Study on the Hygroscopic Properties and Mechanism of Novel Melt-Cast Matrix 3,4-Dinitropyrazole (DNP)
Abstract
1. Introduction
2. Results and Discussion
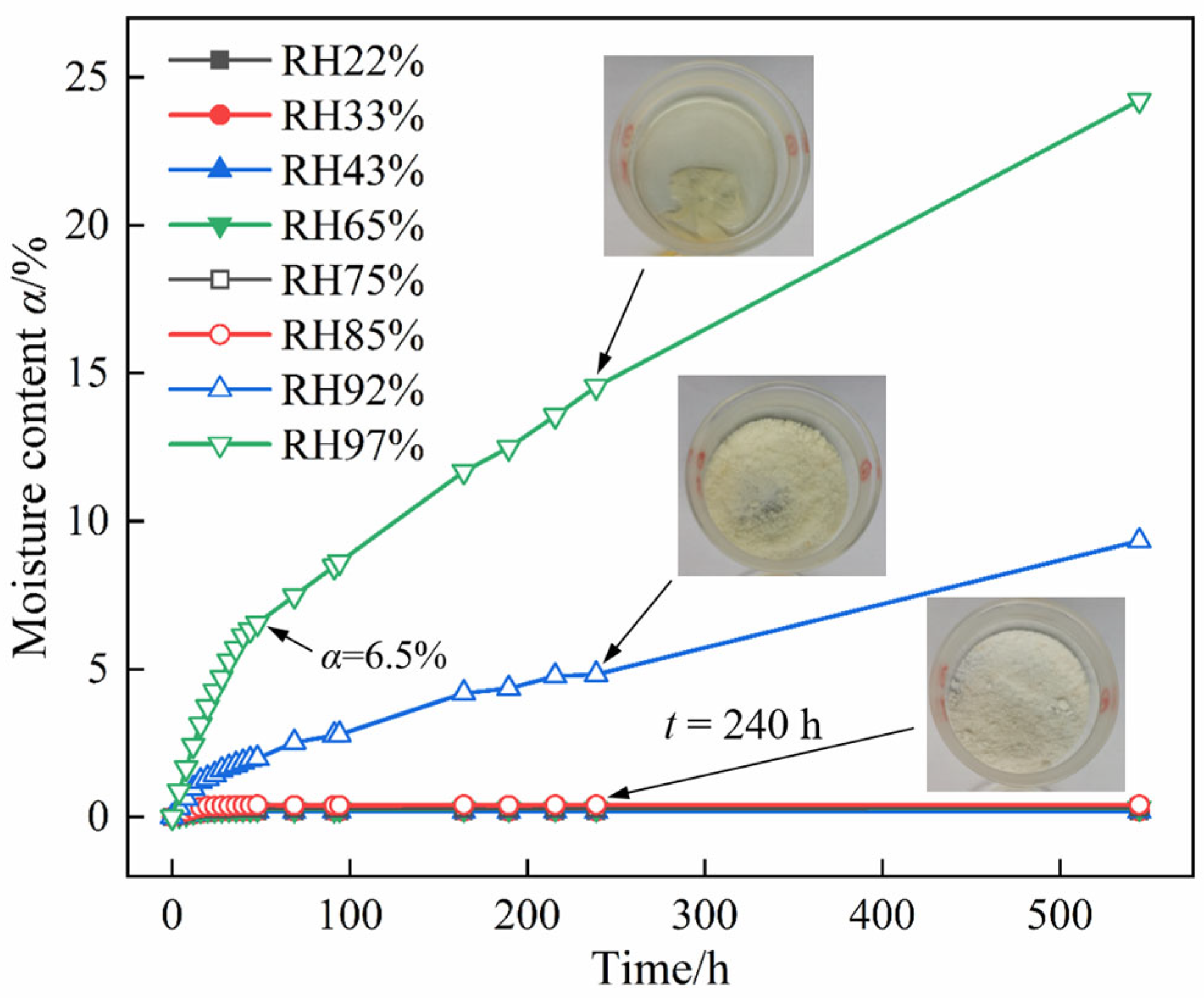
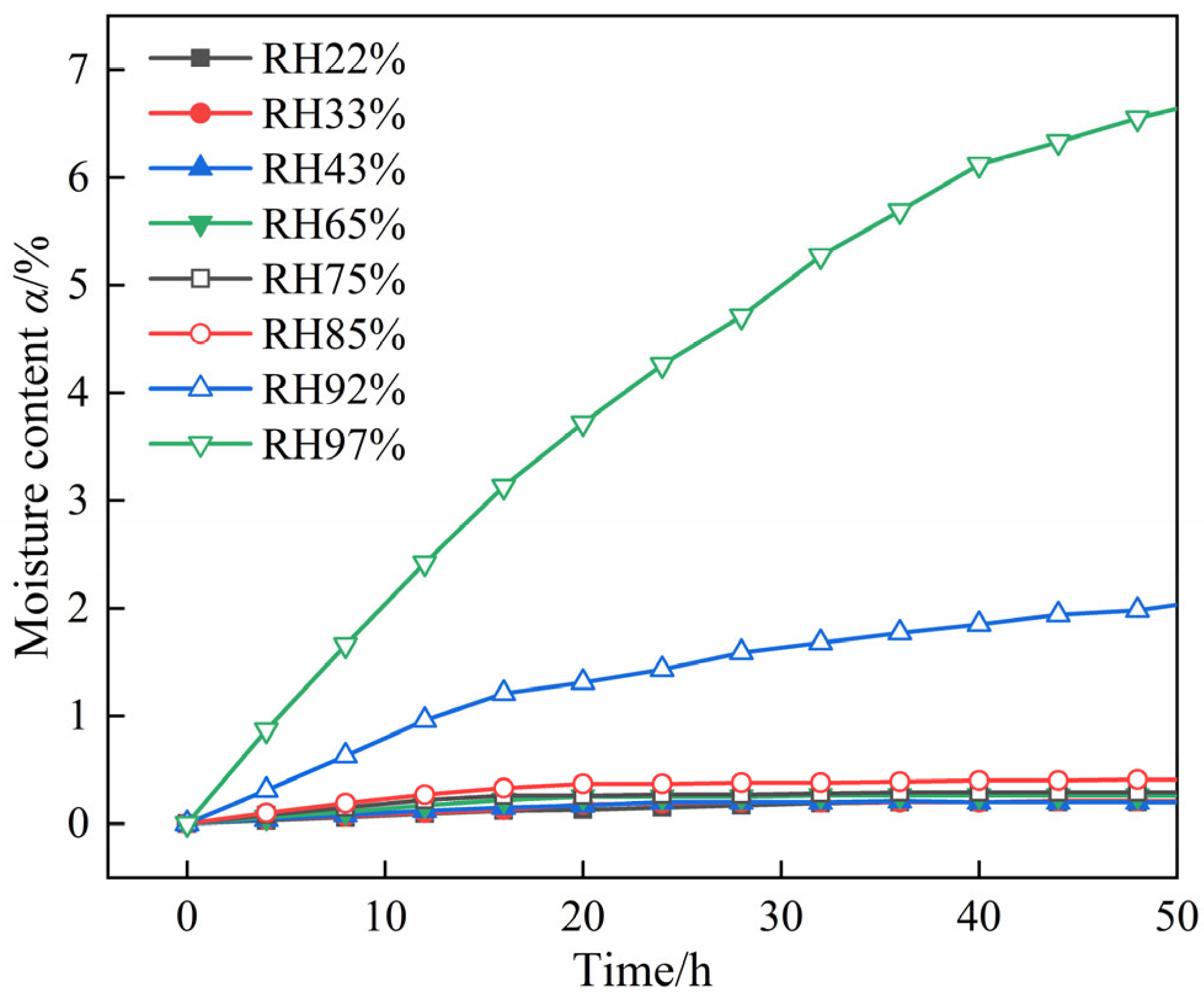
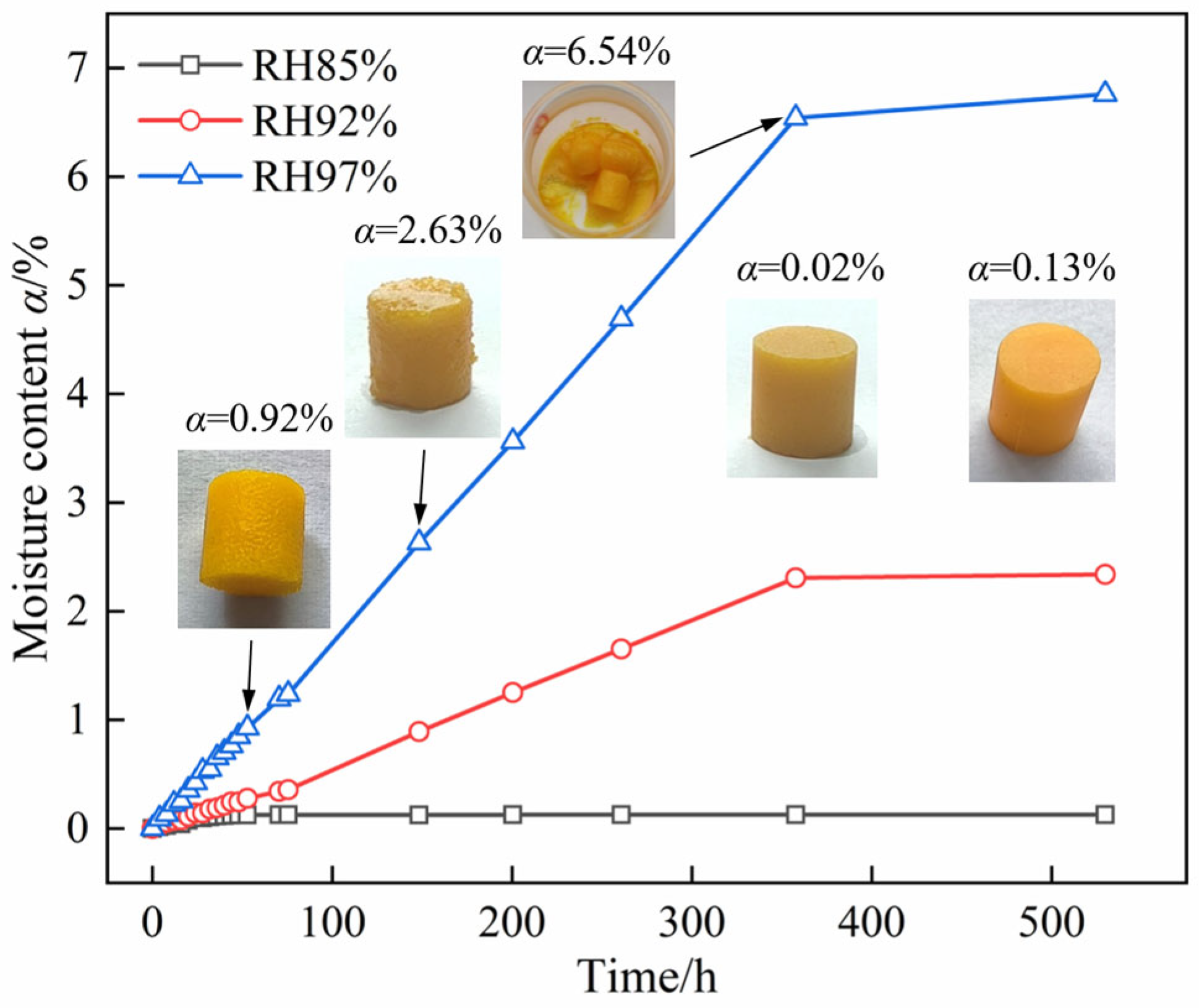
2.1. Macroscopic Hygroscopic Properties of DNP Powder and Charge
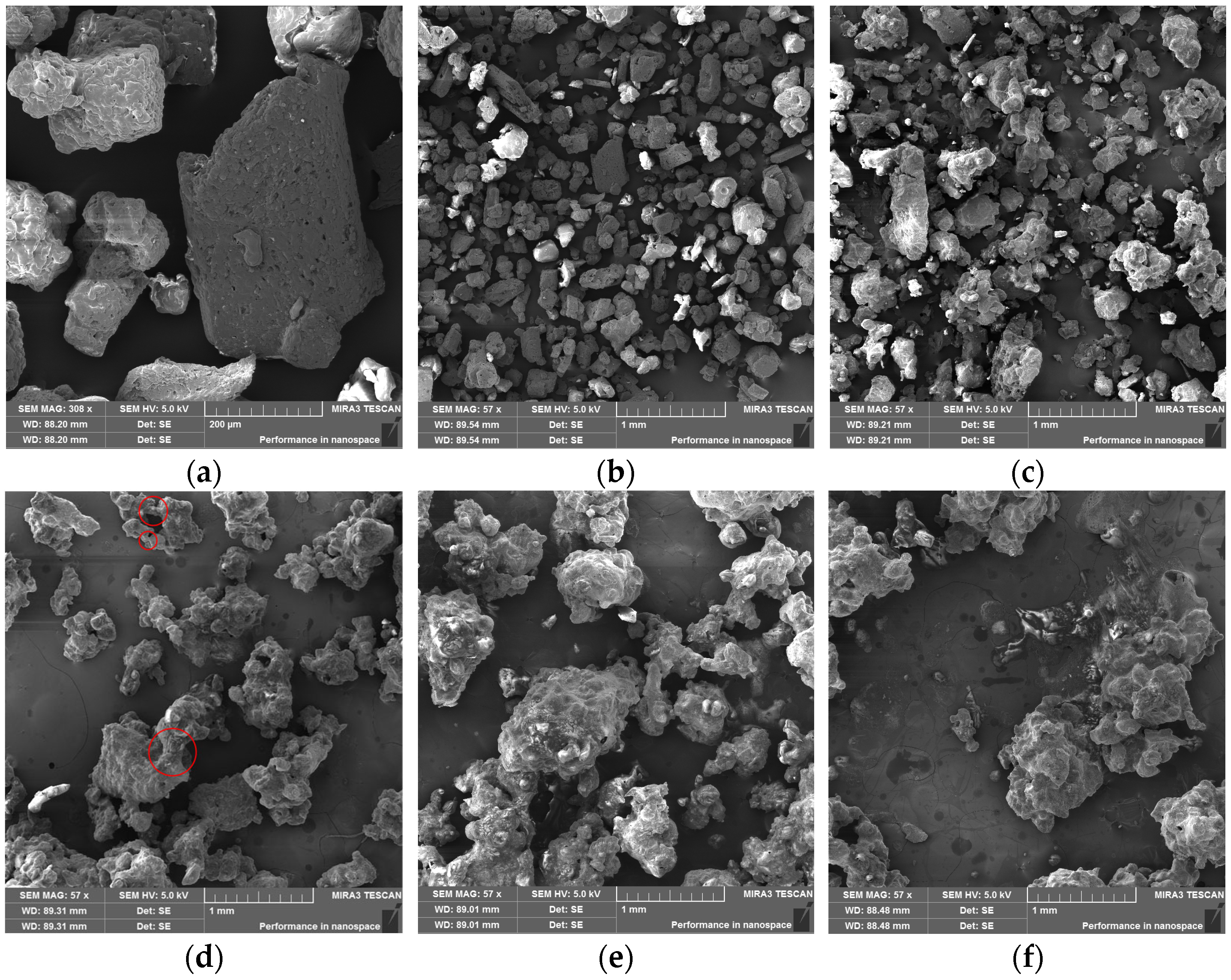
2.2. Morphological Evolution of DNP upon Moisture Absorption
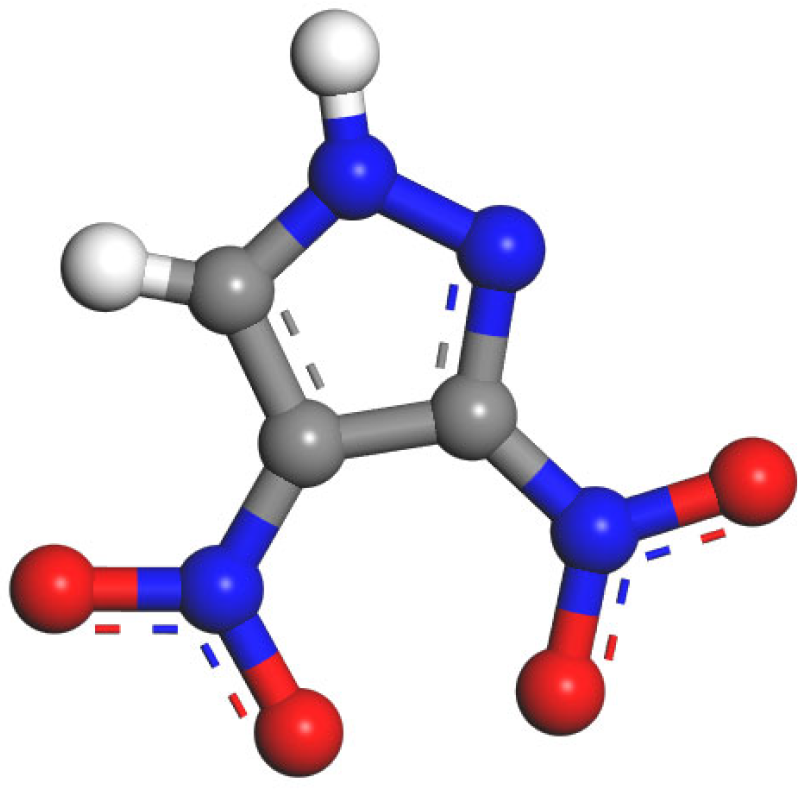
2.3. The Interaction Process Between DNP and Water Molecules
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Initial Moisture Content
3.2.2. Moisture Content
3.2.3. Scanning Electron Microscope (SEM)
3.2.4. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reinhardt, E.; Lenz, T.; Bauer, L.; Stierstorfer, J.; Klapötke, T.M. Synthesis and Characterization of Azido- and Nitratoalkyl Nitropyrazoles as Potential Melt-Cast Explosives. Molecules 2023, 28, 6489. [Google Scholar] [CrossRef]
- Morris, J.; Phillips, J.; Tucker, N.; Wilmoth, R. Synthesis, Formulation, and Testing of 3.4-DNP; BAE Systems: Portland, Oregon, 2018; Available online: https://imemg.org/wp-content/uploads/2019/11/22236-Paper-Synthesis-Formulation-and-Testing-of-34DNP-1.pdf (accessed on 13 November 2025).
- Xie, H. Comparative Researches on Performance of DNP-Based and TNT-Based Melt-Cast Explosives. Ph.D. Thesis, Beijing Institute of Technology, Beijing, China, 2024. [Google Scholar]
- Tariq, Q.-N.; Bi, Y.; Manzoor, S.; Tariq, M.-N.; Cao, W.-L.; Dong, W.-S.; Zhang, J.-G. Synthesis, performance, and thermal behavior of two insensitive 3,4-dinitropyrazole-based energetic cocrystals. Cryst. Growth Des. 2023, 23, 112–119. [Google Scholar] [CrossRef]
- Hervé, G.; Roussel, C.; Graindorge, H. Selective preparation of 3,4,5-trinitro-1 H- pyrazole: A stable all-carbon-nitrated arene. Angew. Chem. Int. Ed. 2010, 49, 3177–3181. [Google Scholar] [CrossRef]
- Liang, J. Research on the Influence of Hygroscopicity on the Performance of Typical Ignition Powder Containing Metallic Powder. Master’s Thesis, Shenyang Ligong University, Shenyang, China, 2021. [Google Scholar]
- Liu, L.; Hu, Y. Effect of moisture content on MANFO performance. Eng. Blasting 2012, 18, 87–90+39. [Google Scholar]
- Allada, R.; Maruthapillai, A.; Palanisamy, K.; Chappa, P. Hygroscopicity categorization of pharmaceutical solids by gravimetric sorption analysis: A systematic approach. Asian J. Pharm. (AJP) 2016, 10, 279–286. [Google Scholar]
- Hu, K.; Huang, W.; Ma, X.; Xu, X. Test and study on the modification of ammonium nitrate by coating its surface. Explos. Mater. 2006, 35, 14–17. [Google Scholar]
- Wang, J.; Zhang, G.; Yan, R.; Hu, L.; Zhang, T. Hygroscopicity of ADN with dynamic method. Chin. J. Energetic Mater. 2012, 20, 86–89. [Google Scholar]
- Elzaki, B.I.; Jun, Z.Y. Relationships between structures of surfactants and their anti-hygroscopicity performance of ammonium nitrate particles. Arab. J. Chem. 2020, 13, 7626–7636. [Google Scholar] [CrossRef]
- Gong, T.; Qin, L.-J.; Yan, R.; Hu, L.; Ji, Y.-P.; Feng, H. Alumina thin film coated ammonium dinitramide fabricated by atomic layer deposition. J. Inorg. Mater. 2014, 29, 869. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, Y.; Cao, L.; Li, L.; Li, C. Determining Critical Relative Humidity by Measuring Air Humidity in Equilibrium Directly. Eur. J. Pharm. Sci. 2010, 41, 383–387. [Google Scholar] [CrossRef]
- Cui, F. Pharmaceutics; People’s Medical Publishing House: Beijing, China, 2006; pp. 372–373. [Google Scholar]
- Kumar, P. An overview on properties, thermal decomposition, and combustion behavior of ADN and ADN based solid propellants. Def. Technol. 2018, 14, 661–673. [Google Scholar] [CrossRef]
- Adams, J.R.; Merz, A.R. Hygroscopicity of fertilizer materials and mixtures. Ind. Eng. Chem. 1929, 21, 305–307. [Google Scholar] [CrossRef]
- Peng, C.; Chen, L.; Tang, M. A database for deliquescence and efflorescence relative humidities of compounds with atmospheric relevance. Fundam. Res. 2022, 2, 578–587. [Google Scholar] [CrossRef]
- Yan, N.; Bian, C.; Li, H.; Wang, J.; Xu, M.; Huang, H. Pickering emulsion-templated encapsulation of ammonium dinitramide by graphene sheets for hygroscopic inhibition. Appl. Surf. Sci. 2021, 537, 147994. [Google Scholar] [CrossRef]
- Cui, J.; Han, J.; Wang, J.; Huang, R. Study on the crystal structure and hygroscopicity of ammonium dinitramide. J. Chem. Eng. Data 2010, 55, 3229–3234. [Google Scholar] [CrossRef]
- Lightstone, J.M.; Onasch, T.B.; Imre, D.; Oatis, S. Deliquescence, efflorescence, and water activity in ammonium nitrate and mixed ammonium nitrate/succinic acid microparticles. J. Phys. Chem. A 2000, 104, 9337–9346. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, B.; Xie, Z.; Hu, Y.; Yang, H.; Yang, J.; Xiao, L.; Zhao, F.; Gao, H.; Jiang, W.; et al. Exploring the hygroscopic behavior of highly energetic oxidizer ammonium dinitramide (ADN) at different temperatures and humidities using an innovative hygroscopic modeling. Def. Technol. 2024, 40, 25–34. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Gong, X.D. A Theoretical study on the structure and hygroscopicity of ammonium dinitramide. Struct. Chem. 2013, 24, 1537–1543. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Z.; Zhang, J.; Yin, X. Crystal structure of 3,4-dinitropyrazole. Chin. J. Energetic Mater. 2016, 24, 965–968. [Google Scholar] [CrossRef]
- Guo, H.; Yu, S.; Li, Y.; Wang, J.; Cao, D.; Qin, Z. Crystal structure of 3,4-dinitropyrazole in water. Mol. Cryst. Liq. Cryst. 2019, 690, 43–49. [Google Scholar] [CrossRef]
- Morteza, M. DFT study of the intermolecular interaction of 3,4-dinitropyrazole (DNP) and H2O. J. Phys. Theor. Chem. 2017, 14, 229–236. [Google Scholar]
- GJB770B; Hygroscopic-Drier Balance Method. People’s Republic of China National Military Standard: Beijing, China, 2005.

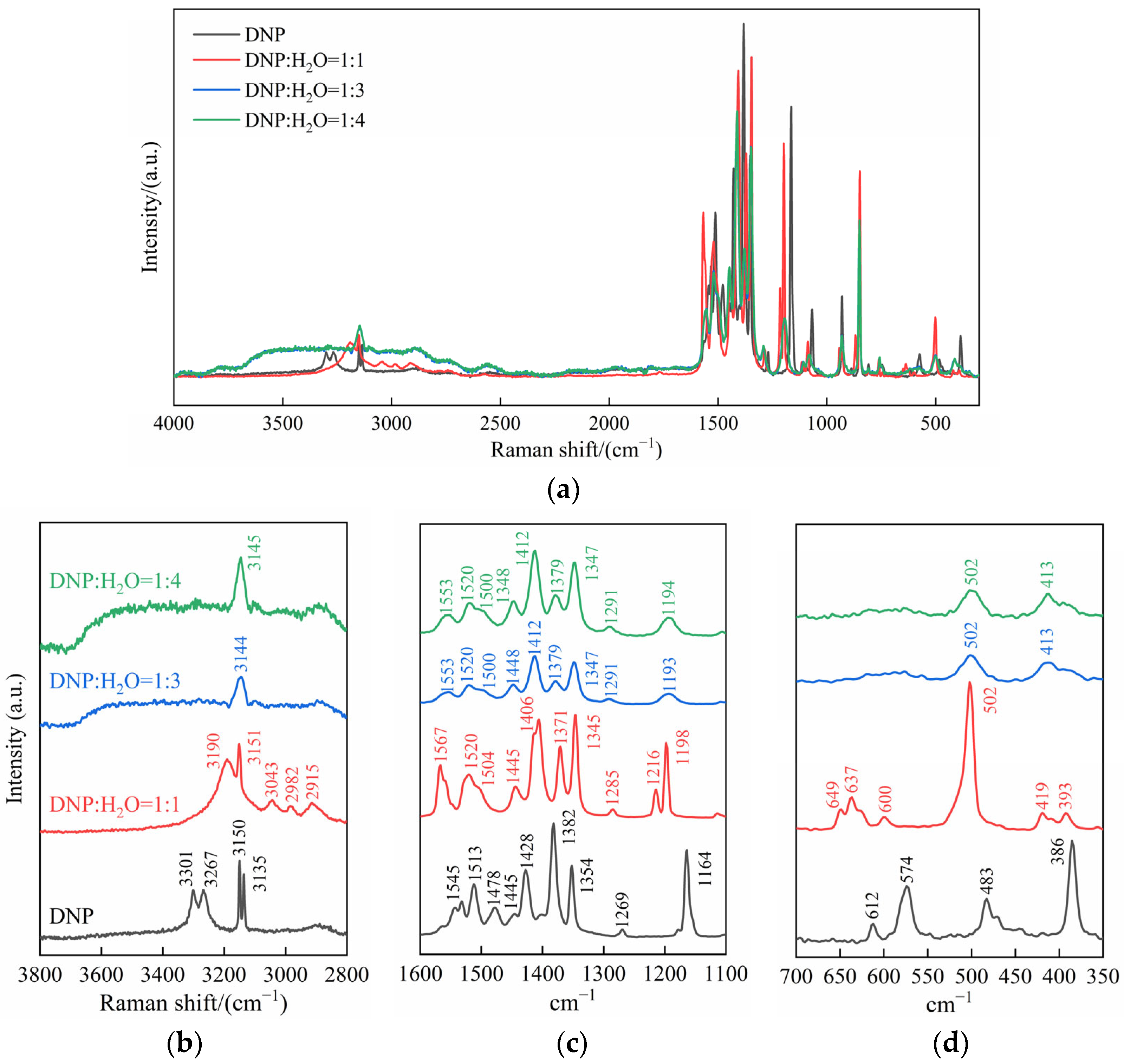
| Vibration Mode | Wavenumber/cm−1 | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| N-H stretching | 3301, 3267 | 3190, 3151 | 3144 | 3144 |
| C-H stretching | 3150, 3135 | 3043, 2982 | - | - |
| O-H stretching (H2O) | - | 2915 | 2800–3700 | 2800–3700 |
| Vibration Mode | Wavenumber/cm−1 | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| -NO2 asymmetric stretching | 1545 | 1567 | 1553 | 1553 |
| C=N stretching | 1513 | 1520 | 1520 | 1520 |
| C=C stretching | 1478 | 1504 | 1500 | 1500 |
| N-H in-plane bending | 1445 | 1445 | 1448 | 1448 |
| C-H in-plane bending | 1428 | 1406 | 1412 | 1412 |
| -NO2 symmetric stretching | 1382, 1354 | 1371, 1345 | 1379, 1347 | 1379, 1347 |
| C-N stretching | 1269 | 1285 | 1291 | 1291 |
| C-C stretching | 1164 | 1216, 1198 | 1193 | 1194 |
| Vibration Mode | Wavenumber/cm−1 | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| pyrazole ring global out-of-plane deformation | 612 | 649, 637 | - | - |
| C=C out-of-plane bending | 574 | 600 | - | - |
| N-H out-of-plane bending | 483 | 502 | 502 | 502 |
| pyrazole ring out-of-plane bending | 386 | 419, 393 | 413 | 413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, T.; Yue, Y.; Ying, W.; Yan, B.; Liu, P.; Zhang, X. Study on the Hygroscopic Properties and Mechanism of Novel Melt-Cast Matrix 3,4-Dinitropyrazole (DNP). Molecules 2025, 30, 4644. https://doi.org/10.3390/molecules30234644
Guan T, Yue Y, Ying W, Yan B, Liu P, Zhang X. Study on the Hygroscopic Properties and Mechanism of Novel Melt-Cast Matrix 3,4-Dinitropyrazole (DNP). Molecules. 2025; 30(23):4644. https://doi.org/10.3390/molecules30234644
Chicago/Turabian StyleGuan, Tong, Yuehui Yue, Wujiang Ying, Bo Yan, Pan Liu, and Xiangrong Zhang. 2025. "Study on the Hygroscopic Properties and Mechanism of Novel Melt-Cast Matrix 3,4-Dinitropyrazole (DNP)" Molecules 30, no. 23: 4644. https://doi.org/10.3390/molecules30234644
APA StyleGuan, T., Yue, Y., Ying, W., Yan, B., Liu, P., & Zhang, X. (2025). Study on the Hygroscopic Properties and Mechanism of Novel Melt-Cast Matrix 3,4-Dinitropyrazole (DNP). Molecules, 30(23), 4644. https://doi.org/10.3390/molecules30234644







