Extraction and Identification of Active Components from Lilium lancifolium Based on NADES-UHPLC-MS/MS Technology
Abstract
1. Introduction
2. Results
2.1. NADES Stability Test Results
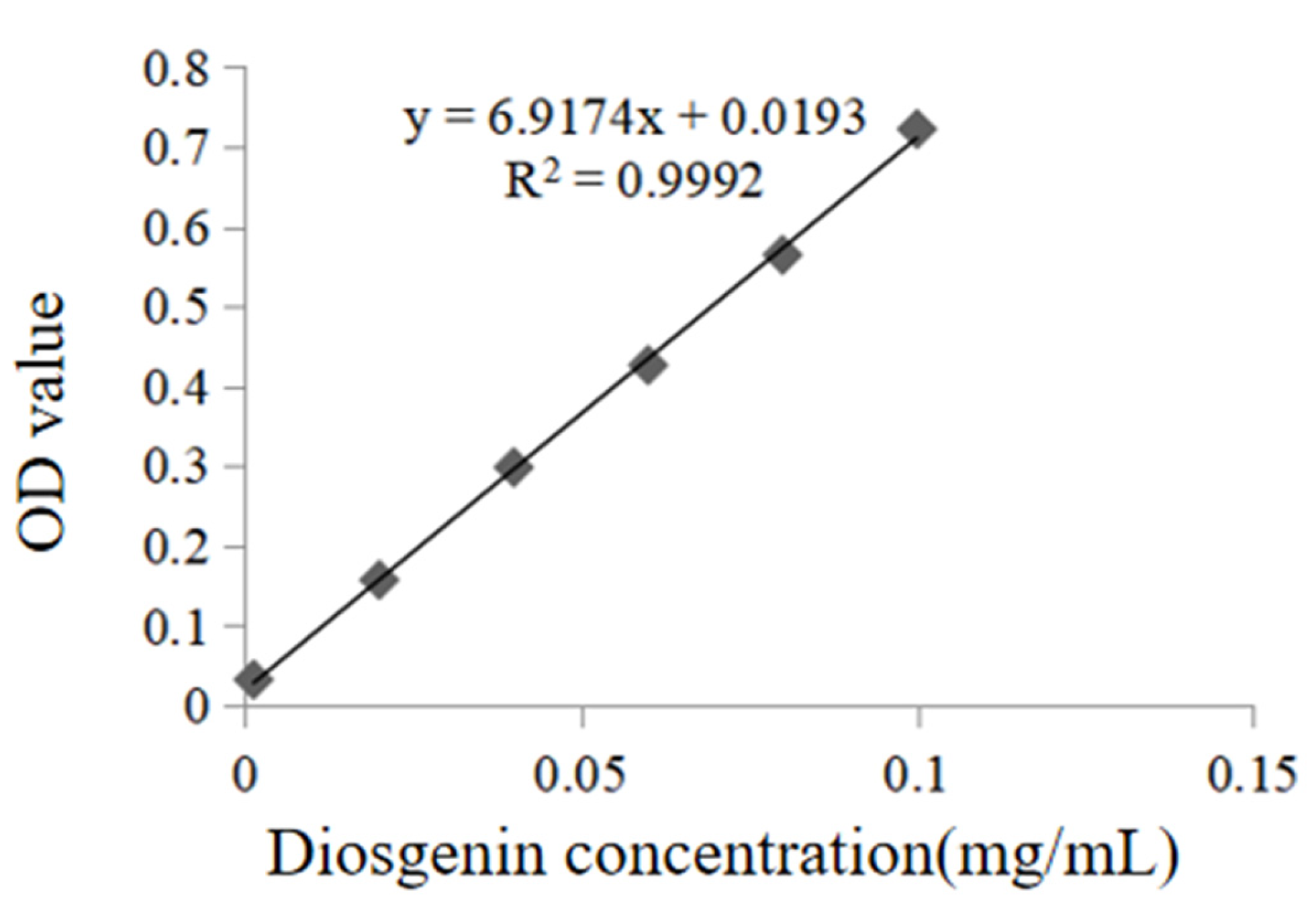
2.2. Drawing Results of the Standard Curve
2.3. Content Determination and Component Identification
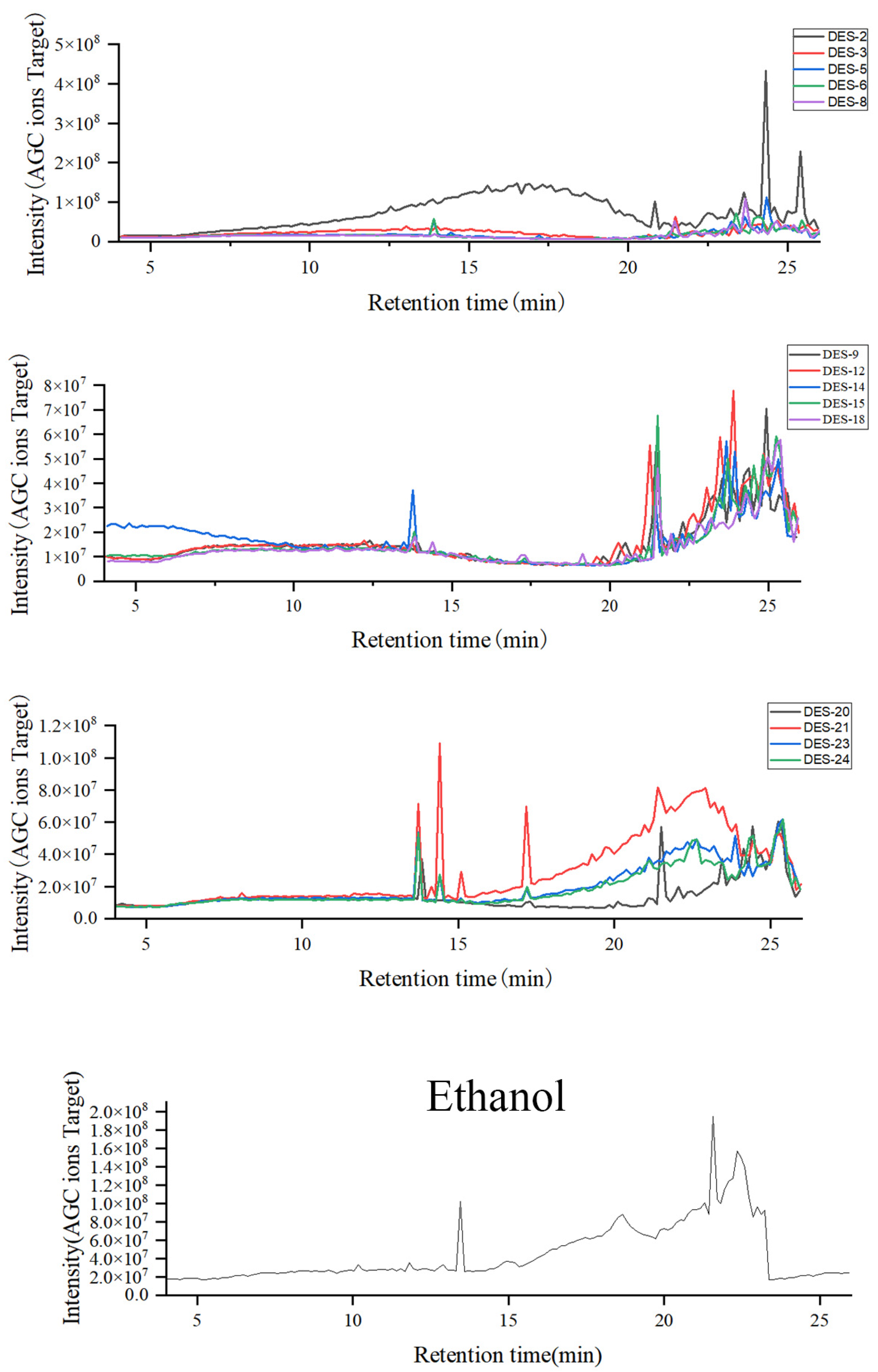
2.3.1. Content Determination Results
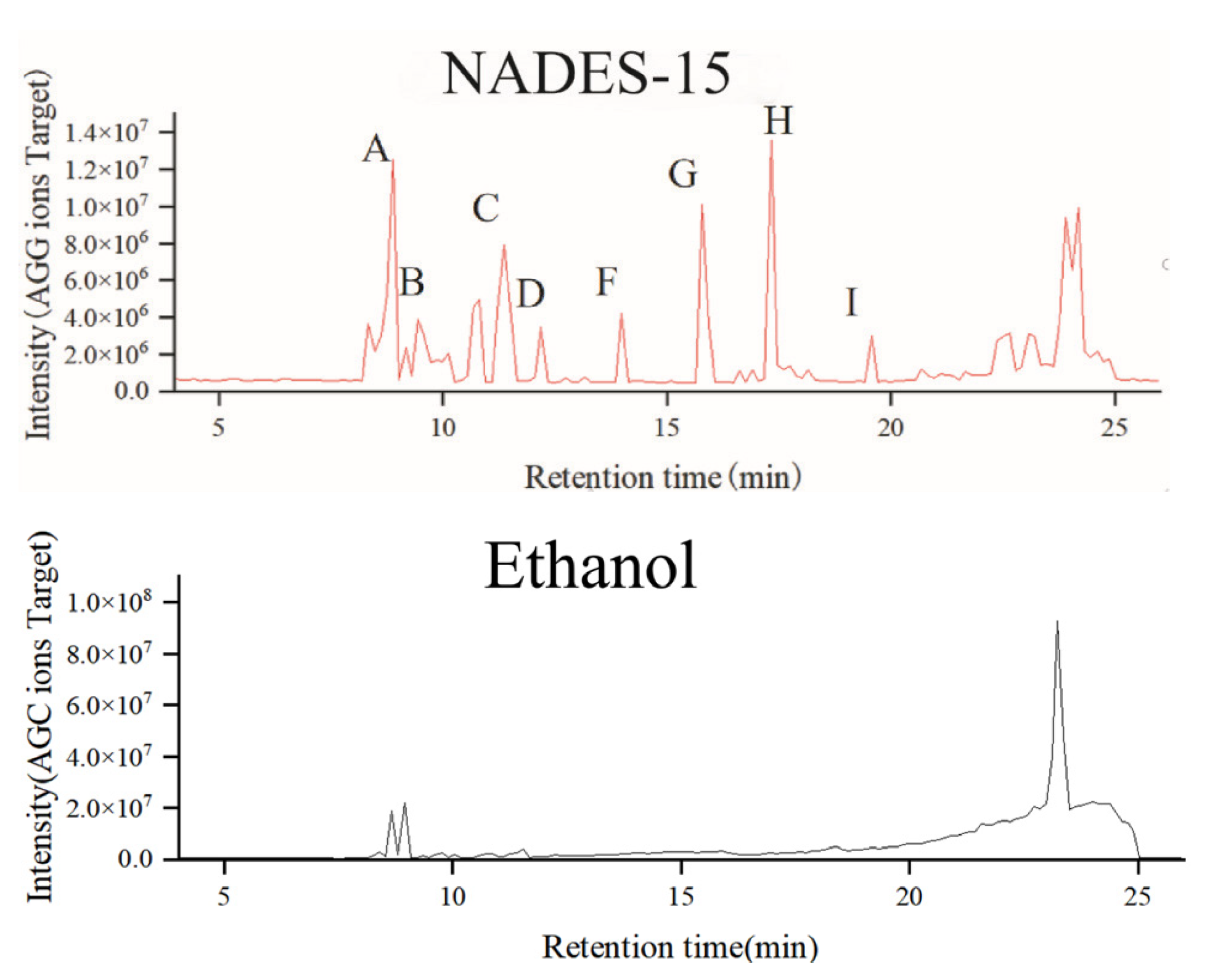
2.3.2. Identification and Comparison of Lilium lancifolium Saponin Components
3. Discussion
4. Materials and Methods
4.1. Chemicals, Instruments and Plant Materials
4.2. Experimental Contents and Methods
4.2.1. Preparation of NADES
4.2.2. Stability Study of NADES
4.2.3. Preparation of the Standard Curve
4.3. Extraction and Identification of Lilium lancifolium Chemical Components
4.3.1. Preparation of NADES Extract
4.3.2. Preparation of Ethanol Extract
4.3.3. Determination of Saponin Content
- C = concentration calculated from the standard curve (mg/mL)
- V = volume of the lily extract (mL)
- N = dilution factor
- M = mass of the lily raw powder (g)
4.4. UHPLC-MS/MS Analysis
4.4.1. Chromatographic Conditions
4.4.2. Mass Spectrometry Conditions
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Wang, H.; Wang, B.; Fu, L.; Yuan, M.; Liu, J.; Zhou, L.; Ding, C. Characterization and antioxidant activities of polysaccharides from the leaves of Lilium lancifolium Thunb. Int. J. Biol. Macromol. 2016, 92, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yun, N.; Jang, Y.P.; Kim, J. Lilium lancifolium Thunb. extract attenuates pulmonary inflammation and air space enlargement in a cigarette smoke-exposed mouse model. J. Ethnopharmacol. 2013, 149, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, X.-M.; An, R.-F.; Li, X.-R.; Wu, K.-T.; Li, S.-M.; Huang, X.-F. Four new steroidal glycosides from Lilium lancifolium Thunb. and their antitumor activity. Fitoterapia 2024, 173, 105808. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.-S.; Kim, N.S.; Song, K.-H. The HPLC–PDA Method for Simultaneous Determination of Regalosides from Bulbs of Lilium lancifolium Thunb. and Their Antioxidant Effects. Plants 2024, 13, 2793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, P.P.; Anmol Suresh, P.S.; Sharma, U. NADES extraction, UHPLC-ELSD-based quantification, and network pharmacolo-gy-guided target identification of fourteen specialised metabolites from Trillium govanianum Wall. ex D.Don. Phytochem. Anal. 2024, 35, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Karpitskiy, D.A.; Bessonova, E.A.; Shishov, A.Y.; Kartsova, L.A. Selective extraction of plant bioactive compounds with deep eutectic solvents: Iris sibirica L. as example. Phytochem. Anal. 2024, 35, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of Bioactive Compounds from Cannabis sativa L. Flowers and/or Leaves Using Deep Eutectic Solvents. Front. Nutr. 2022, 9, 892314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, L.; Liaqat, F.; Khazi, M.I.; Sun, J.; Zhu, D. Natural deep eutectic solvents-based green extraction of vanillin: Optimization, purification, and bioactivity assessment. Front. Nutr. 2024, 10, 1279552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ianni, F.; Scandar, S.; Mangiapelo, L.; Blasi, F.; Marcotullio, M.C.; Cossignani, L. NADES-Assisted Extraction of Polyphenols from Coriander Seeds: A Systematic Optimization Study. Antioxidants 2023, 12, 2048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Alfonso, I.M.; Martínez, S.; Genisheva, Z.; Gullón, B. Deep Eutectic Solvents as a Green Tool for the Extraction of Bioactive Phenolic Compounds from Avocado Peels. Molecules 2022, 27, 6646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Yang, Y.-Y.; Zhou, R.-R.; Fang, L.-Z.; Zhao, D.; Cai, P.; Yu, R.; Zhang, S.-H.; Huang, J.-H. The extraction of phenolic acids and polysaccharides from Lilium lancifolium Thunb. using a deep eutectic solvent. Anal. Methods 2021, 13, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Gray, S. Design of improved deep eutectic solvents using hole theory. Chemphyschem 2006, 7, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, H.; Kurek, M.A. Deep eutectic solvents for the extraction of polyphenols from food plants. Food Chem. 2024, 444, 138629. [Google Scholar] [CrossRef] [PubMed]
- Sportiello, L.; Favati, F.; Condelli, N.; Di Cairano, M.; Caruso, M.C.; Simonato, B.; Tolve, R.; Galgano, F. Hydrophobic deep eutectic solvents in the food sector: Focus on their use for the extraction of bioactive compounds. Food Chem. 2023, 405, 134703. [Google Scholar] [CrossRef] [PubMed]
- Plastiras, O.-E.; Andreasidou, E.; Samanidou, V. Microextraction Techniques with Deep Eutectic Solvents. Molecules 2020, 25, 6026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, X.; Song, K.; Yu, H.; Zhou, X.; Guo, J. Extraction and characterisation of kudzu root residue lignin based on deep eutectic solvents. Phytochem. Anal. 2024, 35, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Albertini, B.; Bertoni, S.; Sangiorgi, S.; Nucci, G.; Passerini, N.; Mezzina, E. NaDES as a green technological approach for the solubility improvement of BCS class II APIs: An insight into the molecular interactions. Int. J. Pharm. 2023, 634, 122696. [Google Scholar] [CrossRef] [PubMed]
- Popović, B.M.; Gligorijević, N.; Aranđelović, S.; Macedo, A.C.; Jurić, T.; Uka, D.; Mocko-Blažek, K.; Serra, A.T. Cytotoxicity profiling of choline chloride-based natural deep eutectic solvents. RSC Adv. 2023, 13, 3520–3527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Palos-Hernández, A.; Sánchez, M.d.N.; Fernández, M.Y.G.; Pérez-Iglesias, J.L.; Santos-Buelga, C.; González-Paramás, A.M. Sustainable valorization of grape pomace peels using NADES: A focus on selective recovery of anthocyanins and flavonols and bioactivity. In Analytical and Bioanalytical Chemistry; Advance Online Publication; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–16. [Google Scholar] [CrossRef]
- Gerçek, Y.C.; Kutlu, N.; Çelik, S.; Bayram, S.; Kırkıncı, S.; Bayram, N.E. Optimized ultrasonic-NaDES extraction of anthocyanins, polyphenolics, and organic acids from chokeberry fruit with blueness and antimicrobial evaluation. Microchem. J. 2025, 210, 113061. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Zhou, J.; Yang, Y.; Li, W.; Zhou, P. Application and mechanism of ultrasonic-assisted extraction of ginkgo flavonol glycosides with natural deep eutectic solvent-based supramolecular solvents. Ultrason. Sonochem. 2025, 123, 107664. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, Z.; Grozeva, N.; Todorova, M.; Kamenova-Nacheva, M.; Staleva, P.; Memdueva, N.; Tzanova, M.T. Comparison of the Potential of “Green” Classical and Natural Deep Eutectic Solvents in the Production of Natural Food Colorant Extracts from the Roots of Alkanna tinctoria (L.). Foods 2025, 14, 584. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Wang, N.; Meng, Y.; Fan, S.; Liu, S.; Fan, X.; Hu, D.; Yin, H.; Wang, H.; Wang, X.; et al. Ultrasound-assisted deep eutectic solvent extraction of polysaccharides from Cercis chinensis bark: Optimization, kinetics and antioxidant activities. Ultrason. Sonochem. 2025, 121, 107535. [Google Scholar] [CrossRef]
- Lanari, D.; Zadra, C.; Negro, F.; Njem, R.; Marcotullio, M.C. Influence of choline chloride-based NADES on the composition of Myristica fragrans Houtt. essential oil. Heliyon 2022, 8, e09531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Rao, C.; Ye, X.; Wang, M.; Yang, B.; Wang, C.; Guo, L.; Xiong, Y.; Cui, X. Applications for natural deep eutectic solvents in Chinese herbal medicines. Front. Pharmacol. 2023, 13, 1104096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picchio, M.L.; Minudri, D.; Mantione, D.; Criado-Gonzalez, M.; Guzmán-González, G.; Schmarsow, R.; Müller, A.J.; Tomé, L.C.; Minari, R.J.; Mecerreyes, D. Natural Deep Eutectic Solvents Based on Choline Chloride and Phenolic Compounds as Efficient Bioadhesives and Corrosion Protectors. ACS Sustain. Chem. Eng. 2022, 10, 8135–8142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teslić, N.; Santos, F.; Oliveira, F.; Stupar, A.; Pojić, M.; Mandić, A.; Pavlić, B.; Kljakić, A.C.; Duarte, A.R.C.; Paiva, A.; et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants 2022, 11, 254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Lukk, T.; Karpichev, Y.; Gathergood, N.; Singh, B.N.; Thakur, V.K.; Tabatabaei, M.; Gupta, V.K. Biobased natural deep eutectic system as versatile solvents: Structure, interaction and advanced applications. Sci. Total Environ. 2023, 881, 163002. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, M.; Hu, T.; Liu, C. Natural deep eutectic solvent—A novel green solvent for protein stabilization. Int. J. Biol. Macromol. 2023, 247, 125477. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Zhang, M.; Wan, Y.; Qiu, H. Utilization of deep eutectic solvents as novel mobile phase additives for improving the separation of bioactive quaternary alkaloids. Talanta 2016, 149, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Rachiero, G.P.; Berton, P.; Shamshina, J. Deep Eutectic Solvents: Alternative Solvents for Biomass-Based Waste Valorization. Molecules 2022, 27, 6606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ivanović, M.; Razboršek, M.I.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, J.G.; Li, L.; Kong, L.Y. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2012, 131, 1056–1062. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Yan, L.; Guo, Y.; Niu, L. Phenolic compounds and antioxidant activity of bulb extracts of six Lilium species native to China. Molecules 2012, 17, 9361–9378. [Google Scholar] [CrossRef]
- Hong, X.-X.; Luo, J.-G.; Guo, C.; Kong, L.-Y. New steroidal saponins from the bulbs of Lilium brownii var. viridulum. Carbohydr. Res. 2012, 361, 19–26. [Google Scholar] [CrossRef]
- Eisenreichová, E.; Haladová, M.; Mucaji, P.; Buděšínský, M.; Ubik, K. A new steroidal saponin from the bulbs of Lilium lanci-folium. Die Pharm. 2000, 55, 549–550. [Google Scholar]
- Mimaki, Y.; Nakamura, O.; Sashida, Y.; Satomi, Y.; Nishino, A.; Nishino, H. Steroidal saponins from the bulbs of Lilium longiflorum and their antitumour-promoter activity. Phytochemistry 1994, 37, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Sashida, Y. Steroidal saponins and alkaloids from the bulbs of Lilium brownii var. colchesteri. Chem. Pharm. Bull. 1990, 38, 3055–3059. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Zhang, Q.; Sun, S.; Liu, B.; Zhang, Y.; Zhang, X.; Sun, C. Study on the technology of efficient extraction of eleutheroside E from Acanthopanax senticosus by green solvent DES. Phytochem. Anal. 2022, 33, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Petrochenko, A.A.; Orlova, A.; Frolova, N.; Serebryakov, E.B.; Soboleva, A.; Flisyuk, E.V.; Frolov, A.; Shikov, A.N. Natural Deep Eutectic Solvents for the Extraction of Triterpene Saponins from Aralia elata var. mandshurica (Rupr. & Maxim.) J. Wen. Molecules 2023, 28, 3614. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, W.; Du, M.; Zhong, H.; Fang, X. Widely targeted metabolomic and KEGG analyses of natural deep eutectic solvent-based saponins extraction from Camellia oleifera Abel.: Effects on composition. Food Chem. 2024, 450, 139333. [Google Scholar] [CrossRef]
- Yang, G.-Y.; Song, J.-N.; Chang, Y.-Q.; Wang, L.; Zheng, Y.-G.; Zhang, D.; Guo, L. Natural Deep Eutectic Solvents for the Extraction of Bioactive Steroidal Saponins from Dioscoreae Nipponicae Rhizoma. Molecules 2021, 26, 2079. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Wang, P.-W.; Zhang, H.; Fan, Y.-Y.; Cao, X.; Luo, Y.-Q.; Li, Q.; Njolibimi, M.; Li, W.-J.; Hong, B.; et al. A high-permeability method for extracting purple yam saponins based on ultrasonic-assisted natural deep eutectic solvent. Food Chem. 2024, 457, 140046. [Google Scholar] [CrossRef]
- Shikov, A.N.; Shikova, V.A.; Whaley, A.O.; Burakova, M.A.; Flisyuk, E.V.; Whaley, A.K.; Terninko, I.I.; Generalova, Y.E.; Gravel, I.V.; Pozharitskaya, O.N. The Ability of Acid-Based Natural Deep Eutectic Solvents to Co-Extract Elements from the Roots of Glycyrrhiza glabra L. and Associated Health Risks. Molecules 2022, 27, 7690. [Google Scholar] [CrossRef]
- Shikov, A.N.; Obluchinskaya, E.D.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Pozharitskaya, O.N. The Impact of Natural Deep Eutectic Solvents and Extraction Method on the Co-Extraction of Trace Metals from Fucus vesiculosus. Mar. Drugs 2022, 20, 324. [Google Scholar] [CrossRef]

| Type | 30 Days | 60 Days | 100 Days |
|---|---|---|---|
| NADES-1 | - | + | ++ |
| NADES-2 | - | - | + |
| NADES-3 | - | - | - |
| NADES-4 | + | ++ | ++ |
| NADES-5 | - | - | - |
| NADES-6 | - | - | - |
| NADES-7 | + | + | ++ |
| NADES-8 | - | - | - |
| NADES-9 | - | - | - |
| NADES-10 | - | - | - |
| NADES-11 | + | ++ | ++ |
| NADES-12 | - | - | - |
| NADES-13 | - | + | ++ |
| NADES-14 | - | - | - |
| NADES-15 | - | - | - |
| NADES-16 | + | ++ | ++ |
| NADES-17 | - | - | + |
| NADES-18 | - | - | - |
| NADES-19 | - | + | + |
| NADES-20 | - | - | - |
| NADES-21 | - | - | - |
| NADES-22 | + | ++ | ++ |
| NADES-23 | - | - | - |
| NADES-24 | - | - | - |
| Sample | Absorbance (n = 3) | Content (mg/g) (Mean ± SD) |
|---|---|---|
| NADES-3 | 0.4198 | 28.9 ± 0.26 ** |
| NADES-5 | 0.1625 | 10.40 ± 0.14 * |
| NADES-6 | 0.0774 | 4.20 ± 0.50 |
| NADES-8 | 0.0296 | 0.70 ± 0.005 |
| NADES-9 | 0.0439 | 1.80 ± 0.03 |
| NADES-10 | 0.1196 | 7.20 ± 0.01 |
| NADES-12 | 0.0780 | 4.20 ± 0.01 |
| NADES-14 | 0.1249 | 7.60 ± 0.01 |
| NADES-15 | 0.6636 | 46.6 ± 0.02 ** |
| NADES-18 | 0.0576 | 2.80 ± 0.04 |
| NADES-20 | 0.0231 | 0.30 ± 0.006 |
| NADES-21 | 0.2096 | 13.80 ± 0.20 ** |
| NADES-23 | 0.028 | 0.60 ± 0.01 |
| NADES-24 | 0.0255 | 0.40 ± 0.006 |
| Ethanol | 0.3012 | 8.20 ± 0.10 |
| Code | HBA | HBD | Molar Ratio |
|---|---|---|---|
| NADES-1 | Choline chloride | L-Proline | 1:1 |
| NADES-2 | Choline chloride | Xylitol | 1:1 |
| NADES-3 | Choline chloride | Citric acid | 1:1 |
| NADES-4 | Choline chloride | D-(+)-Glucose | 1:1 |
| NADES-5 | Choline chloride | Malic acid | 1:1 |
| NADES-6 | Choline chloride | Glycerol | 1:1 |
| NADES-7 | Betaine | L-Proline | 1:1 |
| NADES-8 | Betaine | Xylitol | 1:1 |
| NADES-9 | Betaine | Citric acid | 1:1 |
| NADES-10 | Betaine | D-(+)-Glucose | 1:1 |
| NADES-11 | Betaine | Malic acid | 1:1 |
| NADES-12 | Betaine | Glycerol | 1:1 |
| NADES-13 | Choline chloride | L-Proline | 2:1 |
| NADES-14 | Choline chloride | Xylitol | 2:1 |
| NADES-15 | Choline chloride | Citric acid | 2:1 |
| NADES-16 | Choline chloride | D-(+)-Glucose | 2:1 |
| NADES-17 | Choline chloride | Malic acid | 2:1 |
| NADES-18 | Choline chloride | Glycerol | 2:1 |
| NADES-19 | Betaine | L-Proline | 2:1 |
| NADES-20 | Betaine | Xylitol | 2:1 |
| NADES-21 | Betaine | Citric acid | 2:1 |
| NADES-22 | Betaine | D-(+)-Glucose | 2:1 |
| NADES-23 | Betaine | Malic acid | 2:1 |
| NADES-24 | Betaine | Glycerol | 2:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, Y.; Jiang, Z.; Tang, W.; Wang, C.; Zhao, H.; Zhang, Y. Extraction and Identification of Active Components from Lilium lancifolium Based on NADES-UHPLC-MS/MS Technology. Molecules 2025, 30, 4531. https://doi.org/10.3390/molecules30234531
Wang Y, Ma Y, Jiang Z, Tang W, Wang C, Zhao H, Zhang Y. Extraction and Identification of Active Components from Lilium lancifolium Based on NADES-UHPLC-MS/MS Technology. Molecules. 2025; 30(23):4531. https://doi.org/10.3390/molecules30234531
Chicago/Turabian StyleWang, Yuliang, Yingjie Ma, Zhenxu Jiang, Weiwei Tang, Chaoxing Wang, Hong Zhao, and Yu Zhang. 2025. "Extraction and Identification of Active Components from Lilium lancifolium Based on NADES-UHPLC-MS/MS Technology" Molecules 30, no. 23: 4531. https://doi.org/10.3390/molecules30234531
APA StyleWang, Y., Ma, Y., Jiang, Z., Tang, W., Wang, C., Zhao, H., & Zhang, Y. (2025). Extraction and Identification of Active Components from Lilium lancifolium Based on NADES-UHPLC-MS/MS Technology. Molecules, 30(23), 4531. https://doi.org/10.3390/molecules30234531






