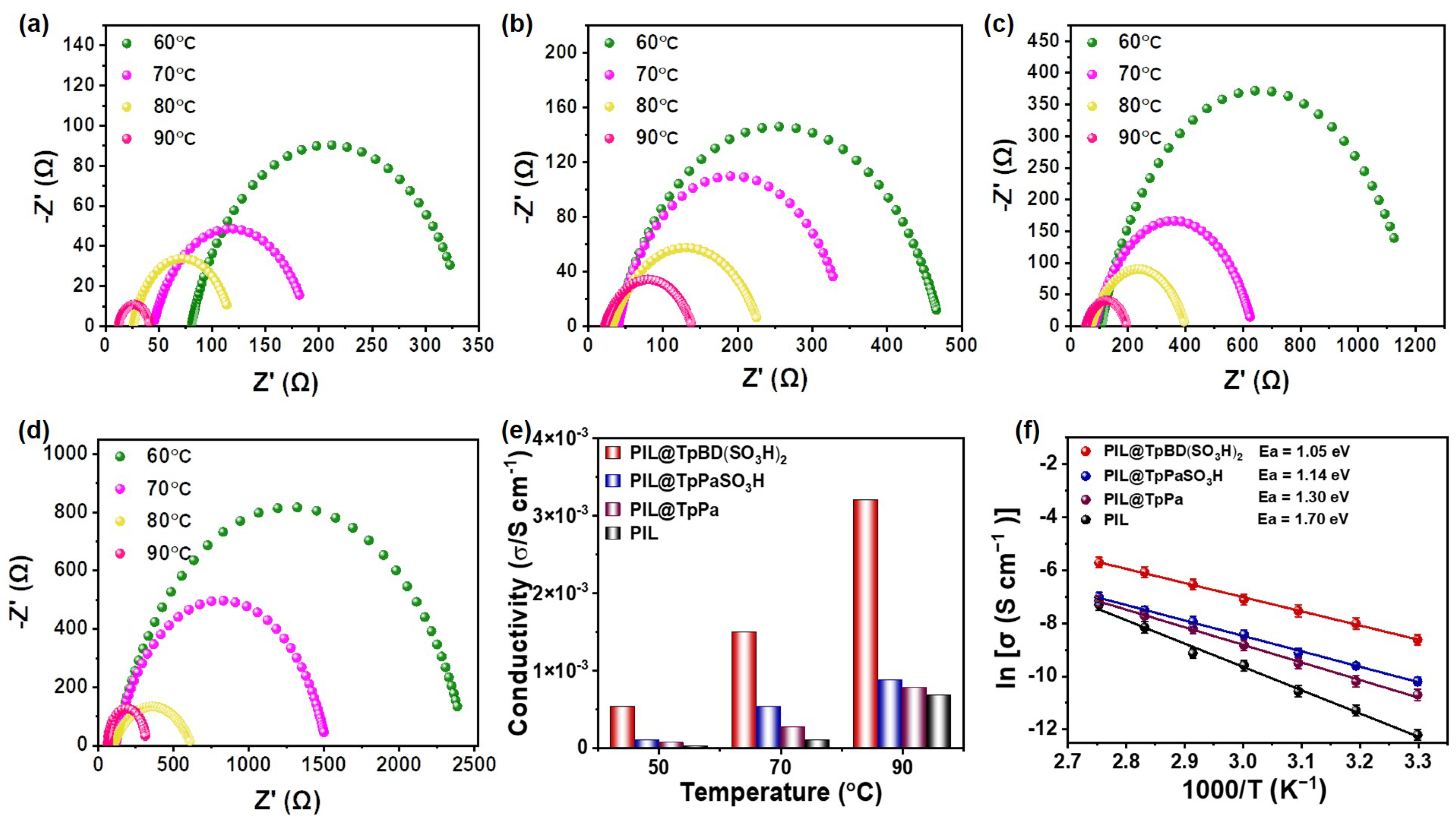
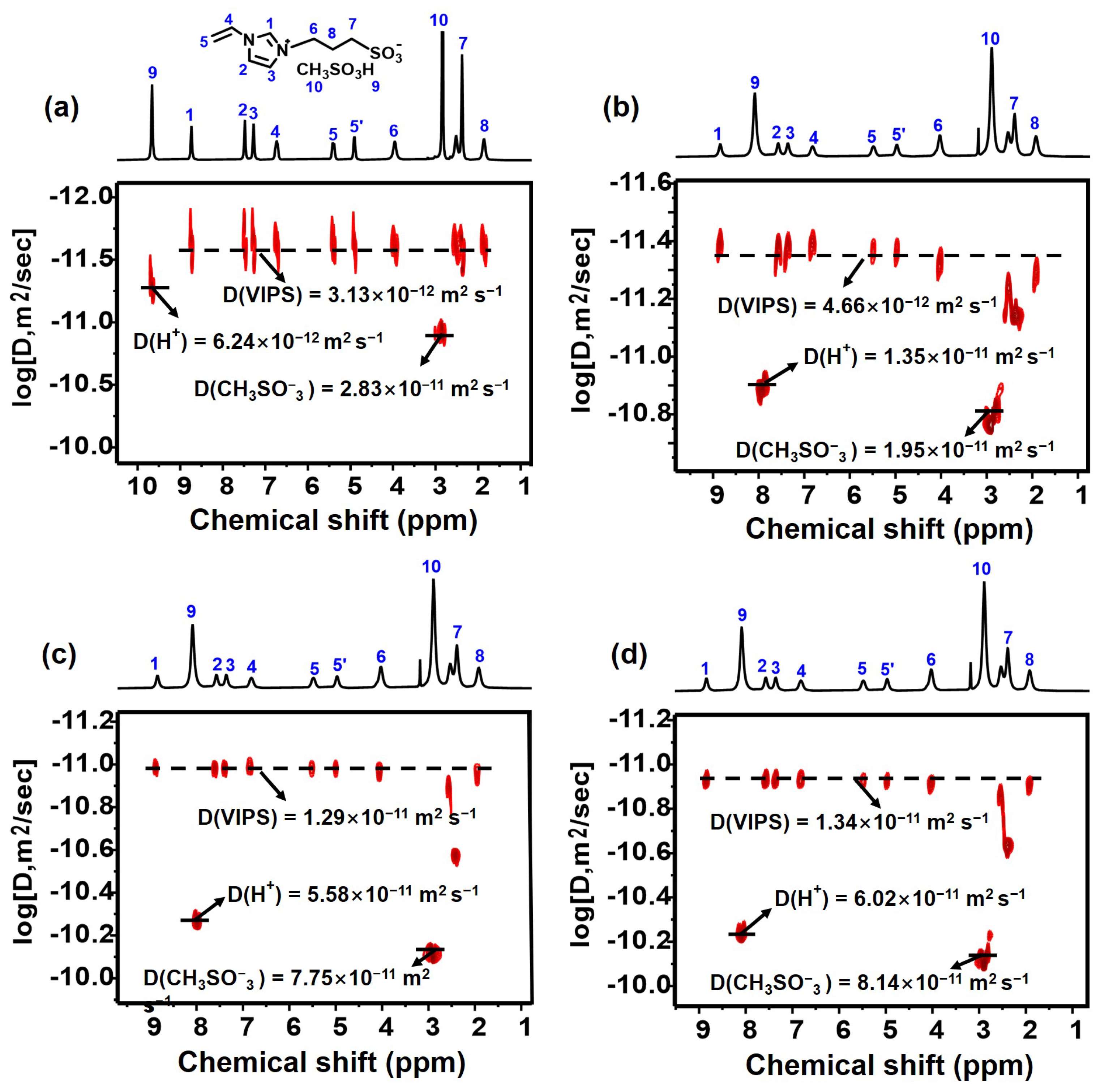
2.3. Proton Conduction Properties of PIL@COF Composite Membranes
3. Materials and Methods
3.1. Materials
3.2. Synthesis of 2,4,6-Triformylphloroglucinol (TFP)
3.3. Synthesis of 1-vinyl-3-(3-Sulfopropyl)-imidazolium Methanesulfonate
3.4. Synthesis of TpPa COF, TpPaSO3H COF and TpBD(SO3H)2 COF
3.4.1. Synthesis of TpBD (SO3H)2 COF
3.4.2. Synthesis of TpPaSO3H COF
3.4.3. Synthesis of TpPa COF
3.5. Production of PIL, PIL@TpPa, PIL@TpPaSO3H, and PIL@TpBD(SO3H)2 Composite Membranes
3.6. Proton Conductivity Measurements
3.7. Diffusion Coefficient Measurements
3.8. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Zhao, X.; Zhang, T.; Pei, P. The reactant starvation of the proton exchange membrane fuel cells for vehicular applications: A review. Energy Convers. Manag. 2019, 182, 282–298. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, Z.; Tao, W.-Q.; Wang, X.; Wu, L.; Wu, S.; Xie, X.; Tongsh, C.; Huo, W.; Bao, Z.; et al. Porous Flow Field for Next-Generation Proton Exchange Membrane Fuel Cells: Materials, Characterization, Design, and Challenges. Chem. Rev. 2023, 123, 989–1039. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the Air and Improving Health with Hydrogen Fuel-Cell Vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.-Y.; Li, X.-M.; Zhao, F.; Bai, X.-T.; Cao, L.-H. Enhanced proton conduction of crystalline organic salt hybrid membranes and the performance of fuel cells. Mater. Chem. Front. 2022, 6, 3402–3408. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in hybrid composite Nafion®-based membranes for proton exchange fuel cell application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Zeng, L.; Lu, X.; Yuan, C.; Yuan, W.; Chen, K.; Guo, J.; Zhang, X.; Wang, J.; Liao, Q.; Wei, Z. Self-Enhancement of Perfluorinated Sulfonic Acid Proton Exchange Membrane with Its Own Nanofibers. Adv. Mater. 2024, 36, 2305711. [Google Scholar] [CrossRef] [PubMed]
- Berber, M.R.; Hafez, I.H. Boosting the proton conductivity, chemical stability, and fuel cell performance of nafion membrane at high operating temperatures and low humidity levels by incorporating phytic acid. Int. J. Hydrogen Energy 2024, 57, 1126–1138. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Deng, Q.; Chen, W.; Zhang, Y.; Zhou, Y.; Zou, Z. Recent Progress of Amorphous Porous Organic Polymers as Heterogeneous Photocatalysts for Organic Synthesis. Adv. Funct. Mater. 2023, 33, 2307179. [Google Scholar] [CrossRef]
- Meng, X.; Wang, H.-N.; Song, S.-Y.; Zhang, H.-J. Proton-conducting crystalline porous materials. Chem. Soc. Rev. 2017, 46, 464–480. [Google Scholar] [CrossRef]
- Yoon, S.; Talin, A.A.; Stavila, V.; Mroz, A.M.; Bennett, T.D.; He, Y.; Keen, D.A.; Hendon, C.H.; Allendorf, M.D.; So, M.C. From n- to p-Type Material: Effect of Metal Ion on Charge Transport in Metal–Organic Materials. ACS Appl. Mater. Interfaces 2021, 13, 52055–52062. [Google Scholar] [CrossRef]
- Chai, M.; Chen, R.; Xu, K.; Chen, Y.; Ma, S.; Lin, R.; Chen, V.; Hou, J. Ion transport and conduction in metal–organic framework glasses. J. Mater. Chem. A 2023, 11, 20302–20314. [Google Scholar] [CrossRef]
- Liang, X.; Tian, Y.; Yuan, Y.; Kim, Y. Ionic Covalent Organic Frameworks for Energy Devices. Adv. Mater. 2021, 33, 2105647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, H.; Wang, L.; Lei, J.; Liu, J. Efficient proton conduction in porous and crystalline covalent-organic frameworks (COFs). J. Energy Chem. 2023, 82, 198–218. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.; Zhang, P.; Andaloussi, Y.H.; Zhang, H.; Jiang, Z.; Chen, Y.; Ma, S.; Cheng, P.; Zhang, Z. Combined Intrinsic and Extrinsic Proton Conduction in Robust Covalent Organic Frameworks for Hydrogen Fuel Cell Applications. Angew. Chem. Int. Ed. 2020, 59, 3678–3684. [Google Scholar] [CrossRef]
- Liu, L.; Yin, L.; Cheng, D.; Zhao, S.; Zang, H.-Y.; Zhang, N.; Zhu, G. Surface-Mediated Construction of an Ultrathin Free-Standing Covalent Organic Framework Membrane for Efficient Proton Conduction. Angew. Chem. Int. Ed. 2021, 60, 14875–14880. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, Y. Continuous Ultrathin Zwitterionic Covalent Organic Framework Membrane Via Surface-Initiated Polymerization Toward Superior Water Retention. Small 2024, 20, 2308499. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hong, Y.-L.; Xu, B.; Nishiyama, Y.; Jiang, W.; Zhu, J.; Zhang, G.; Kitagawa, S.; Horike, S. Perfluoroalkyl-Functionalized Covalent Organic Frameworks with Superhydrophobicity for Anhydrous Proton Conduction. J. Am. Chem. Soc. 2020, 142, 14357–14364. [Google Scholar] [CrossRef]
- Chandra, S.; Kundu, T.; Kandambeth, S.; BabaRao, R.; Marathe, Y.; Kunjir, S.M.; Banerjee, R. Phosphoric Acid Loaded Azo (−N═N−) Based Covalent Organic Framework for Proton Conduction. J. Am. Chem. Soc. 2014, 136, 6570–6573. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Y.-R.; Liu, Y.; Luo, H.-B.; Zou, Y.; Zang, S.-Q.; Ren, X.-M. Superprotonic Conduction of Acidified Benzimidazole-Linked Covalent Organic Framework. ACS Mater. Lett. 2022, 4, 2597–2603. [Google Scholar] [CrossRef]
- Sasmal, H.S.; Aiyappa, H.B.; Bhange, S.N.; Karak, S.; Halder, A.; Kurungot, S.; Banerjee, R. Superprotonic Conductivity in Flexible Porous Covalent Organic Framework Membranes. Angew. Chem. Int. Ed. 2018, 57, 10894–10898. [Google Scholar] [CrossRef]
- Cao, L.; Wu, H.; Cao, Y.; Fan, C.; Zhao, R.; He, X.; Yang, P.; Shi, B.; You, X.; Jiang, Z. Weakly Humidity-Dependent Proton-Conducting COF Membranes. Adv. Mater. 2020, 32, 2005565. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.; Ren, Y.; Lei, J.; Wang, L.; Liu, J. Subnanometer Nanowire-Reinforced Construction of COF-Based Membranes with Engineering Biomimetic Texture for Efficient and Stable Proton Conduction. Adv. Funct. Mater. 2023, 34, 2313844. [Google Scholar] [CrossRef]
- Tao, S.; Zhai, L.; Wonanke, A.D.D.; Addicoat, M.A.; Jiang, Q.; Jiang, D. Confining H3PO4 network in covalent organic frameworks enables proton super flow. Nat. Commun. 2020, 11, 1981. [Google Scholar] [CrossRef]
- Avid, A.; Ochoa, J.L.; Huang, Y.; Liu, Y.; Atanassov, P.; Zenyuk, I.V. Revealing the role of ionic liquids in promoting fuel cell catalysts reactivity and durability. Nat. Commun. 2022, 13, 6349. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Stettner, T.; Balducci, A. Protic ionic liquids in energy storage devices: Past, present and future perspective. Energy Storage Mater. 2021, 40, 402–414. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Gao, X.; Sun, P.; Zheng, L. Nanostructured proton-conducting membranes based on polymerizable zwitterionic ionic liquid microemulsions. New J. Chem. 2016, 40, 7580–7586. [Google Scholar] [CrossRef]
- Wu, B.; Kuroda, K.; Takahashi, K.; Castner, E.W., Jr. Structural analysis of zwitterionic liquids vs. homologous ionic liquids. J. Chem. Phys. 2018, 148, 193807. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lu, F.; Dong, B.; Zhou, T.; Liu, Y.; Zheng, L. Temperature-responsive proton-conductive liquid crystals formed by the self-assembly of zwitterionic ionic liquids. RSC Adv. 2015, 5, 63732–63737. [Google Scholar] [CrossRef]
- Smith, D.E.; Walsh, D.A. The Nature of Proton Shuttling in Protic Ionic Liquid Fuel Cells. Adv. Energy Mater. 2019, 9, 1900744. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, D.; Xu, Y.; Lan, M.; Jiang, L.; Wang, Z.; Tang, B.; Liu, H. Enhanced Proton Transfer in Proton-Exchange Membranes with Interconnected and Zwitterion-Functionalized Covalent Porous Material Structures. ChemSusChem 2023, 16, e202202279. [Google Scholar] [CrossRef] [PubMed]
- Codescu, M.-A.; Kunze, T.; Weiß, M.; Brehm, M.; Kornilov, O.; Sebastiani, D.; Nibbering, E.T.J. Ultrafast Proton Transfer Pathways Mediated by Amphoteric Imidazole. J. Phys. Chem. Lett. 2023, 14, 4775–4785. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef]
- Kalikin, N.N.; Kolesnikov, A.L.; Budkov, Y.A. Polymerized ionic liquids on charged electrodes: New prospects for electrochemistry. Curr. Opin. Electrochem. 2022, 36, 101134. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, L.; Zhang, W.; Wang, H. Large-Scale and Controllable Syntheses of Covalently-Crosslinked Poly(ionic liquid) Nanoporous Membranes. Angew. Chem. Int. Ed. 2023, 62, e202302168. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, H.; Douglin, J.C.; Chen, Y.; Yin, S.; Zhang, J.; Deng, X.; Wu, H.; Yin, Y.; Dekel, D.R.; et al. Single Solution-Phase Synthesis of Charged Covalent Organic Framework Nanosheets with High Volume Yield. Angew. Chem. Int. Ed. 2023, 62, e202209306. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Tang, S. Ionic liquid-impregnated covalent organic framework/silk nanofibril composite membrane for efficient proton conduction. Chem. Eng. J. 2021, 415, 129021. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zhou, M.; Zhou, J.; Liu, X.; Yu, X.; Gao, J.; Liang, E.; Chen, X.; Zhang, Y.; et al. N-doping of β-ketoenamine based covalent organic frameworks for catalytic conversion of CO2 to cyclic carbonate and Knoevenagel condensation. Microporous Mesoporous Mater. 2024, 364, 112872. [Google Scholar] [CrossRef]
- Guo, Y.; Zou, X.; Li, W.; Hu, Y.; Jin, Z.; Sun, Z.; Gong, S.; Guo, S.; Yan, F. High-density sulfonic acid-grafted covalent organic frameworks with efficient anhydrous proton conduction. J. Mater. Chem. A 2022, 10, 6499–6507. [Google Scholar] [CrossRef]
- Chang, X.; Geng, Y.; Cao, H.; Zhou, J.; Tian, Y.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Dual-Crosslink Physical Hydrogels with High Toughness Based on Synergistic Hydrogen Bonding and Hydrophobic Interactions. Macromol. Rapid Commun. 2018, 39, 1700806. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, K.; Zuo, Y.; Chen, X.; Xiong, Y.; Yuan, Y. Supramolecular assembly of poly(ionic liquid) nanogel driven by host-stabilized charge transfer interaction. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2251–2259. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Liu, Z.; Zheng, S.; Li, Q.; Yan, F. Supramolecular Ionogels Tougher than Metals. Adv. Mater. 2023, 35, 2301383. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, K.; Wan, X.; Wang, S.; Fan, S.; Ye, Z.; Wang, Y.; Yan, Y.; Peng, X. Stable Two-dimensional Nanoconfined Ionic Liquids with Highly Efficient Ionic Conductivity. Small 2022, 18, 2108026. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liang, X.; Wang, M.; Wang, J.; Gao, Y.; Lu, F. The Incorporation of Nanoconfined Poly(ionic liquid)s with Two-Dimensional Covalent Organic Frameworks to Enhance Proton Conduction. Molecules 2025, 30, 1004. https://doi.org/10.3390/molecules30051004
Wang Y, Liang X, Wang M, Wang J, Gao Y, Lu F. The Incorporation of Nanoconfined Poly(ionic liquid)s with Two-Dimensional Covalent Organic Frameworks to Enhance Proton Conduction. Molecules. 2025; 30(5):1004. https://doi.org/10.3390/molecules30051004
Chicago/Turabian StyleWang, Yonghong, Xiaoxiao Liang, Ming Wang, Jiahui Wang, Yanan Gao, and Fei Lu. 2025. "The Incorporation of Nanoconfined Poly(ionic liquid)s with Two-Dimensional Covalent Organic Frameworks to Enhance Proton Conduction" Molecules 30, no. 5: 1004. https://doi.org/10.3390/molecules30051004
APA StyleWang, Y., Liang, X., Wang, M., Wang, J., Gao, Y., & Lu, F. (2025). The Incorporation of Nanoconfined Poly(ionic liquid)s with Two-Dimensional Covalent Organic Frameworks to Enhance Proton Conduction. Molecules, 30(5), 1004. https://doi.org/10.3390/molecules30051004







