Multifunctional Chitosan/Mn(II) Complexes: Preparation, Catalytic Activity in Imine Synthesis and Aldol Reaction, and Effect on Milk Fermentation/Post-Acidification
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.1.1. Synthesis of Complexes
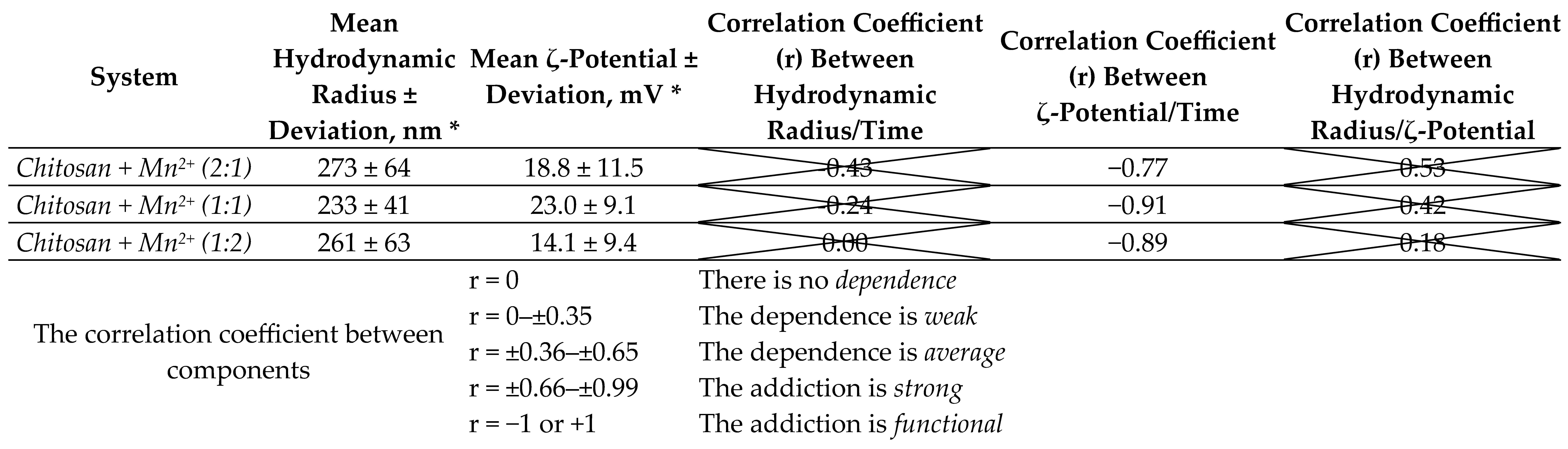
2.1.2. Dynamic and Electrophoretic Light Scattering Studies
2.1.3. FTIR Analysis
2.1.4. X-Ray Diffraction Studies
2.1.5. Differential Thermal and Thermogravimetric Analysis
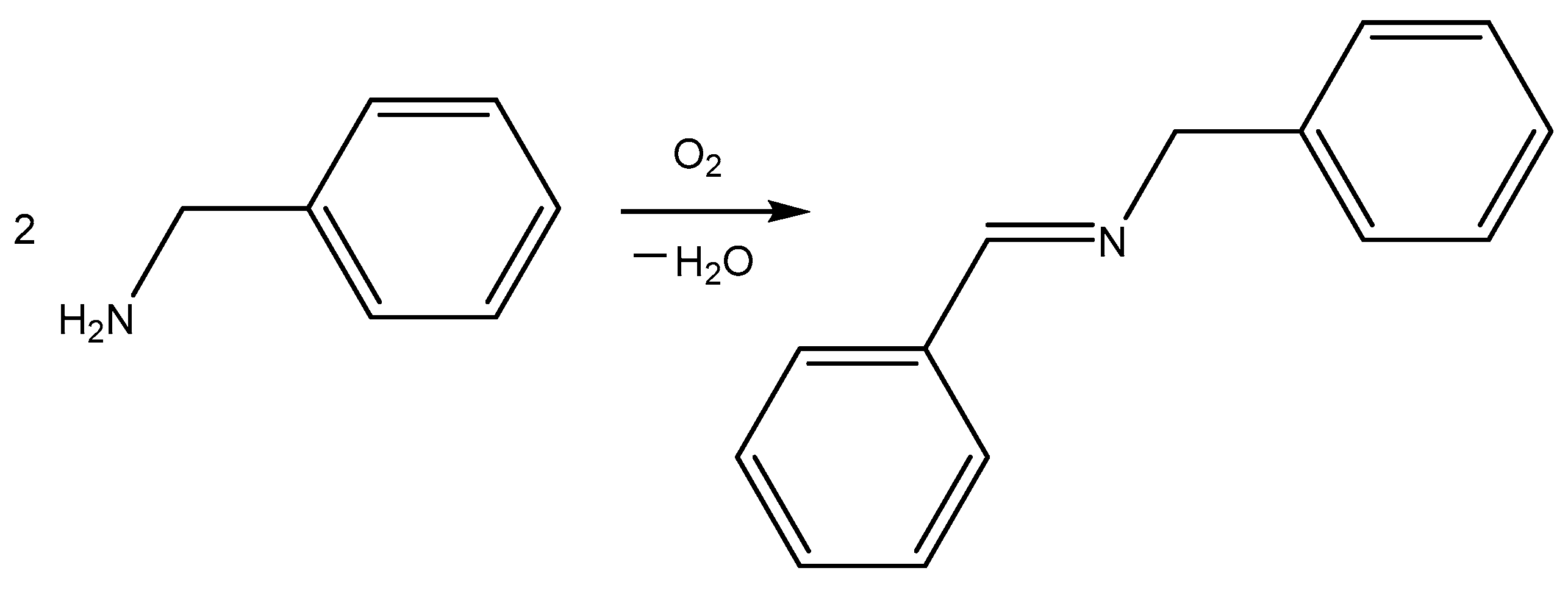
2.1.6. Catalytic Activity of Chitosan–Mn2+ Systems in Oxidative Coupling of Benzylamine
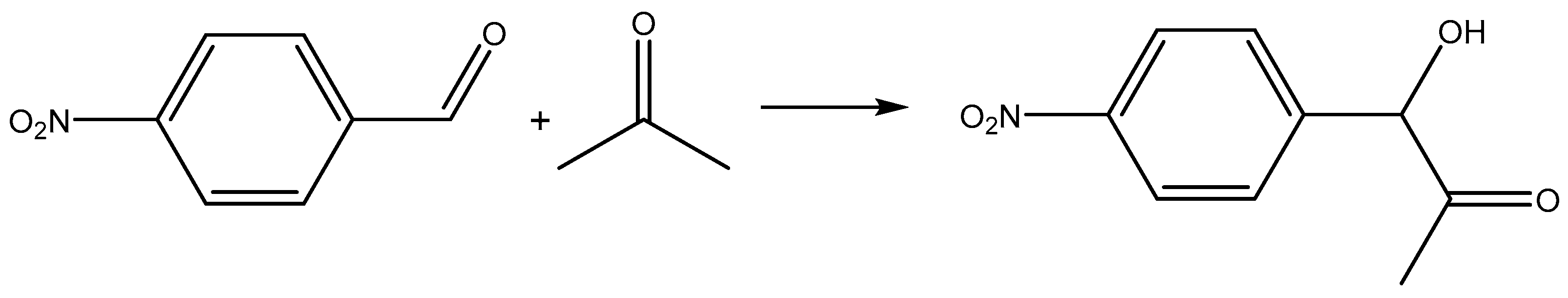
2.1.7. Catalytic Activity of Chitosan–Mn2+ Systems in Aldol Reaction
2.2. Biology
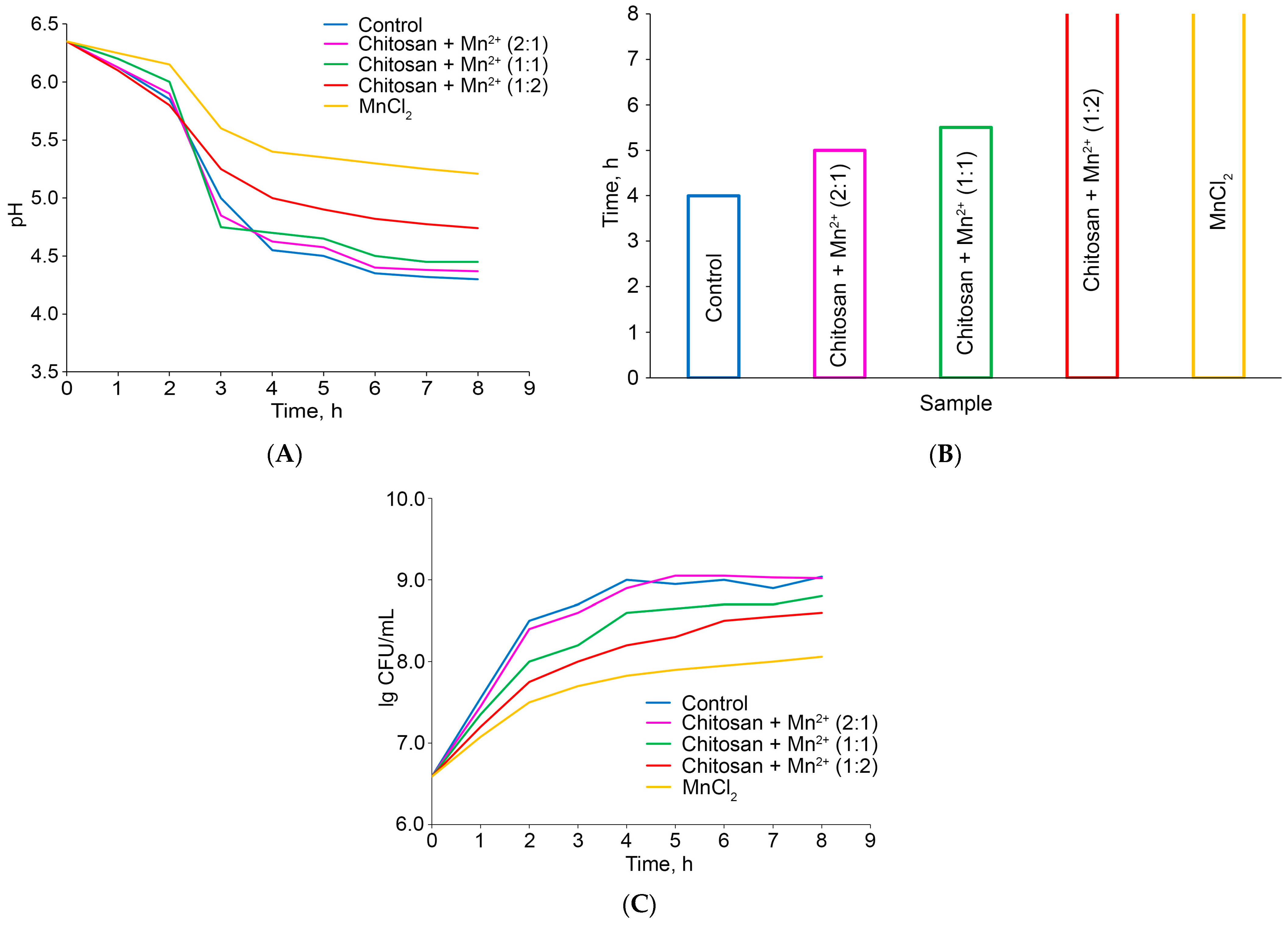
2.2.1. Effect of Chitosan–Mn2+ Systems on Fermentation: Kinetics, pH Changes, and Dynamics of CFU Number Changes
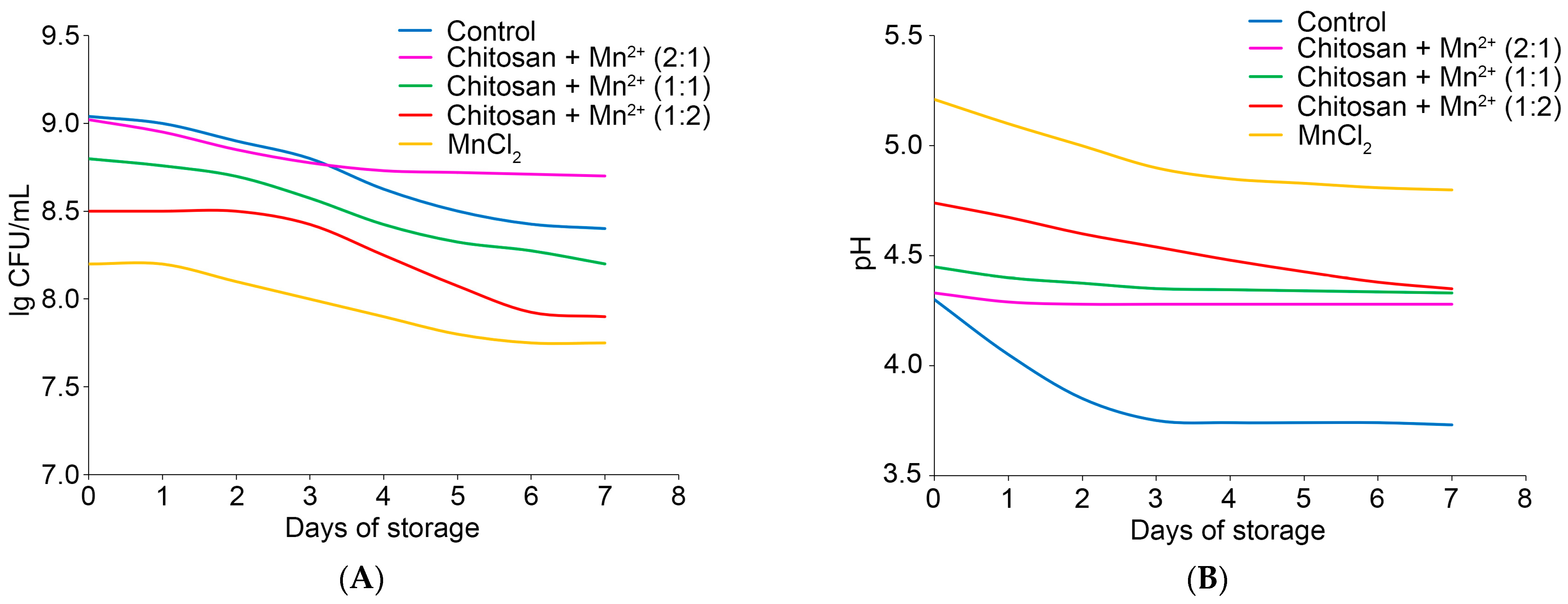
2.2.2. Effect of Chitosan–Mn2+ Systems on Post-Acidification: Dynamics of Changes in the Number of CFU and pH of the Fermented Product During Storage
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. Instrumentation
3.3. Preparation of Chitosan/Manganese(II) Complexes
3.4. Catalytic Synthesis of Imine by Oxidative Coupling of Benzylamine
3.5. Catalytic Aldol Reaction
3.6. Fermentation Study
3.7. Post-Acidification Study
3.8. Statistics
4. Conclusions
- We successfully synthesized and fully characterized nanoparticles based on chitosan and manganese(II) ions in various molar ratios: Chitosan + Mn2+ (1:2), Chitosan + Mn2+ (1:1), and Chitosan + Mn2+ (2:1);
- These nanoparticles exhibited microscale sizes and a progressively decreasing zeta potential in aqueous medium, indicating sedimentation instability;
- ICP analysis verified the quantitative manganese content, while IR spectroscopy demonstrated the coordination of manganese(II) to the chitosan matrix.
- Nanoparticles Chitosan + Mn2+ (1:1) high catalytic activity in oxidative coupling of benzylamine resulting the imine formation. The reaction proceeds selectively under green solvent-free conditions and affords quantitative yields of the product;
- Moreover, Chitosan + Mn2+ (1:1) nanoparticles are efficient catalysts for selective aldol reaction at room temperature in the greenest solvent system H2O/EtOH 5/1 (volume/volume);
- Catalyst Chitosan + Mn2+ (1:1) is very easy to prepare and convenient to use. The catalyst is separated from the reaction mixture by nanoporous filter or centrifugation and does not lose catalytic activity after at least ten uses.
- Chitosan + Mn2+ (2:1) complex significantly reduced the milk fermentation time, demonstrating its efficiency in promoting faster fermentation;
- Over a 7-day storage period, the Chitosan + Mn2+ (2:1) system exhibited the least pronounced decline in colony-forming units (CFUs) of Streptococcus thermophilus;
- The pH of the fermented milk products treated with this system remained nearly constant, with only a slight decrease, indicating enhanced stability;
- Using the Chitosan + Mn2+ (2:1) system nearly doubled the shelf life of fermented milk products compared to the control.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chicea, D.; Nicolae-Maranciuc, A. A Review of Chitosan-Based Materials for Biomedical, Food, and Water Treatment Applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef]
- Wiącek, A.E. (Ed.) Chitosan, Chitosan Derivatives and Their Applications; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2024. [Google Scholar]
- Kumari, S.; Kishor, R. Chapter 1—Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Mabrouk, M.; Hammad, S.F.; Mansour, F.R.; Abdella, A.A. A Critical Review of Analytical Applications of Chitosan as a Sustainable Chemical with Functions Galore. Crit. Rev. Anal. Chem. 2024, 54, 840–856. [Google Scholar] [CrossRef]
- Reshad, R.; Jishan, T.; Chowdhury, N. Chitosan and its Broad Applications: A Brief Review. J. Clin. Trials Exp. Investig. 2021, 12, em00779. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Ainyanbhor, I.E.; Edo, G.I.; Akpoghelie, P.O.; Owheruo, J.O.; Sumer Gaaz, T.S.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Essaghah, A.E.A.; et al. A review on manganese and its effect on health and distribution in selected African countries. J. Trace Elem. Med. Biol. 2025, 91, 127707. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Iqbal, M.A. Coordination Complexes of Manganese and Their Biomedical Applications. ChemistrySelect 2017, 2, 1586–1604. [Google Scholar] [CrossRef]
- Daksh, S.; Kaul, A.; Deep, S.; Datta, A. Current advancement in the development of manganese complexes as magnetic resonance imaging probes. J. Inorg. Biochem. 2022, 237, 112018. [Google Scholar] [CrossRef] [PubMed]
- Vanden Broeck, S.M.P.; Cazin, C.S.J. Manganese-N-heterocyclic carbene (NHC) complexes—An overview. Polyhedron 2021, 205, 115204. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Kronenberger, S.; Naumann, R.; Förster, C.; East, N.R.; Klett, J.; Heinze, K. A manganese(I) complex with a 190 ns metal-to-ligand charge transfer lifetime. Nat. Commun. 2025, 16, 7850. [Google Scholar] [CrossRef]
- Kozieł, S.; Wojtala, D.; Szmitka, M.; Sawka, J.; Komarnicka, U.K. Can Mn coordination compounds be good candidates for medical applications? Front. Chem. Biol. 2024, 3, 1337372. [Google Scholar] [CrossRef]
- Kluczka, J. Removal of boron and manganese ions from wet-flue gas desulfurizationwastewater by hybrid chitosan-zirconium sorbent. Polymers 2020, 12, 635. [Google Scholar] [CrossRef]
- Febriana, E.; Pitriani, P.; Handayani, M.; Irawan, J.; Prasetyo, A.B.; Sulistiyono, E.; Firdiyono, F. Adsorption of Metal Ion Manganese (Mn) in Sodium Silicate Solution using Chitosan. J. Phys. Conf. Ser. 2021, 1912, 012037. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, C.; Shen, P.; Liu, Z.; Hu, J.; Zhang, Y. Facile synthesis of recyclable Mn-crosslinked chitosan schiff base composites as heterogeneous catalysts for selective oxidation of methyl phenyl sulfide with H2O2. Int. J. Biol. Macromol. 2025, 311, 144119. [Google Scholar] [CrossRef]
- Mo, L.Q.; Huang, X.F.; Wang, G.C.; Huang, G.; Liu, P. Full use of factors promoting catalytic performance of chitosan supported manganese porphyrin. Sci. Rep. 2020, 10, 14132. [Google Scholar] [CrossRef]
- Huang, G.; Yan, C.; Cai, J.L.; Mo, L.Q.; Zhao, S.K.; Guo, Y.A.; Wei, S.J.; Shen, Y.L. Practicably efficient ethylbenzene oxidation catalyzed by manganese tetrakis(4-sulfonatophenyl)porphyrin grafted to powdered chitosan. J. Porph. Phthalocyanines 2018, 22, 481–490. [Google Scholar] [CrossRef]
- IşIk, C.; Teke, M. Polycaprolactone/chitosan/manganese (II) oxide nanofiber: Improving pH stability of arginase enzyme. Polym. Bull. 2025, 82, 7827–7850. [Google Scholar] [CrossRef]
- Mohammad Hosseini, N.; Sheshmani, S.; Shahvelayati, A.S. Manganese ferrite-graphite oxide-chitosan nanocomposite for efficient dye removal from aqueous and textile wastewater under UV and sunlight irradiation. Sci. Rep. 2025, 15, 866. [Google Scholar] [CrossRef] [PubMed]
- Dysin, A.P.; Egorov, A.R.; Godzishevskaya, A.A.; Kirichuk, A.A.; Tskhovrebov, A.G.; Kritchenkov, A.S. Biologically Active Supplements Affecting Producer Microorganisms in Food Biotechnology: A Review. Molecules 2023, 28, 1413. [Google Scholar] [CrossRef] [PubMed]
- Badsha, I.; Namasivayam, S.K.R.; Jayaprakash, C.; Nachiyar, C.V.; Bharani, R.S.A. Polylysine. In Handbook of Nutraceuticals and Natural Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 257–272. [Google Scholar]
- Yousefi, M.; Khanniri, E.; Khorshidian, N.; Sohrabvandi, S.; Mortazavian, A.M. Development of Probiotic Apple Juice using Encapsulated Probiotics in Xanthan-Chitosan Based Hydrogels. Appl. Food Biotechnol. 2023, 10, 205–213. [Google Scholar]
- Tamura, Y.; Hayashi, H.; Nishimura, Y.; Ikeda, M. Reactions of 1-alkylbenzimidazolium 3-imines with acetylenic-compounds and benzaldehyde. J. Heterocycl. Chem. 1975, 12, 225–230. [Google Scholar] [CrossRef]
- Hill, A.J.; Walton, A.; Mazzeo, F.A. Suspension Stability; Why Particle Size, Zeta Potential and Rheology are Important. Ann. Trans. Nord. Rheol. Soc. 2011, 20, 209–214. [Google Scholar]
- Belenkii, D.; Balakhanov, D.; Lesnikov, E. Measurement of the zeta potential. Brief review of the main methods. Analytics 2017, 34, 82–89. [Google Scholar] [CrossRef]
- Sustmann, R. A simple model for substituent effects in cycloaddition reactions. I. 1,3-dipolar cycloadditions. Tetrahedron Lett. 1971, 12, 2717–2720. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Liu, H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohydr. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: Hoboken, NJ, USA, 1986; p. 536. [Google Scholar]
- Sustmann, R. A simple model for substituent effects in cycloaddition reactions. II. The diels-alder reaction. Tetrahedron Lett. 1971, 12, 2721–2724. [Google Scholar] [CrossRef]
- Moyano, A.; Pericas, M.A.; Valenti, E. A theoretical-study on the mechanism of the thermal and the acid-catalyzed decarboxylation of 2-oxetanones (beta-lactones). J. Org. Chem. 1989, 54, 573–582. [Google Scholar] [CrossRef]
- Lecea, B.; Arrieta, A.; Roa, G.; Ugalde, J.M.; Cossio, F.P. Catalytic and solvent effects on the cycloaddition reaction between ketenes and carbonyl-compounds to form 2-oxetanones. J. Am. Chem. Soc. 1994, 116, 9613–9619. [Google Scholar] [CrossRef]
- Ritthidej, G.C.; Phaechamud, T.; Koizumi, T. Moist heat treatment on physicochemical change of chitosan salt films. Int. J. Pharm. 2002, 232, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.Z.A.; Harun, M.K.; Ali, A.M.M.; Mohammat, M.F.; Hanafiah, M.A.K.M.; Ibrahim, S.C.; Mustaffa, M.; Darus, Z.M.; Latif, F. XRD and Surface Morphology Studies on Chitosan-Based Film Electrolytes. J. Appl. Sci. 2006, 6, 3150–3154. [Google Scholar] [CrossRef]
- Nieto, J.M.; Peniche-Covas, C.; Padro’n, G. Characterization of chitosan by pyrolysis-mass spectrometry, thermal analysis and differential scanning calorimetry. Thermochim. Acta 1991, 176, 63–68. [Google Scholar] [CrossRef]
- López, F.A.; Mercê, A.L.R.; Alguacil, F.J.; López-Delgado, A. A kinetic study on the thermal behaviour of chitosan. J. Therm. Anal. Calorim. 2007, 91, 633–639. [Google Scholar] [CrossRef]
- de Britto, D.; Campana-Filho, S.P. Kinetics of the thermal degradation of chitosan. Thermochim. Acta 2007, 465, 73–82. [Google Scholar] [CrossRef]
- Wanjun, T.; Cunxin, W.; Donghua, C. Kinetic studies on the pyrolysis of chitin and chitosan. Polym. Degrad. Stabil. 2005, 87, 389–394. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Park, L.H.; Leitao, E.M.; Weber, C.C. Green imine synthesis from amines using transition metal and micellar catalysis. Org. Biomol. Chem. 2023, 22, 202–227. [Google Scholar] [CrossRef]
- Singh, G.S. Chapter 15—Green chemistry of evergreen imines in the synthesis of nitrogen-containing heterocycles. In Green Synthetic Approaches for Biologically Relevant Heterocycles, 2nd ed.; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 655–687. [Google Scholar]
- Chetty, L.C.; Kruger, H.G.; Arvidsson, P.I.; Naicker, T.; Govender, T. Investigating the efficacy of green solvents and solvent-free conditions in hydrogen-bonding mediated organocatalyzed model reactions. RSC Adv. 2024, 14, 7992–7998. [Google Scholar] [CrossRef]
- Cho, J.; Weck, M.; Hwang, S.; Jang, S.S. Multiscale Modeling Approach for the Aldol Addition Reaction in Multicompartment Micelle-Based Nanoreactor. J. Phys. Chem. B 2023, 127, 10067–10076. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, S.; Ghosh, S.K.; Ghosh, A.; Saha, R.; Banerjee, S.; Saha, B. Review of the aldol reaction. Synth. Commun. 2016, 46, 1327–1342. [Google Scholar] [CrossRef]
- Cordes, M.; Kalesse, M. Very recent advances in vinylogous mukaiyama aldol reactions and their applications to synthesis. Molecules 2019, 24, 3040. [Google Scholar] [CrossRef]
- Tekin, K.; Hao, N.; Karagoz, S.; Ragauskas, A.J. Ethanol: A Promising Green Solvent for the Deconstruction of Lignocellulose. ChemSusChem 2018, 11, 3559–3575. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Tiwari, S.; Kumar, A.; Raman, R.K.; Kadyan, S. Review on factors affecting and control of post-acidification in yoghurt and related products. Trends Food Sci. Technol. 2021, 109, 499–512. [Google Scholar] [CrossRef]
- Guenard-Lampron, V.; St-Gelais, D.; Villeneuve, S.; Turgeon, S.L. Short communication: Effect of stirring operations on changes in physical and rheological properties of nonfat yogurts during storage. J. Dairy Sci. 2020, 103, 210–214. [Google Scholar] [CrossRef]
- Ibarra, A.; Acha, R.; Calleja, M.T.; Chiralt-Boix, A.; Wittig, E. Optimization and shelf life of a low-lactose yogurt with Lactobacillus rhamnosus HN001. J. Dairy Sci. 2012, 95, 3536–3548. [Google Scholar] [CrossRef]
- Julijana, T.; Nikola, G.; Borche, M. Examination of pH, Titratable Acidity and Antioxidant Activity in Fermented Milk. J. Mater. Sci. Eng. A 2016, 6, 326–333. [Google Scholar] [CrossRef]
- Shene, C.; Canquil, N.; Bravo, S.; Rubilar, M. Production of the exopolysaccharides by Streptococcus thermophilus: Effect of growth conditions on fermentation kinetics and intrinsic viscosity. Int. J. Food Microbiol. 2008, 124, 279–284. [Google Scholar] [CrossRef]
- Han, M.; Wu, Y.; Guo, X.; Jiang, L.; Wang, X.; Gai, Z. Milk fermentation by monocultures or co-cultures of Streptococcus thermophilus strains. Front. Bioeng. Biotechnol. 2022, 10, 1097013. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, T.; O’Mahony, J.A.; McCarthy, N.A. pH, the Fundamentals for Milk and Dairy Processing: A Review. Dairy 2023, 4, 395–409. [Google Scholar] [CrossRef]
- Popescu, L.; Bulgaru, V.; Siminiuc, R. Effect of Temperature, pH and Amount of Enzyme Used in the Lactose Hydrolysis of Milk. Food Nutr. Sci. 2021, 12, 1243–1254. [Google Scholar] [CrossRef]

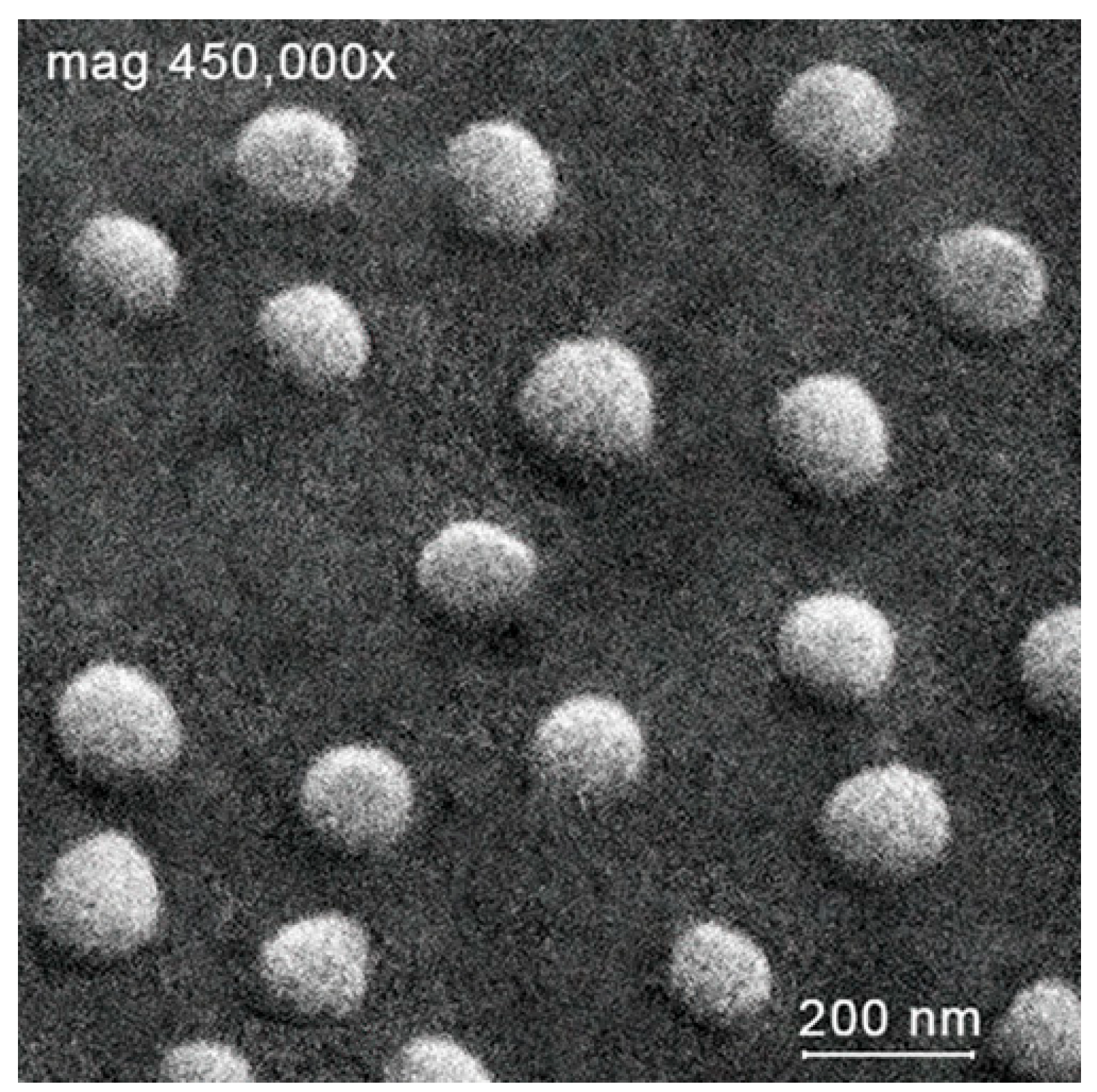

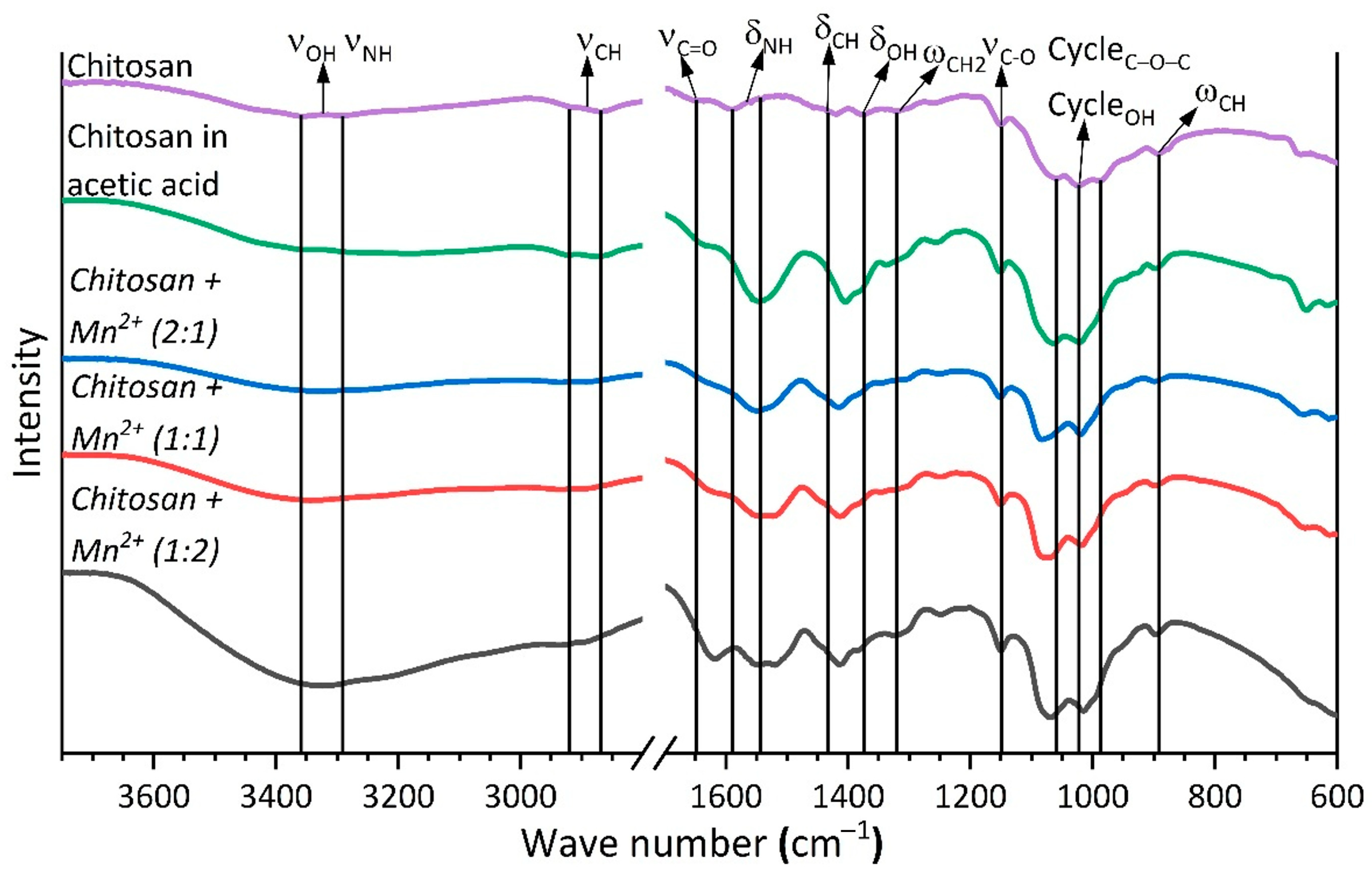
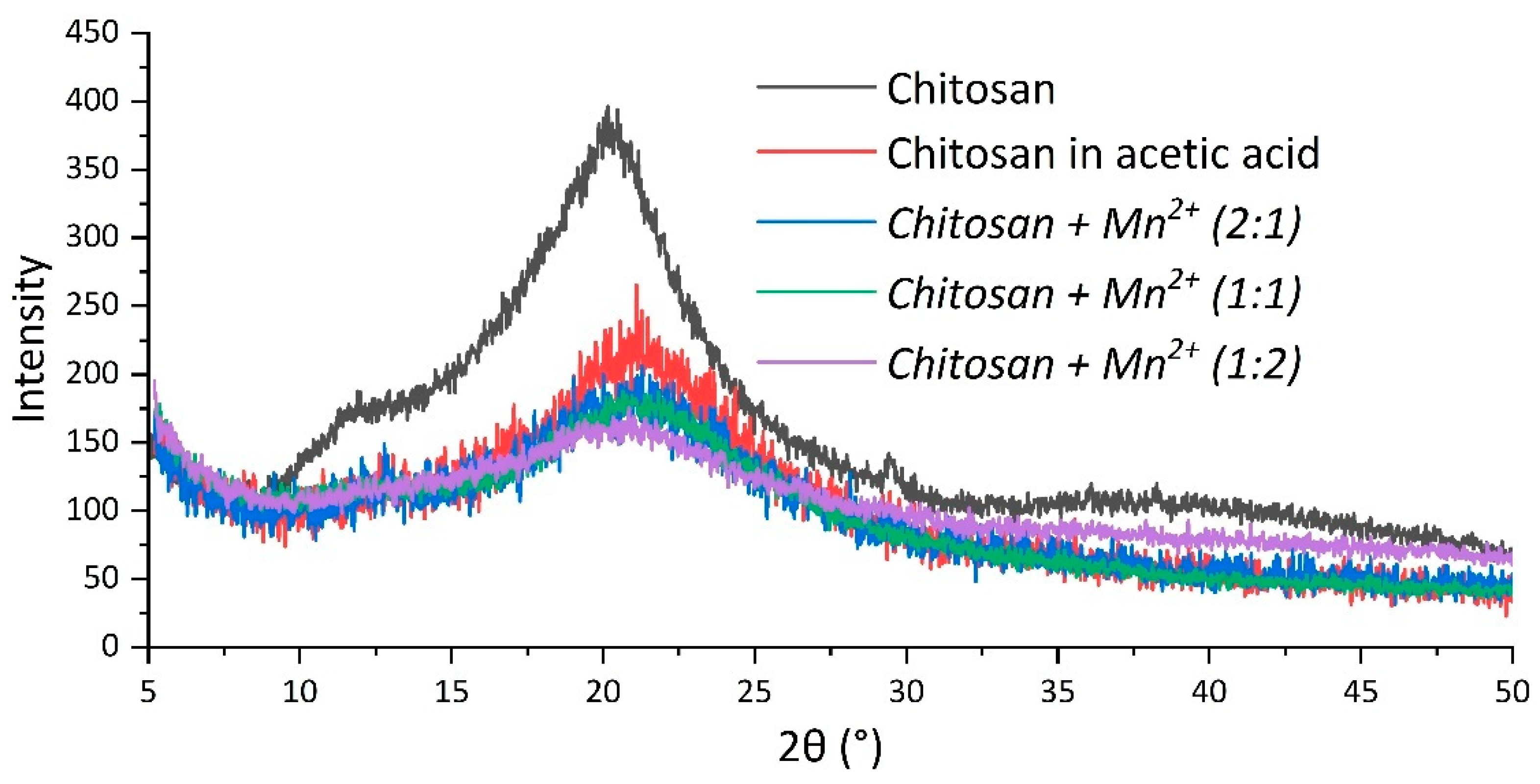

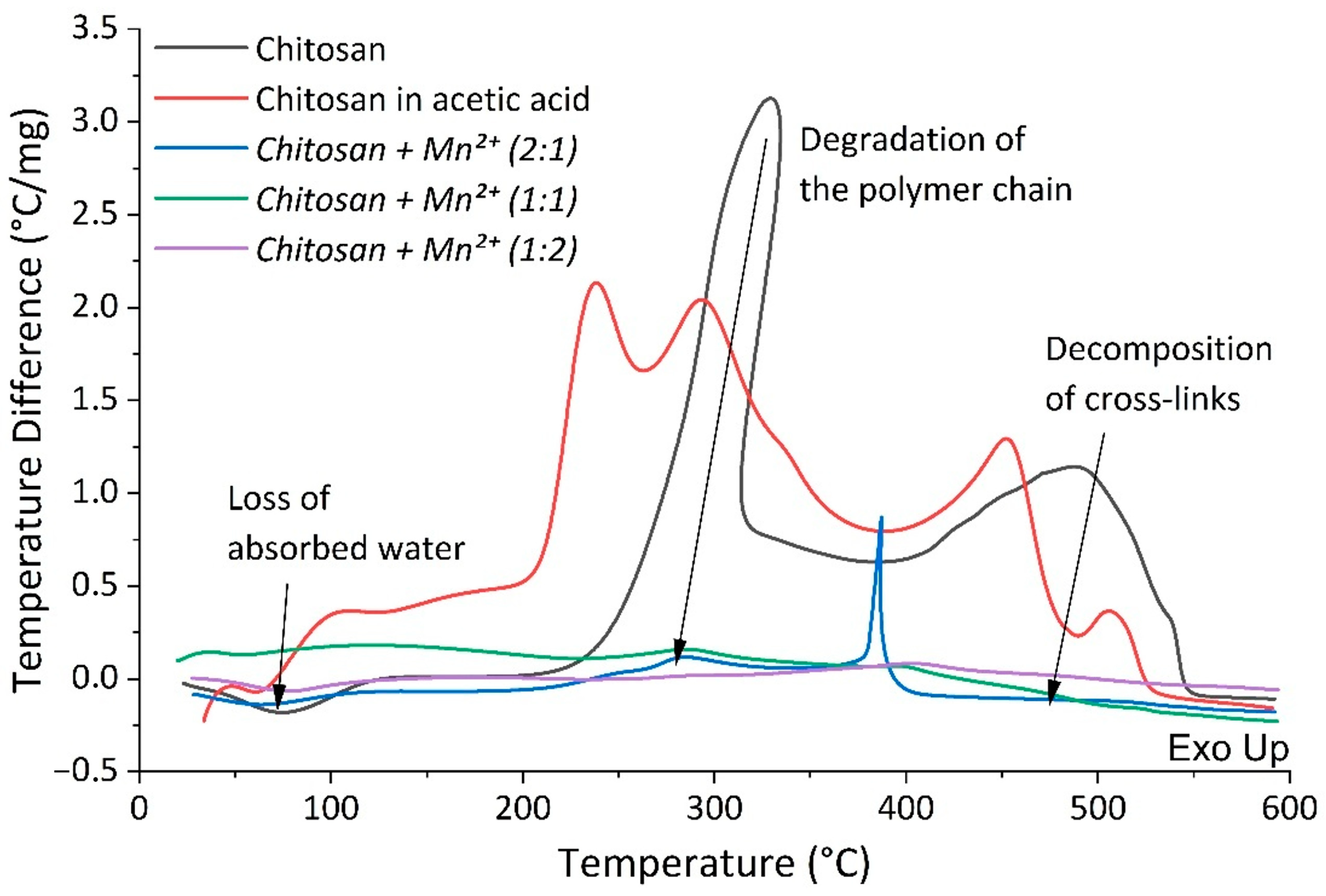
 |
| Chitosan | Chitosan in Acetic Acid | Chitosan + Mn2+ (2:1) | Chitosan + Mn2+ (1:1) | Chitosan + Mn2+ (1:2) | |
|---|---|---|---|---|---|
| ωC-H | 893 | 893 | 899 | 899 | 897 |
| Cycle C–O–C Cycle –OH | 985, 1024, 1059 | 988, 1023, 1065 | 995, 1020, 1082 | 994, 1019, 1070 | 995, 1016, 1070 |
| νC–O | 1150 | 1151 | 1152 | 1150 | 1150 |
| ωCH2 | 1320 | 1302, 1338 | 1304, 1344 | 1304, 1326 | 1299, 1324 |
| δO–H | 1375 | 1378 | 1379 | 1384 | 1383 |
| δC–H | 1419, 1454 | 1404 | 1415, 1451 | 1413, 1451 | 1413, 1453 |
| δN–H | 1542, 1589 | 1548 | 1549 | 1545, 1579 | 1548 |
| νC=O | 1649 | 1634 | 1624 | 1617 | 1616 |
| νC–H | 2868, 2914 | 2877, 2924 | 2890, 2934 | 2894, 2934 | 2894, 2936 |
| νO–H + νN–H | 3291, 3352 | 3185, 3267, 3356 | 3331 | 3218, 3343 | 3212, 3325 |
| № | Sample | Endoeffect (°C)—Loss of Absorbed Water | Weight Loss (%) of Absorbed Water | Exoeffect (°C)—Thermal Degradation of the Polymer Chain, Cleavage of Glycosidic Bonds | Exoeffect (°C)—Decomposition of Chitosan Crosslinks | Weight Loss (%) of Thermal Decomposition | Total Mass Loss (%) |
|---|---|---|---|---|---|---|---|
| 1 | Chitosan | 76 | 7.09 | 329 | 489 | 91.79 | 98.88 |
| 1′ | Chitosan in acetic acid | 62 | 7.00 | 238, 294 | 453, 507 | 92.31 | 99.31 |
| 2 | Chitosan + Mn2+ (2:1) | 65 | 13.14 | 284 | 387, 513 | 73.29 | 86.43 |
| 3 | Chitosan + Mn2+ (1:1) | 54 | 15.15 | 286 | 396, 420, 501 | 67.95 | 83.10 |
| 4 | Chitosan + Mn2+ (1:2) | 78 | 19.75 | 285 | 403, 479 | 50.81 | 70.56 |
| Entry | Catalyst | Solvent | mol% of Catalyst (Based on Mn) | Yield, % ** |
|---|---|---|---|---|
| 1 | Chitosan + Mn2+ (2:1) | H2O | 1 | 20 |
| 2 | Chitosan + Mn2+ (1:1) | H2O | 1 | 22 |
| 3 | Chitosan + Mn2+ (1:2) | H2O | 1 | 20 |
| 4 | Chitosan + Mn2+ (2:1) | MeOH | 1 | traces |
| 5 | Chitosan + Mn2+ (1:1) | MeOH | 1 | traces |
| 6 | Chitosan + Mn2+ (1:2) | MeOH | 1 | traces |
| 7 | Chitosan + Mn2+ (2:1) | MePh | 1 | 27 |
| 8 | Chitosan + Mn2+ (1:1) | MePh | 1 | 34 |
| 9 | Chitosan + Mn2+ (1:2) | MePh | 1 | 25 |
| 10 | Chitosan + Mn2+ (2:1) | – | 1 | 53 |
| 11 | Chitosan + Mn2+ (1:1) | – | 1 | 80 |
| 12 | Chitosan + Mn2+ (1:2) | – | 1 | 63 |
| 13 | – | – | – | 10 |
| 14 | Chitosan + Mn2+ (1:1) | – | 2 | 93 |
| 15 | Chitosan + Mn2+ (1:1) | – | 3 | 100 |
| 16 | Mn2+ | – | 1 | 15 |
| 17 | Chitosan | – | 1 | 6 |
| Entry | Catalyst | Solvent | Conversion of 4-Nitrobenzaldehyde into the Aldol, % ** | |||
|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 60 min | |||
| 1 | Chitosan + Mn2+ (2:1) | H2O | 0 | 0 | 0 | 6 |
| 2 | Chitosan + Mn2+ (1:1) | H2O | 0 | 0 | 8 | 12 |
| 3 | Chitosan + Mn2+ (1:2) | H2O | 0 | 0 | 0 | 7 |
| 4 | Chitosan + Mn2+ (2:1) | H2O/EtOH5/1 (v/v) | 14 | 26 | 41 | 48 |
| 5 | Chitosan + Mn2+ (1:1) | H2O/EtOH5/1 (v/v) | 43 | 85 | 89 | 100 |
| 6 | Chitosan + Mn2+ (1:2) | H2O/EtOH5/1 (v/v) | 20 | 38 | 51 | 63 |
| 7 | – | H2O/EtOH | 0 | 0 | 0 | 0 |
| 8 | Mn2+ | 5/1 (v/v) | 0 | 0 | 0 | 0 |
| 9 | Chitosan | H2O/EtOH | 0 | 0 | 0 | 0 |
| 10 | Chitosan + Mn2+ (1:1) + triethylbenzylammonium chloride (10%) | H2O | 9 | 20 | 38 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubev, R.A.; Nikolaev, A.A.; Semenkova, D.I.; Egorov, A.R.; Nguyen, L.V.; Nazarov, R.H.; Kirichuk, A.A.; Rubanik, V.V.; Shakola, T.V.; Garkushina, I.S.; et al. Multifunctional Chitosan/Mn(II) Complexes: Preparation, Catalytic Activity in Imine Synthesis and Aldol Reaction, and Effect on Milk Fermentation/Post-Acidification. Molecules 2025, 30, 4522. https://doi.org/10.3390/molecules30234522
Golubev RA, Nikolaev AA, Semenkova DI, Egorov AR, Nguyen LV, Nazarov RH, Kirichuk AA, Rubanik VV, Shakola TV, Garkushina IS, et al. Multifunctional Chitosan/Mn(II) Complexes: Preparation, Catalytic Activity in Imine Synthesis and Aldol Reaction, and Effect on Milk Fermentation/Post-Acidification. Molecules. 2025; 30(23):4522. https://doi.org/10.3390/molecules30234522
Chicago/Turabian StyleGolubev, Roman A., Andrey A. Nikolaev, Daria I. Semenkova, Anton R. Egorov, Linh V. Nguyen, Rovshan H. Nazarov, Anatoly A. Kirichuk, Vasili V. Rubanik, Tatsiana V. Shakola, Irina S. Garkushina, and et al. 2025. "Multifunctional Chitosan/Mn(II) Complexes: Preparation, Catalytic Activity in Imine Synthesis and Aldol Reaction, and Effect on Milk Fermentation/Post-Acidification" Molecules 30, no. 23: 4522. https://doi.org/10.3390/molecules30234522
APA StyleGolubev, R. A., Nikolaev, A. A., Semenkova, D. I., Egorov, A. R., Nguyen, L. V., Nazarov, R. H., Kirichuk, A. A., Rubanik, V. V., Shakola, T. V., Garkushina, I. S., Liu, W., Tskhovrebov, A. G., & Kritchenkov, A. S. (2025). Multifunctional Chitosan/Mn(II) Complexes: Preparation, Catalytic Activity in Imine Synthesis and Aldol Reaction, and Effect on Milk Fermentation/Post-Acidification. Molecules, 30(23), 4522. https://doi.org/10.3390/molecules30234522








