Reaction Behavior and Kinetic Model of Hydroisomerization and Hydroaromatization of Fluid Catalytic Cracking Gasoline
Abstract
1. Introduction
2. Results and Discussion
2.1. Reaction Behavior of Hydroisomerization and Aromatization of FCC Gasoline
2.1.1. Reaction Behavior of Olefin Model Compound
2.1.2. Reaction Behavior of FCC Middle Gasoline
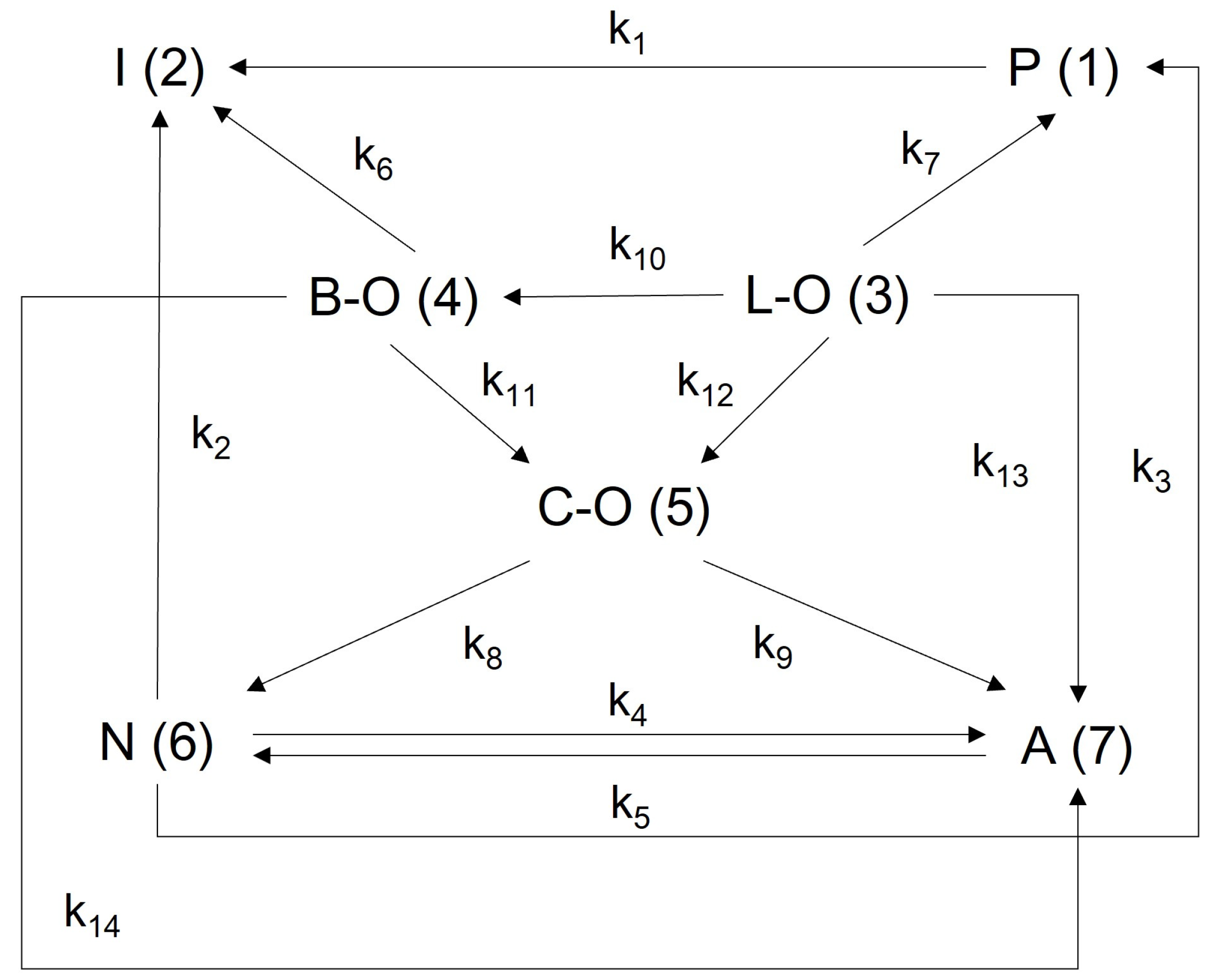
2.2. Lumped Kinetic Model for FCC Middle Gasoline Hydro-Upgrading
2.2.1. Establishment of a Lumped Reaction Kinetics Model
2.2.2. Calculation and Analysis of Kinetic Parameters
2.3. Validation of the Kinetics Model
3. Experimental
3.1. Materials and Chemicals
3.2. Experimental Apparatus and Catalyst
3.3. Feedstock and Product Analysis Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, T.; Li, W.; Chang, X.; Xiang, Y.; Bao, X. Advances in fluid catalytic cracking naphtha cleaning technology. Chem. Ind. Eng. Prog. 2019, 38, 196–207. [Google Scholar]
- Zhang, R.; Li, T.; Zhang, Y.; Hu, Y.; Zhao, Q.; Ju, Y.; Ge, S. Advances in processes and catalysts for reducing olefin content of FCC gasoline. Ind. Catal. 2019, 27, 12–19. [Google Scholar]
- Li, T.; Li, J.; Ren, K.; Zhu, P.; Wang, Y. Characteristics of FCC gasoline hydrogenation technology M-PHG and its application. Pet. Process Petrochem. 2020, 51, 98–101. [Google Scholar]
- Ju, Y.; Mei, J.; Lan, L.; Zhao, Q.; Zhong, H.; Ge, S. Commercial application of selective hydrodesulfurization technology for fluid catalytic cracking gasoline hydro-upgrading. Pet. Tech. Appl. 2019, 37, 112–115. [Google Scholar]
- Wen, B.; Gao, H.; Li, Y.; Tang, S.; Wang, D. Commercial application of GARDES-II external fully-vulcanized state catalyst in FCC gasoline hydrodesulfurization unit. Mod. Chem. Ind. 2021, 4, 214–217. [Google Scholar]
- Zhang, Y.; Xiang, Y.; Wang, T.; Yao, W.; Li, J.; Chang, X.; Gao, H.; Wang, G. Development and application of FCC gasoline hydroupgrading technology GARDES-II. Pet. Process Petrochem. 2021, 52, 38–44. [Google Scholar]
- Yu, M.; Pan, T.; Ju, Y.; Zhang, R.; Wu, P.; Wu, Z. Effect of Zeolite Crystal Size on the Catalytic Performance of Zn/ZSM-5Zeolite for Olefin Conversion. Acta Petrol. Sin. Pet. Process Sect. 2024, 40, 23–36. [Google Scholar]
- Song, S.; Li, T.; Ju, Y.; Li, Y.; Lv, Z.; Zhang, P.; Duan, A.-J.; Wu, P.; Wang, X. Lanthanum/Gallium-Modified Zn/ZSM-5 Zeolite for Efficient Isomerization/Aromatization of FCC Light Gasoline. Ind. Eng. Chem. Res. 2022, 61, 9667–9677. [Google Scholar] [CrossRef]
- Zhang, R.; Ju, Y.; Wu, P.; Chen, J.; Lv, Z.; Zhang, Y.; Song, S.; Zhang, Z.; Ma, C.; Zhang, R.; et al. Efficiently reducing olefin content of FCC gasoline over ZSM-5 zeolite based catalyst via hydro-upgrading. Catal. Today 2022, 405, 57–65. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Z.; Zhou, K. In-situ incorporating Zn into hierarchical ZSM-5 zeolites for olefin hydroisomerization. Ind. Eng. Chem. Res. 2020, 59, 12371–12380. [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.; Nie, H.; Shi, Y.; Li, D. Study and Application of Hydrocarbon Isomerization. Sci. Tech. Rev. 2005, 23, 46–51. [Google Scholar]
- Gao, D.; Cao, L.; Zhi, Y.; Zhao, L.; Gao, J.; Xu, C. Study of Metal Modified Zeolites as Highly Efficient Light Olefin Aromatization Catalysts. Chem. React. Eng. Technol. 2022, 38, 362–374. [Google Scholar]
- Long, H.; Jin, F.; Xiong, G.; Wang, X. Effect of lanthanum and phosphorus on the aromatization activity of Zn/ZSM-5 in FCC gasoline upgrading. Micropor. Mesopor. Mater. 2014, 198, 29–34. [Google Scholar] [CrossRef]
- Lu, Q.; Lin, X.; Wang, L.; Gao, J.; Bao, X. On-stream stability enhancement of HZSM-5 based fluid catalytic cracking naphtha hydro-upgrading catalyst via magnesium modification. Catal. Commun. 2016, 83, 31–34. [Google Scholar] [CrossRef]
- Gao, D.; Zhi, Y.; Cao, L.; Zhao, L.; Gao, J.; Xu, X. Optimizing the Acid Properties of the HZSM-5 Catalyst for Increasing the p-Xylene Yield in 1-Hexene Aromatization. Ind. Eng. Chem. Res. 2022, 61, 3539–3549. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Zhou, K.; Ju, Y.; Li, T.; Ge, S. Synthesin, Modification and Catalytic Properties of Aggregated ZSM-5 Zeolite Nanocrystals for Olefin Conversion. Acta Petrol. Sin. Pet. Process Sect. 2020, 36, 899–908. [Google Scholar]
- Gao, D.; Zhi, Y.; Cao, L.; Zhao, L.; Gao, J.; Xu, C.; Ma, M.; Hao, P. Influence of zinc state on the catalyst properties of Zn/HZSM-5 zeolite in 1-hexene aromatization and cyclohexane dehydrogenation. Chin. J. Chem. Eng. 2022, 43, 124–134. [Google Scholar] [CrossRef]
- Pan, T.; Ge, S.; Yu, M.; Ju, Y.; Zhang, R.; Wu, P.; Zhou, K.; Wu, Z. Synthesis and consequence of Zn modified ZSM-5 zeolite supported Ni catalyst for catalytic aromatization of olefin/paraffin. Fuel 2022, 311, 122629. [Google Scholar] [CrossRef]
- Huang, G.; Sun, Z.; Liu, Y.; Yu, Z.; Wang, Y.; Liu, J.; Wang, A.; Hu, Y. Bifunctional Ni2P/SAPO-11 catalyst for simultaneous hydroisomerization of 1-hexene and hydrodesulfurization of thiophene. Chem. Eng. Sci. 2025, 301, 120714. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Ling, L.; Zhang, Y.; Zhang, R.; Shen, X.; Li, X.; Wang, B. The active site for dehydrogenation and cyclization on Zn2+/HZSM-5 catalyst aiming at long-chain C6 mono-olefins aromatization. Fuel 2024, 366, 131362. [Google Scholar] [CrossRef]
- Wei, C.; Gao, D.; Zhang, G.; Zhao, L.; Gao, J.; Xu, C. Effect of mesopore spatial distribution of HZSM-5 catalyst on zinc state and product distribution in 1-hexene aromatization. Chin. J. Chem. Eng. 2024, 67, 16–26. [Google Scholar] [CrossRef]
- Fan, Y.; Yin, J.; Shi, G.; Liu, H.; Bao, X. Mechanistic Pathways for Olefin Hydroisomerization and Aromatization in Fluid Catalytic Cracking Gasoline Hydro-upgrading. Energy Fuels 2009, 23, 3016–3023. [Google Scholar] [CrossRef]
- Fan, Y.; Yin, J.; Shi, G.; Liu, H.; Gao, J.; Bao, X. A Six-Lump Kinetic Model for Olefin Hydrogenation, Hydroisomerization and Aromatization in FCC Gasoline Hydro-Upgrading. Catal. Lett. 2009, 129, 181–188. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, X.; Qi, Y.; Liu, S. Study on Kinetics and Reaction Mechanism of FCC Gasoline Hydroisomerization over Pt/HZSM-5 Dual Functional Catalyst. J. Mol. Catal. 2003, 17, 111–117. [Google Scholar]
- Chen, Z.; Xu, J.; Shi, G.; Fan, Y.; Bao, X. Lumping Kinetic Model for Fluid Catalytic Cracking Gasoline Hydroisomerization and Aromatization. Chem. React. Eng. Technol. 2013, 29, 510–518. [Google Scholar]
- Ouyang, F.; Shen, J.; Wang, Y.; Xu, Q. Study on the Lumped Kinetic Model for FCC Gasoline Hydro-Upgrading Process. J. Chem. Eng. Chin. Univ. 2018, 32, 124–130. [Google Scholar]
- Xiang, Y.; Shen, J.; Ouyang, F. A Lumped Kinetic Model of M-DSO Process for Fluid Catalytic Cracking Gasoline Hydro-Upgrading. Pet. Chem. 2021, 61, 465–471. [Google Scholar] [CrossRef]
- Li, D.; Li, M.; Chu, Y.; Nie, H.; Shi, Y. Skeletal isomerization of light FCC naphtha. Catal. Today 2003, 81, 65–73. [Google Scholar] [CrossRef]
- Abbot, J.; Wojciechowski, B.W. The Mechanism of Catalytic Cracking of n-Alkenes on ZSM-5 Zeolite. Can. J. Chem. Eng. 1985, 63, 462–469. [Google Scholar] [CrossRef]
- Abbot, J.; Wojciechowski, B.W. The Catalytic Cracking and Skeletal Isomerization of n-Hexene on ZSM-5 Zeolite. Can. J. Chem. Eng. 1985, 63, 451–461. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, J.; Bao, X. Studies on the reaction mechanism of light olefin isomerization and aromatization. Chem. Ind. Eng. Prog. 2015, 34, 617–623. [Google Scholar]
- Huang, X.; Aihemaitijiang, D.; Xiao, W. Reaction pathway and kinetics of C3-C7 olefin transformation over high-silicon HZSM-5 zeolite at 400-490 °C. Chem. Eng. J. 2015, 280, 222–232. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Wang, Z. Lumps and kinetics for the secondary reactions in catalytically cracked gasolin. Chem. Eng. J. 2005, 109, 1–9. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]

| Reaction Temperature and LHSV | Conversion (%) | ≤C3 in Product (wt%) | Group Composition in Product (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | I | L-O | B-O | C-O | N | A | |||
| 300 °C + 1.5 h−1 | 100 | 1.89 | 10.28 | 10.73 | 16.42 | 60.77 | 0.07 | 1.73 | 0 |
| 340 °C + 1.5 h−1 | 100 | 7.66 | 27.05 | 42.99 | 0.66 | 4.63 | 5.80 | 5.28 | 13.59 |
| 380 °C + 1.5 h−1 | 100 | 14.18 | 29.98 | 39.18 | 0.54 | 0.76 | 0.03 | 3.86 | 25.65 |
| 340 °C + 3.0 h−1 | 100 | 3.95 | 13.91 | 27.07 | 5.19 | 28.13 | 9.31 | 12.00 | 4.39 |
| Carbon Number | Group Composition in Product (wt%) (T = 300 °C) | Group Composition in Product (wt%) (T = 380 °C) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | I | L-O | B-O | C-O | N | A | P | I | L-O | B-O | C-O | N | A | |
| ≤C3 | 1.89 | 0 | 0 | 0 | 0 | 0 | 0 | 14.03 | 0 | 0.15 | 0 | 0 | 0 | 0 |
| C4 | 0.54 | 0.37 | 2.57 | 1.99 | 0 | 0 | 0 | 9.65 | 14.37 | 0.29 | 0.42 | 0 | 0 | 0 |
| C5 | 0.48 | 0.38 | 1.10 | 4.07 | 0 | 1.47 | 0 | 4.43 | 10.96 | 0.03 | 0.24 | 0 | 0.06 | 0 |
| C6 | 6.10 | 6.18 | 5.23 | 38.44 | 0 | 0 | 0 | 1.42 | 7.50 | 0 | 0.10 | 0.01 | 0.04 | 0.04 |
| C7 | 0.33 | 1.87 | 0.52 | 5.63 | 0 | 0.02 | 0 | 0.23 | 4.67 | 0 | 0 | 0.02 | 1.88 | 4.99 |
| C8 | 0 | 0.27 | 0.05 | 5.27 | 0.07 | 0.05 | 0 | 0.22 | 1.27 | 0 | 0 | 0 | 1.26 | 7.96 |
| C9 | 0.18 | 0.47 | 0.57 | 3.14 | 0 | 0.07 | 0 | 0 | 0.38 | 0.07 | 0 | 0 | 0.46 | 5.78 |
| ≥C10 | 0.76 | 1.19 | 6.38 | 2.23 | 0 | 0.12 | 0 | 0 | 0.03 | 0 | 0 | 0 | 0.16 | 6.88 |
| Carbon Number | Group Composition in Product (wt%) | Total (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| P | I | L-O | B-O | C-O | N | A | ||
| C4 | 0.01 | 0.01 | 0.02 | 0 | 0 | 0 | 0 | 0.04 |
| C5 | 0.10 | 0.15 | 0.22 | 0.58 | 0 | 0 | 0 | 1.05 |
| C6 | 2.93 | 15.67 | 3.93 | 7.13 | 1.89 | 4.33 | 1.18 | 37.06 |
| C7 | 1.88 | 15.31 | 1.57 | 5.92 | 0.50 | 8.84 | 6.74 | 40.76 |
| C8 | 0 | 9.51 | 0.55 | 2.64 | 0 | 4.21 | 1.41 | 18.32 |
| C9 | 0 | 1.29 | 0.45 | 0.89 | 0 | 0.02 | 0 | 2.65 |
| C10 | 0 | 0 | 0 | 0.03 | 0 | 0 | 0.07 | 0.10 |
| C11 | 0 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0.02 |
| Total | 4.92 | 41.96 | 6.74 | 17.19 | 2.39 | 17.40 | 9.40 | 100 |
| Reaction Temperature | Group Composition Changes in Product (wt%) | RON Changes | ||||||
|---|---|---|---|---|---|---|---|---|
| P | I | L-O | B-O | C-O | N | A | ||
| 320 °C | 2.48 | 1.96 | −1.83 | −5.20 | −1.84 | 2.07 | 2.36 | −0.42 |
| 340 °C | 2.94 | 3.99 | −4.17 | −6.82 | −2.04 | 2.15 | 3.94 | −0.83 |
| 360 °C | 3.10 | 5.64 | −5.12 | −9.99 | −2.21 | 1.37 | 7.22 | −0.29 |
| 380 °C | 2.64 | 4.29 | −5.93 | −10.80 | −2.32 | 0.73 | 11.40 | 2.60 |
| Component | Hydrocarbon Group Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| P | I | L-O | B-O | C-O | N | A | ||
| 320 °C | yie | 7.40 | 43.92 | 4.92 | 11.99 | 0.55 | 19.47 | 11.76 |
| yic | 7.17 | 43.60 | 4.90 | 12.00 | 0.54 | 19.59 | 12.20 | |
| RE (%) | −3.11 | −0.73 | −0.41 | 0.08 | −1.82 | 0.62 | 3.74 | |
| 340 °C | yie | 7.86 | 45.95 | 2.57 | 10.37 | 0.35 | 19.55 | 13.34 |
| yic | 7.91 | 45.80 | 2.54 | 10.48 | 0.36 | 19.56 | 13.35 | |
| RE (%) | 0.64 | −0.33 | −1.17 | 1.06 | 2.86 | 0.05 | 0.07 | |
| 360 °C | yie | 8.02 | 47.60 | 1.62 | 7.20 | 0.18 | 18.77 | 16.62 |
| yic | 7.99 | 47.27 | 1.64 | 7.54 | 0.18 | 18.70 | 16.70 | |
| RE (%) | −0.37 | −0.69 | 1.23 | 4.72 | 0 | −0.37 | 0.48 | |
| 380 °C | yie | 7.56 | 46.25 | 0.81 | 6.39 | 0.07 | 18.13 | 20.80 |
| yic | 7.72 | 47.50 | 0.80 | 6.12 | 0.07 | 17.27 | 20.53 | |
| RE (%) | 2.12 | 2.70 | −1.23 | −4.23 | 0 | −4.74 | −1.30 | |
| Carbon Number | Group Composition in Product (wt%) (LHSV = 1.5 h−1) | Group Composition in Product (wt%) (LHSV = 3.0 h−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | I | L-O | B-O | C-O | N | A | P | I | L-O | B-O | C-O | N | A | |
| ≤C3 | 7.66 | 0 | 0.04 | 0 | 0 | 0 | 0 | 3.95 | 0 | 0.01 | 0 | 0 | 0 | 0 |
| C4 | 7.90 | 11.24 | 0.41 | 0.62 | 0 | 0 | 0 | 2.23 | 4.12 | 3.11 | 4.74 | 0 | 0 | 0 |
| C5 | 6.01 | 10.45 | 0.09 | 0.68 | 0 | 0.12 | 0 | 2.30 | 4.41 | 1.06 | 8.30 | 0.00 | 1.40 | 0 |
| C6 | 3.80 | 10.15 | 0.12 | 0.27 | 0.19 | 1.25 | 0 | 3.57 | 7.27 | 0.99 | 5.32 | 0.51 | 0.99 | 0 |
| C7 | 1.04 | 5.16 | 0 | 0.32 | 1.00 | 1.43 | 2.02 | 0.23 | 3.83 | 0.02 | 4.73 | 0.45 | 2.97 | 0.26 |
| C8 | 0.35 | 2.90 | 0 | 0.37 | 1.78 | 1.37 | 3.44 | 1.12 | 2.52 | 0 | 1.20 | 5.52 | 2.21 | 0.50 |
| C9 | 0.18 | 1.41 | 0 | 0.58 | 2.35 | 0.58 | 4.47 | 0.18 | 1.76 | 0 | 1.85 | 1.85 | 1.60 | 0.47 |
| ≥C10 | 0.11 | 1.68 | 0 | 1.79 | 0.48 | 0.53 | 3.66 | 0.33 | 3.16 | 0 | 1.99 | 0.98 | 2.83 | 3.16 |
| Carbon Number | 320 °C | 340 °C | 360 °C | 380 °C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | I | N | A | P | I | N | A | P | I | N | A | P | I | N | A | |
| C4 | 0.13 | 0.13 | 0 | 0 | 0.23 | 0.25 | 0 | 0 | 0.48 | 0.49 | 0 | 0 | 1.01 | 1.26 | 0 | 0 |
| C5 | 0.28 | 0.36 | 0 | 0 | 0.45 | 0.62 | 0 | 0 | 0.81 | 1.13 | 0 | 0 | 1.37 | 1.89 | 0 | 0 |
| C6 | −0.13 | −0.80 | 0.01 | −0.96 | 0.02 | 0.01 | 0.21 | −0.97 | 0.06 | 0.36 | 0.23 | −0.98 | −0.24 | 0.25 | −0.13 | −1.06 |
| C7 | 0.18 | 0.79 | 0.93 | 0.12 | 0.19 | 1.27 | 0.59 | −0.01 | 0.03 | 1.26 | 0.74 | 0.06 | −0.53 | 0.38 | 0.26 | 0.61 |
| C8 | 1.72 | 1.10 | 0.28 | 2.71 | 1.58 | 1.10 | 0.47 | 3.28 | 1.42 | 1.95 | −0.42 | 4.97 | 1.04 | 0.26 | −0.05 | 7.59 |
| C9 | 0.19 | −0.06 | 0.77 | 0.17 | 0.23 | 0.27 | 0.89 | 0.58 | 0.09 | 0.20 | 0.81 | 0.91 | 0 | 0.01 | 0.66 | 1.38 |
| C10 | 0.12 | 0 | 0 | 0.33 | 0.25 | 0.10 | 0 | 0.67 | 0.21 | 0 | 0 | 1.77 | 0 | 0 | 0 | 2.42 |
| C11 | 0 | 0.05 | 0 | 0 | 0 | 0.04 | 0 | 0 | 0 | −0.02 | 0 | 0 | 0 | −0.02 | 0 | 0.27 |
| C12 | 0 | 0.39 | 0.08 | 0 | 0 | 0.34 | 0 | 0.39 | 0 | 0.28 | 0 | 0.50 | 0 | 0.12 | 0 | 0.19 |
| C13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.15 | 0 | 0 |
| Carbon Number | 320 °C | 340 °C | 360 °C | 380 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-O | B-O | C-O | L-O | B-O | C-O | L-O | B-O | C-O | L-O | B-O | C-O | |
| C4 | 0.88 | 0 | 0 | 0.82 | 0 | 0 | 0.63 | 0 | 0 | 0.41 | 0 | 0 |
| C5 | 0.42 | 1.73 | 0 | 0.31 | 1.21 | 0 | 0.11 | 0.57 | 0 | −0.03 | −0.05 | 0 |
| C6 | −3.47 | −5.46 | −1.44 | −3.64 | −6.06 | −1.62 | −3.84 | −6.55 | −1.71 | −3.89 | −6.85 | −1.82 |
| C7 | −0.86 | −3.36 | −0.40 | −1.06 | −3.71 | −0.42 | −1.27 | −4.54 | −0.50 | −1.46 | −4.63 | −0.50 |
| C8 | 1.47 | 1.31 | 0 | −0.32 | 1.22 | 0 | −0.47 | 0.63 | 0 | −0.55 | −0.32 | 0 |
| C9 | −0.27 | 0.05 | 0 | −0.27 | −0.28 | 0 | −0.28 | −0.46 | 0 | −0.41 | −0.73 | 0 |
| C10 | 0 | 0.06 | 0 | 0 | 0.10 | 0 | 0 | −0.03 | 0 | 0 | 1.25 | 0 |
| C11 | 0 | 0.48 | 0 | 0 | 0.70 | 0 | 0 | 0.38 | 0 | 0 | 0.42 | 0 |
| Carbon Number | Olefins Type | Olefins Name | Feed (wt%) | Product (wt%) | |||
|---|---|---|---|---|---|---|---|
| 320 °C | 340 °C | 360 °C | 380 °C | ||||
| C6 | L-O | 1-hexene | 0.37 | 0 | 0 | 0 | 0 |
| 2-hexene,3-hexene | 3.56 | 0.46 | 0.29 | 0.09 | 0.05 | ||
| B-O | 2-methyl-1-pentene 4-methyl-1-pentene | 1.71 | 0.32 | 0.21 | 0.13 | 0.06 | |
| methyl-2-pentene 4-methyl-2-pentene methyl-3-pentene | 5.28 | 1.35 | 0.86 | 0.46 | 0.22 | ||
| 2,3-dimethyl-1-butene | 0.15 | 0 | 0 | 0 | 0 | ||
| C-O | 1-methylcyclopentene 3-methylcyclopentene | 1.89 | 0.45 | 0.27 | 0.18 | 07 | |
| C7 | L-O | 2-heptene, 3-heptene | 1.57 | 0.71 | 0.51 | 0.30 | 0.11 |
| B-O | 3-methyl-1-hexene 4-methyl-1-hexene | 0.24 | 0.07 | 0.06 | 0 | 0 | |
| methyl-3-hexene 3-methyl-2-hexene | 2.76 | 1.22 | 0.80 | 0.38 | 0.16 | ||
| 2-ethyl-1-pentene 3-ethyl-1-pentene | 1.55 | 1.18 | 0.99 | 0.93 | 1.07 | ||
| ethyl-2-pentene 4,4-dimethyl-2-pentene | 0.41 | 0.10 | 0.06 | 0 | 0 | ||
| 2.3.3-trimethylbutene 2-ethyl-3-methyl-1-butene | 0.11 | 0 | 0.06 | 0.07 | 0.07 | ||
| Others | 0.85 | 0 | 0.14 | 0 | 0 | ||
| C-O | 3-ethylcyclopentene 1-ethylcyclopentene | 0.25 | 0.10 | 0.08 | 0 | 0 | |
| 1-methylcyclohexene | 0.24 | 0 | 0 | 0 | 0 | ||
| No. | Reaction Path | Rate Constant k (cm3·g−1·s −1) | Ea (kJ·mol−1) | A (cm3·g−1·h−1) | |||
|---|---|---|---|---|---|---|---|
| 320 °C | 340 °C | 360 °C | 380 °C | ||||
| 1 | P → I | 0.30 | 2.54 | 4.77 | 6.41 | 160.17 | 6.08 × 1013 |
| 2 | N → I | 0.27 | 1.51 | 3.15 | 4.47 | 148.22 | 4.28 × 1012 |
| 3 | N → P | 3.76 | 4.98 | 5.79 | 6.12 | 33.65 | 3.52 × 103 |
| 4 | N → A | 0.46 | 2.24 | 9.64 | 28.98 | 224.03 | 2.61 × 1019 |
| 5 | A → N | 16.22 | 21.09 | 24.80 | 34.06 | 38.31 | 3.80 × 104 |
| 6 | B-O → I | 8.45 | 19.55 | 30.07 | 32.84 | 73.14 | 2.76 × 107 |
| 7 | L-O → P | 18.59 | 38.31 | 50.50 | 63.50 | 64.18 | 9.47 × 106 |
| 8 | C-O → N | 60.40 | 80.28 | 113.70 | 163.40 | 53.48 | 3.00 × 106 |
| 9 | C-O → A | 58.39 | 75.45 | 109.32 | 160.72 | 54.63 | 3.61 × 106 |
| 10 | L-O → B-O | 0.50 | 16.45 | 26.21 | 48.90 | 90.35 | 7.94 × 108 |
| 11 | B-O → C-O | 0.14 | 0.20 | 0.23 | 0.39 | 53.08 | 6.37 × 103 |
| 12 | L-O → C-O | 0.11 | 0.15 | 0.24 | 0.46 | 74.94 | 4.06 × 105 |
| 13 | L-O → A | 5.72 | 24.04 | 41.00 | 69.99 | 88.79 | 8.77 × 108 |
| 14 | B-O → A | 19.64 | 25.25 | 46.06 | 67.01 | 68.70 | 2.05 × 107 |
| Item | Value | Unit |
|---|---|---|
| packing density | 0.60 | g/mL |
| micropore surface area a | 225 | m2/g |
| external surface area b | 48 | m2/g |
| micropore volume c | 0.10 | mL/g |
| mesopore volume d | 0.15 | mL/g |
| average pore diameter e | 3.63 | nm |
| weak Lewis acidity | 94.6 | µmol/g |
| weak Brönsted acidity | 22.7 | µmol/g |
| medium and strong Lewis acidity | 80.7 | µmol/g |
| medium and strong Brönsted acidity | 9.5 | µmol/g |
| Metal oxide content f | 1.1%La2O3/4.8%ZnO/5.5%NiO | wt% |
| Carbon Number | Group Composition in Product (wt%) | Total (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| P | I | L-O | B-O | C-O | N | A | ||
| C4 | 0.31 | 0.08 | 1.26 | 0 | 0 | 0 | 0 | 1.65 |
| C5 | 1.61 | 9.96 | 3.65 | 6.52 | 0.04 | 0 | 0 | 21.78 |
| C6 | 1.37 | 10.83 | 1.89 | 3.77 | 0.93 | 2.07 | 0.56 | 21.42 |
| C7 | 0.79 | 6.16 | 0.89 | 2.54 | 0.07 | 3.49 | 2.84 | 16.78 |
| C8 | 0.78 | 3.98 | 0.38 | 1.42 | 0 | 1.69 | 6.53 | 14.78 |
| C9 | 0.34 | 2.26 | 0.10 | 0.48 | 0 | 1.35 | 7.72 | 12.25 |
| C10 | 0.32 | 1.19 | 0 | 0.27 | 0 | 0.06 | 4.79 | 6.63 |
| C11 | 0.24 | 1.51 | 0.19 | 0.35 | 0 | 0.04 | 0.75 | 3.08 |
| C12 | 0.10 | 0.92 | 0 | 0 | 0 | 0 | 0.61 | 1.63 |
| Total | 5.86 | 36.89 | 8.36 | 15.35 | 1.04 | 8.70 | 23.80 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Song, X.; He, S.; Zhang, X.; Li, Q.; Xiao, H.; Hu, X.; Wang, Y.; Chen, B.; Li, W. Reaction Behavior and Kinetic Model of Hydroisomerization and Hydroaromatization of Fluid Catalytic Cracking Gasoline. Molecules 2025, 30, 783. https://doi.org/10.3390/molecules30040783
Zhong H, Song X, He S, Zhang X, Li Q, Xiao H, Hu X, Wang Y, Chen B, Li W. Reaction Behavior and Kinetic Model of Hydroisomerization and Hydroaromatization of Fluid Catalytic Cracking Gasoline. Molecules. 2025; 30(4):783. https://doi.org/10.3390/molecules30040783
Chicago/Turabian StyleZhong, Haijun, Xiwen Song, Shuai He, Xuerui Zhang, Qingxun Li, Haicheng Xiao, Xiaowei Hu, Yue Wang, Boyan Chen, and Wangliang Li. 2025. "Reaction Behavior and Kinetic Model of Hydroisomerization and Hydroaromatization of Fluid Catalytic Cracking Gasoline" Molecules 30, no. 4: 783. https://doi.org/10.3390/molecules30040783
APA StyleZhong, H., Song, X., He, S., Zhang, X., Li, Q., Xiao, H., Hu, X., Wang, Y., Chen, B., & Li, W. (2025). Reaction Behavior and Kinetic Model of Hydroisomerization and Hydroaromatization of Fluid Catalytic Cracking Gasoline. Molecules, 30(4), 783. https://doi.org/10.3390/molecules30040783





