Influence of the Cellulose Purification Method on the Properties of PVA Composites with Almond Shell Fibres
Abstract
1. Introduction
2. Results and Discussion
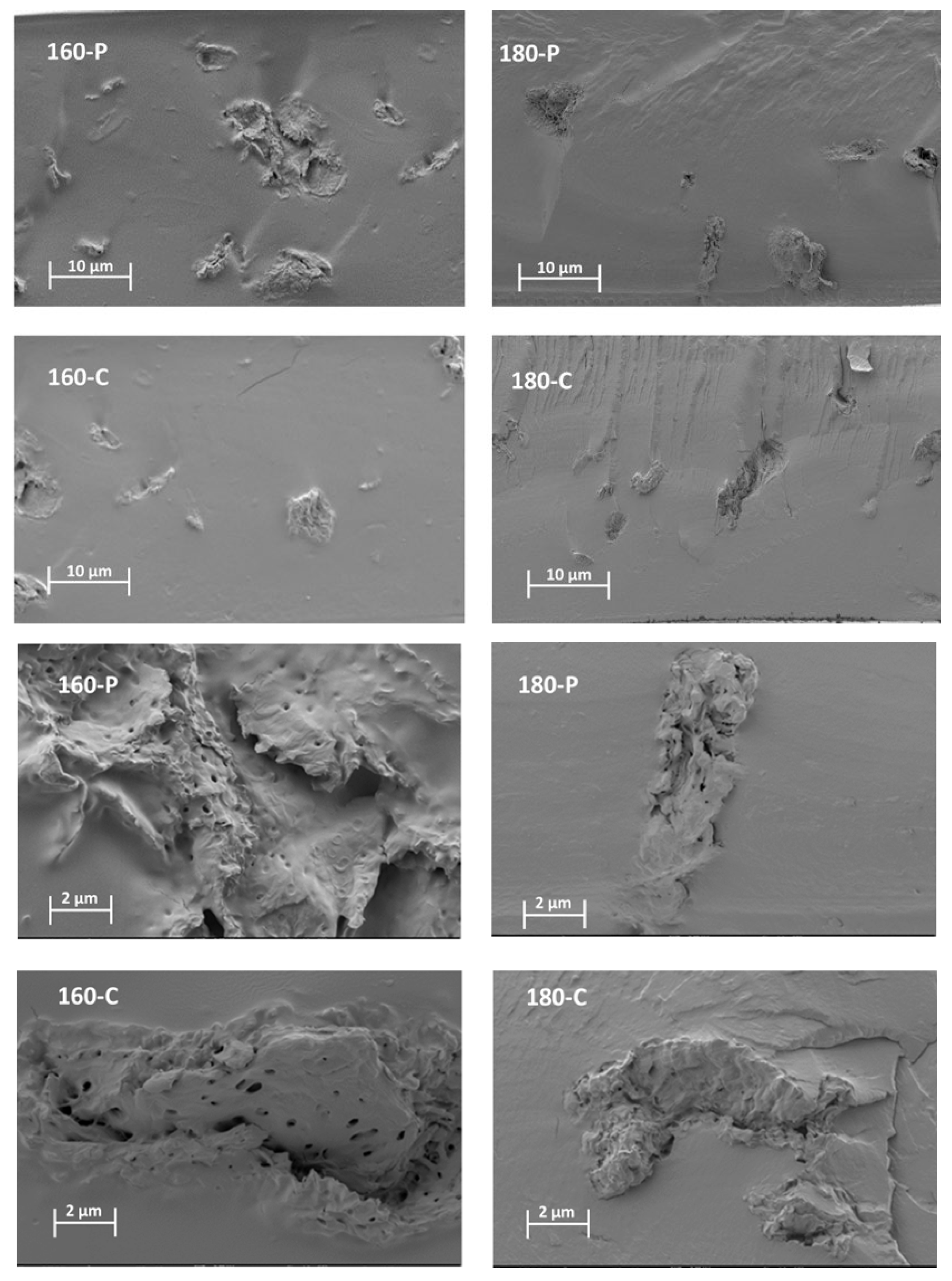
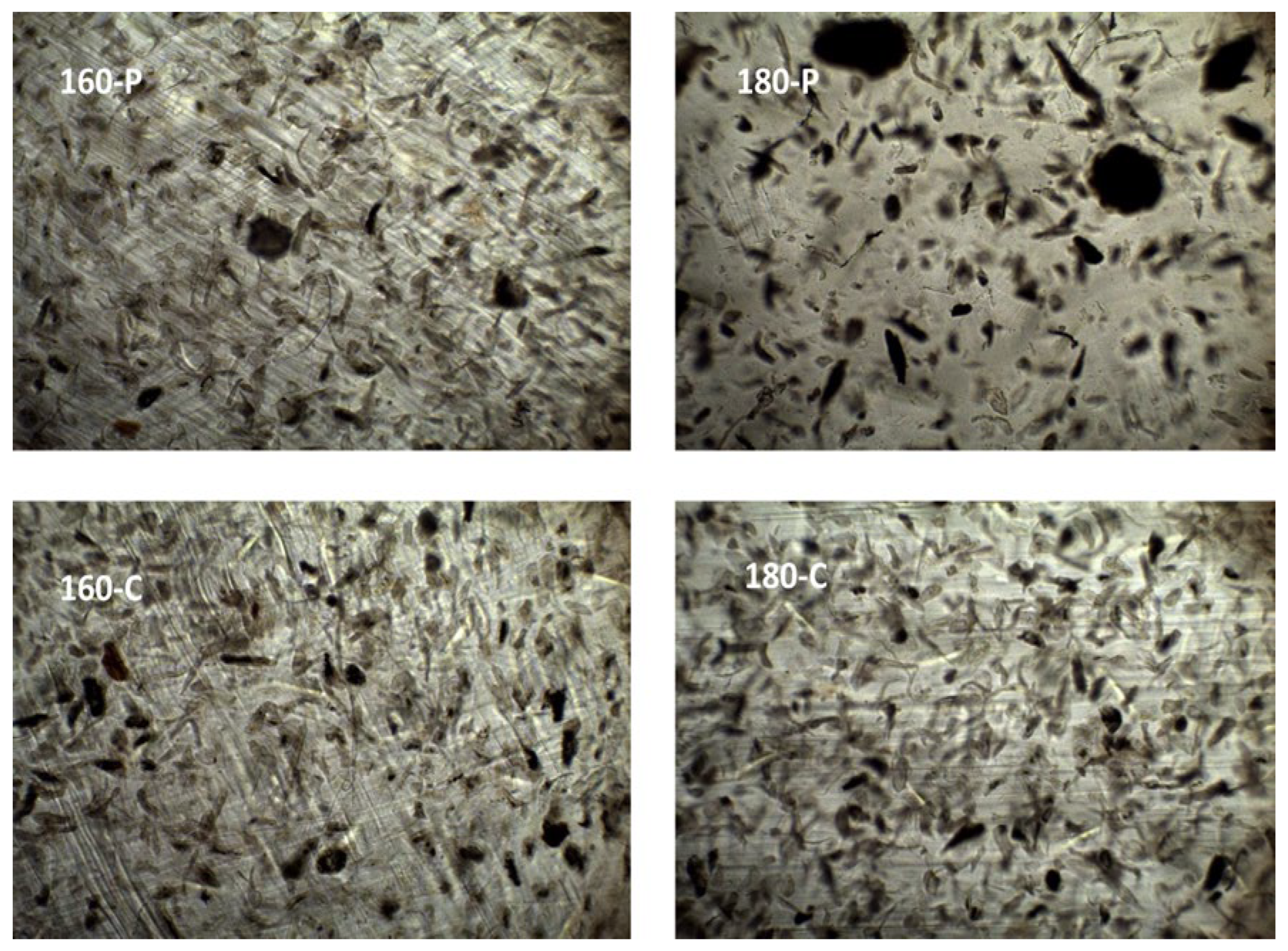
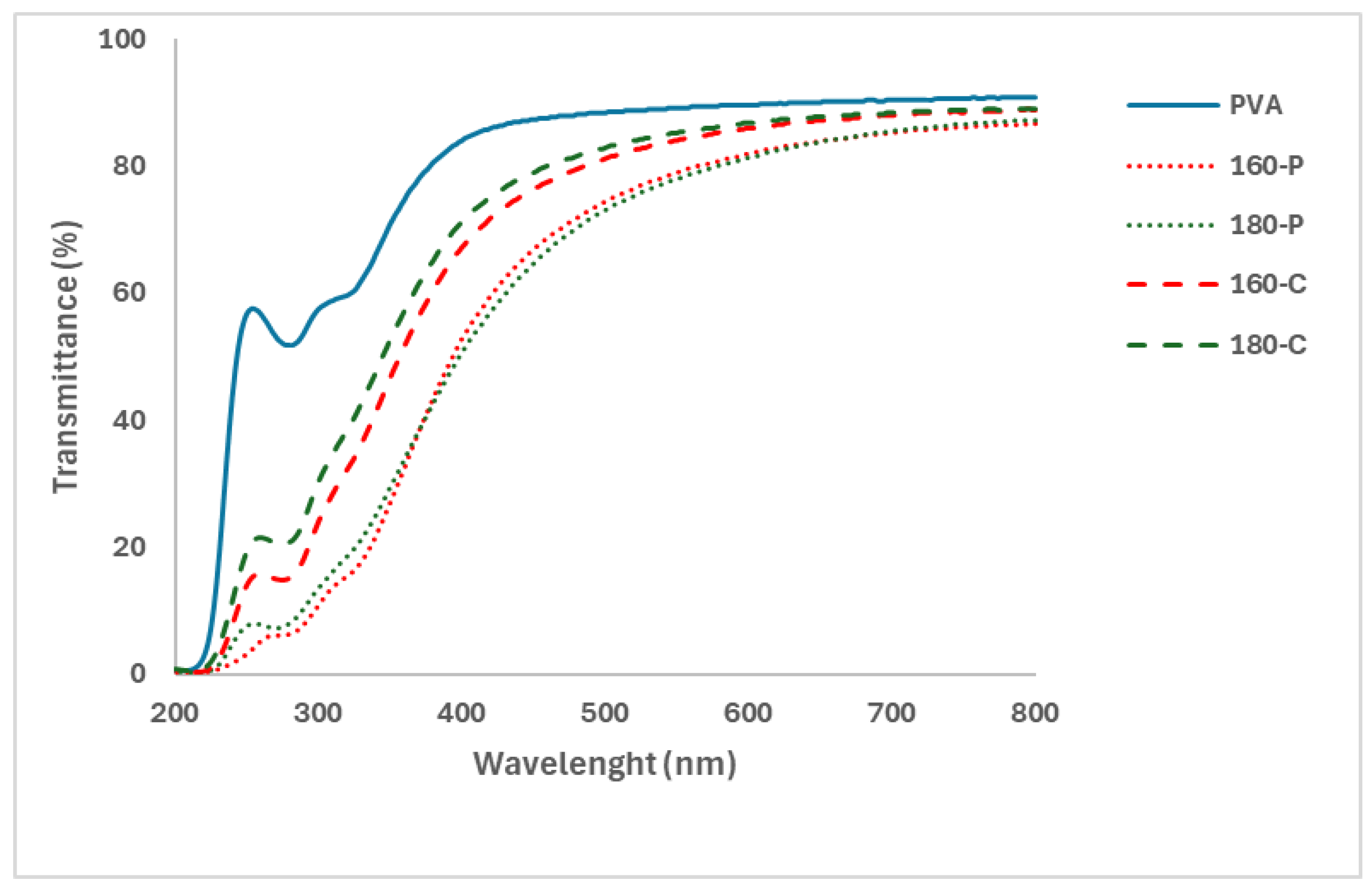
2.1. Structural and Optical Properties of the Films
2.2. Barrier Properties of the Films
2.3. Tensile Properties of the Films
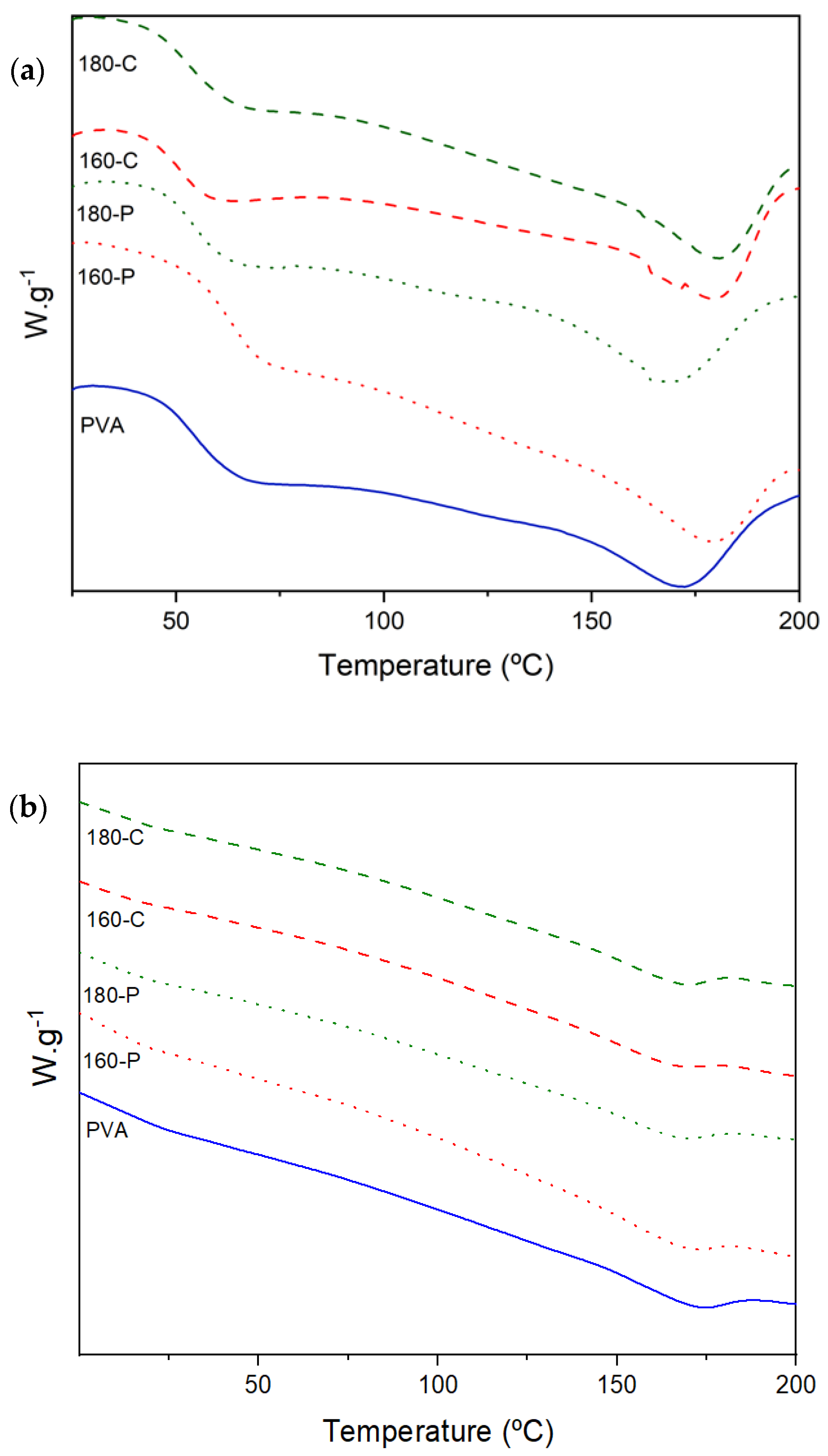
2.4. Glass Transition and Crystallisation Behaviour
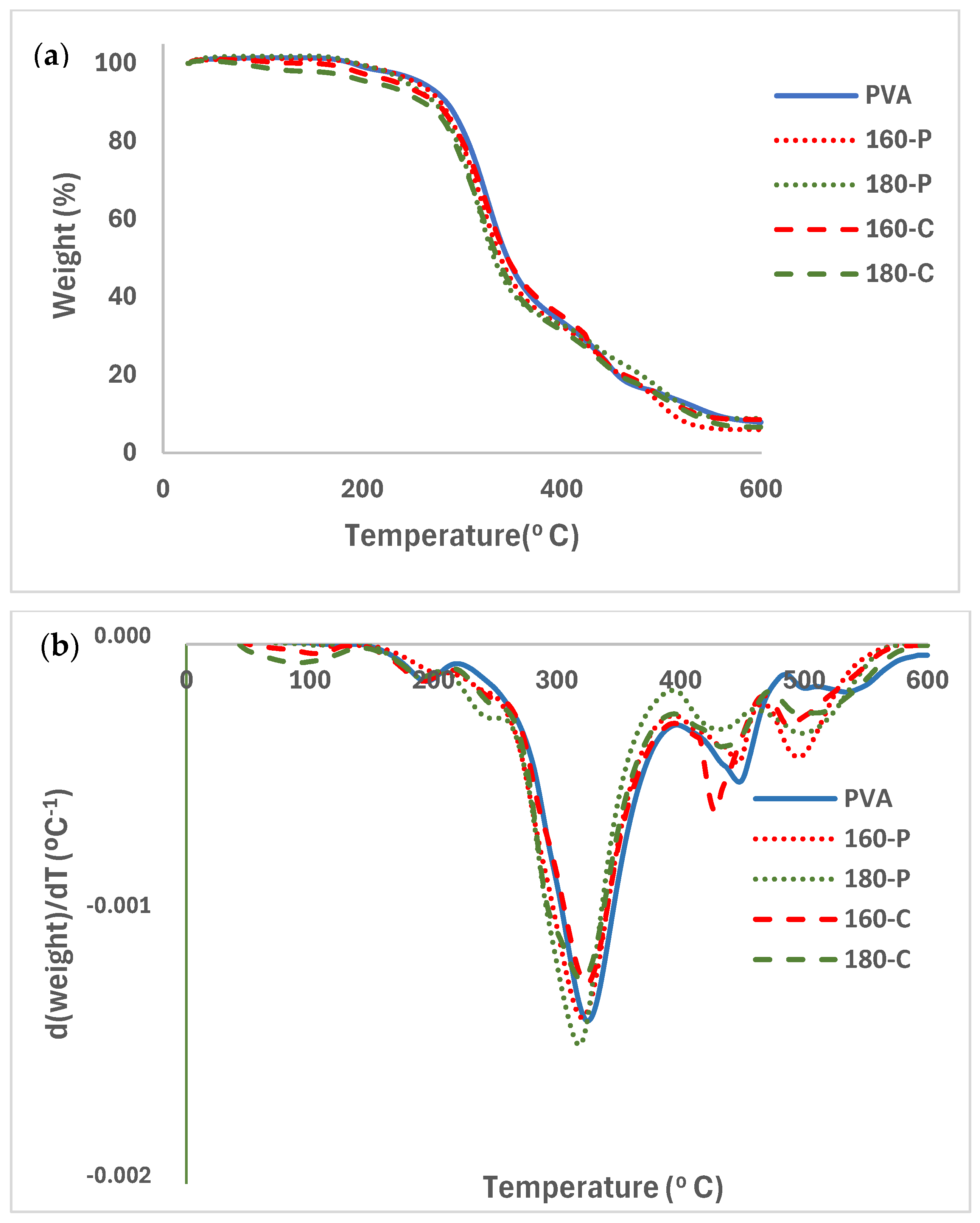
2.5. Thermal Stability of the Films
3. Materials and Methods
3.1. Materials
3.2. Obtaining Composites
3.3. Composite Properties
3.3.1. Microstructure
3.3.2. Optical Properties: Colour and Transparency
3.3.3. Thermal Behaviour (DSC and TGA)
3.3.4. Mechanical Properties
3.3.5. Barrier Properties
3.3.6. Moisture Content
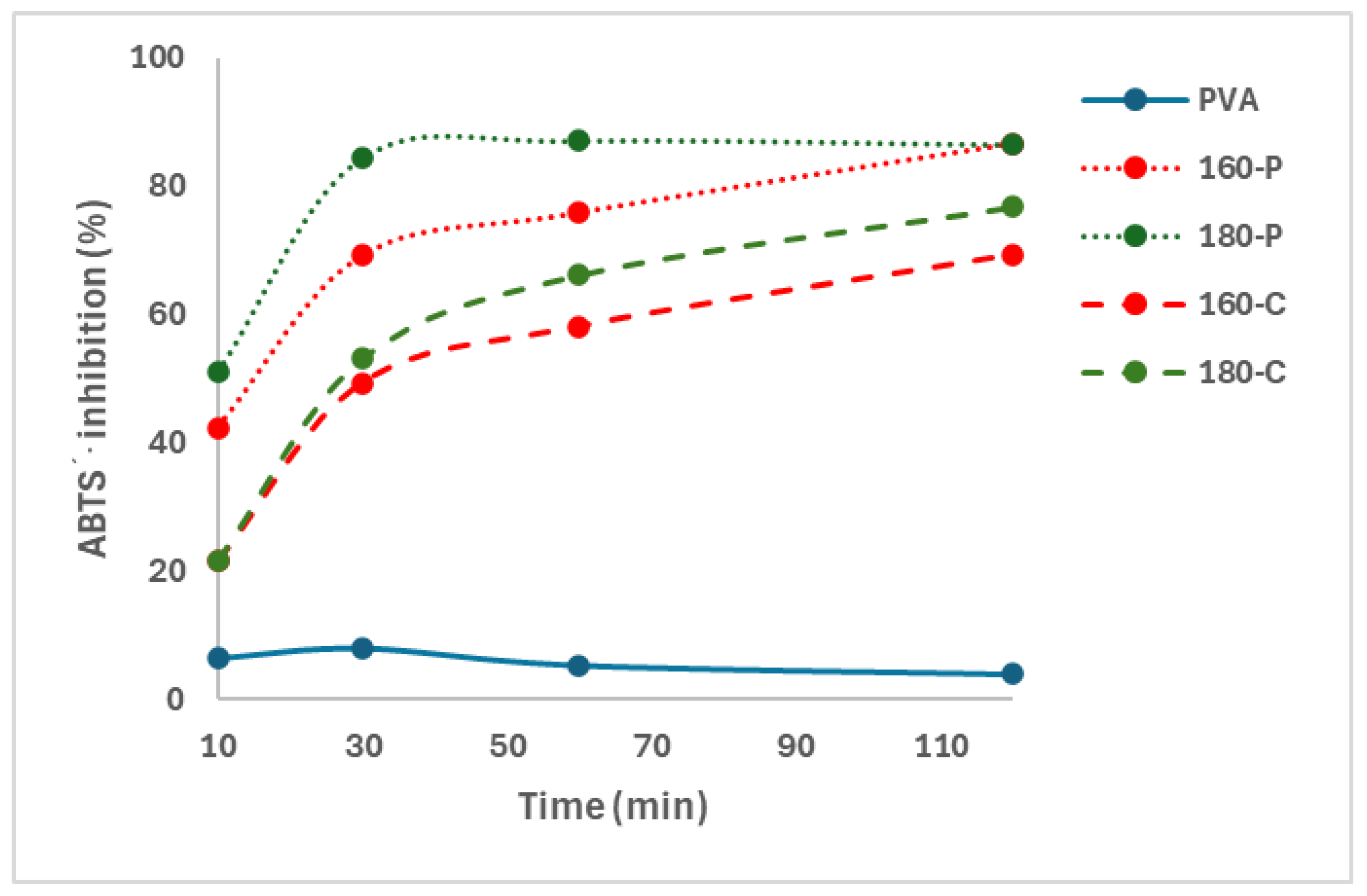
3.3.7. Antioxidant Activity
3.3.8. Contact Angle
3.3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INC International Nut and Dried Fruit Council. Nut and Dried Fruit Statistical Yearbook 2022–23; INC International Nut and Dried Fruit Council: Tarragona, Spain, 2023. [Google Scholar]
- de Hoyos-Martínez, P.L.; Erdocia, X.; Charrier-El Bouhtoury, F.; Prado, R.; Labidi, J. Multistage treatment of almonds waste biomass: Characterization and assessment of the potential applications of raw material and products. Waste Manag. 2018, 80, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzmàn, C.; Summo, C.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef]
- Ibáñez García, A.; Martínez García, A.; Ferrándiz Bou, S. Study of the influence of the almond shell variety on the mechanical properties of starch-based polymer biocomposites. Polymers 2020, 12, 2049. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Phosphonium ionic liquid as interfacial agent of layered double hydroxide: Application to a pectin matrix. Carbohydr. Polym. 2018, 182, 142–148. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Chen, M.; Wei, Y.; Liu, C. Functional packaging films originating from hemicelluloses laurate by direct transesterification in ionic liquid. Carbohydr. Polym. 2020, 229, 115336. [Google Scholar] [CrossRef]
- Dias, M.; Pinto, J.; Henriques, B.; Figueira, P.; Fabre, E.; Tavares, D.; Pereira, E. Nutshells as efficient biosorbents to remove cadmium, lead, and mercury from contaminated solutions. Int. J. Environ. Res. Public Health 2021, 18, 1580. [Google Scholar] [CrossRef] [PubMed]
- Senturk, H.B.; Ozdes, D.; Duran, C. Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent. Desalination 2010, 252, 81–87. [Google Scholar] [CrossRef]
- Essabir, H.; Nekhlaoui, S.; Malha, M.; Bensalah, M.O.; Arrakhiz, F.Z.; Qaiss, A.; Bouhfid, R. Bio-composites based on polypropylene reinforced with Almond Shells particles: Mechanical and thermal properties. Mater. Des. 2013, 51, 225–230. [Google Scholar] [CrossRef]
- Lashgari, A.; Eshghi, A.; Farsi, M. A study on some properties of polypropylene based nanocomposites made using almond shell flour and organoclay. Asian J. Chem. 2013, 25, 1043–1049. [Google Scholar] [CrossRef]
- Veeman, D.; Palaniyappan, S. Process optimisation on the compressive strength property for the 3D printing of PLA/almond shell composite. J. Thermoplast. Compos. Mater. 2023, 36, 2435–2458. [Google Scholar] [CrossRef]
- Valdés, A.; Martínez, C.; Garrigos, M.C.; Jimenez, A. Multilayer films based on poly (lactic acid)/gelatin supplemented with cellulose nanocrystals and antioxidant extract from almond shell by-product and its application on hass avocado preservation. Polymers 2021, 13, 3615. [Google Scholar] [CrossRef] [PubMed]
- Radotić, K.; Mićić, M. Methods for extraction and purification of lignin and cellulose from plant tissues. In Sample Preparation Techniques for Soil, Plant, and Animal Samples; Springer: Berlin/Heidelberg, Germany, 2016; pp. 365–376. [Google Scholar]
- Rao, L.N.; Lakavat, M.; Gandi, S.; Sri, G.K. Extraction and characterization of cellulose from agricultural waste materials. Mater. Today Proc. 2023, 80, 2740–2743. [Google Scholar]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Atiqah, A.; Ansari, M.N.M.; Norrrahim, M.N.F. Production, processes and modification of nanocrystalline cellulose from agro-waste: A review. In Nanocrystalline Materials; Intech Open: Rijeka, Croatia, 2019; pp. 3–32. [Google Scholar]
- Modica, A.; Rosselli, S.; Catinella, G.; Sottile, F.; Catania, C.A.; Cavallaro, G.; Bruno, M. Solid state 13C-NMR methodology for the cellulose composition studies of the shells of Prunus dulcis and their derived cellulosic materials. Carbohydr. Polym. 2020, 240, 116290. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Shahi, A.K.; Dutta, H.; Sahu, J.K. Extraction and characterization of cellulose and cellulose nanowhiskers from almond shell biomass, metal removal and toxicity analysis. Biointerface Res. Appl. Chem. 2022, 12, 1705–1720. [Google Scholar]
- Morales, A.; Hernández-Ramos, F.; Sillero, L.; Fernández-Marín, R.; Dávila, I.; Gullón, P.; Labidi, J. Multiproduct biorefinery based on almond shells: Impact of the delignification stage on the manufacture of valuable products. Bioresour. Technol. 2020, 315, 123896. [Google Scholar] [CrossRef]
- Alhaji Mohammed, M.; Basirun, W.J.; Abd Rahman, N.M.M.; Salleh, N. The effect of particle size of almond shell powders, temperature and time on the extraction of cellulose. J. Nat. Fibers 2022, 19, 5577–5587. [Google Scholar] [CrossRef]
- Maaloul, N.; Arfi, R.B.; Rendueles, M.; Ghorbal, A.; Diaz, M. Dialysis-free extraction and characterization of cellulose crystals from almond (Prunus dulcis) shells. J. Mater. Environ. Sci. 2017, 8, 4171–4181. [Google Scholar]
- Fukuda, J.; Hsieh, Y.L. Almond shell nanocellulose: Characterization and self-assembling into fibers, films, and aerogels. Ind. Crops Prod. 2022, 186, 115188. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Valdés, A.; Mondragon, G.; Garrigós, M.C.; Eceiza, A.; Jiménez, A. Microwave-assisted extraction of cellulose nanocrystals from almond (Prunus amygdalus) shell waste. Front. Nutr. 2023, 9, 1071754. [Google Scholar] [CrossRef] [PubMed]
- Gil-Guillén, I.; Freitas, P.A.; González-Martínez, C.; Chiralt, A. Obtaining Cellulose Fibers from Almond Shell by Combining Subcritical Water Extraction and Bleaching with Hydrogen Peroxide. Molecules 2024, 29, 3284. [Google Scholar] [CrossRef] [PubMed]
- Versino, F.; López, O.V.; García, M.A. Green Biocomposites for Packaging Applications. In Biocomposite Materials: Design and Mechanical Properties Characterization; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–30. [Google Scholar]
- Azammi, A.N.; Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Asrofi, M.; Atiqah, A. Characterization studies of biopolymeric matrix and cellulose fibres-based composites related to functionalized fibre-matrix interface. In Interfaces in Particle and Fibre Reinforced Composites; Woodhead Publishing: Cambridge, UK, 2020; pp. 29–93. [Google Scholar]
- Palanichamy, K.; Anandan, M.; Sridhar, J.; Natarajan, V.; Dhandapani, A. PVA and PMMA nano-composites: A review on strategies, applications and future prospects. Mater. Res. Express 2023, 10, 022002. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-cellulose composites: A review of recent studies on structure, properties and applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Das, L.; Das, P.; Bhowal, A.; Bhattachariee, C. Synthesis of hybrid hydrogel nano-polymer composite using Graphene oxide, Chitosan and PVA and its application in wastewater treatment. Environ. Technol. Innov. 2020, 18, 100664. [Google Scholar] [CrossRef]
- Ghourbanpour, J.; Sabzi, M.; Shafagh, N. Effective dye adsorption behavior of poly (vinyl alcohol)/chitin nanofiber/Fe (III) complex. Int. J. Biol. Macromol. 2019, 137, 296–306. [Google Scholar] [CrossRef]
- Yazik, A.; Tukiran, N.A. Characterization of polyvinyl alcohol (PVA) as antimicrobial biocomposite film: A review. Technology 2021, 7, 79–85. [Google Scholar]
- Sagade Muktar Ahmed, R.F.; Sagade Mokthar Ahamed, M.T.; Madanahalli Ankanathappa, S.; Sannathammegowda, K. Flower Extract–Polyvinyl Alcohol-Based Biocomposites for Sustainable Food Packaging Applications. Phys. Status Solidi 2021, 221, 2300985. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, M.J. Removal of heavy metal ions by hydrogels composed of poly(vinyl alcohol) and carboxymethylcellulose prepared by a freeze-thaw method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Shen, D.; Liu, J.; Gan, L.; Huang, N.; Long, M. Green synthesis of Fe3O4/cellulose/polyvinyl alcohol hybride aerogel and its application for dye removal. J. Polym. Environ. 2018, 26, 2234–2242. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Rowe, A.A.; Tajvidi, M.; Gardner, D.J. Thermal stability of cellulose nanomaterials and their composites with polyvinyl alcohol (PVA). J. Therm. Anal. Calorim. 2016, 126, 1371–1386. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K.; Nassar, M.A. Synthesis and characterization of polyvinyl alcohol/nanospherical cellulose particle films. Carbohydr. Polym. 2010, 79, 694–699. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Luzi, F.; Santulli, C.; Kenny, J.M.; Torre, L. Binary PVA bio-nanocomposites containing cellulose nanocrystals extracted from different natural sources: Part I. Carbohydr. Polym. 2013, 97, 825–836. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Influences of degree of hydrolysis and molecular weight of poly (vinyl alcohol) (PVA) on properties of fish myofibrillar protein/PVA blend films. Food Hydrocoll. 2012, 29, 226–233. [Google Scholar] [CrossRef]
- Andrade, J.; González-Martínez, C.; Chiralt, A. Effect of carvacrol in the properties of films based on poly (vinyl alcohol) with different molecular characteristics. Polym. Degrad. Stab. 2020, 179, 109282. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Rosa, M.D.F.; Cioffi, M.O.H.; Benini, K.C.C.D.C.; Milanese, A.C.; Voorwald, H.J.C.; Mulinari, D.R. Vegetal fibers in polymeric composites: A review. Polym. J. 2015, 25, 9–22. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food and Colour Appearance, Chapman and Hall Food Science Book, 2nd ed.; Springer: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; González-Martínez, C.; Chiralt, A. Physical and active properties of poly (vinyl alcohol) films with phenolic acids as affected by the processing method. Food Packag. Shelf Life 2022, 33, 100855. [Google Scholar] [CrossRef]
- Jayasekara, R.; Harding, I.; Bowater, I.; Christie, G.B.Y.; Lonergan, G.T. Preparation, surface modification and characterisation of solution cast starch PVA blended films. Polym. Test. 2004, 23, 17–27. [Google Scholar] [CrossRef]
- Nuruddin, M.; Chowdhury, R.A.; Szeto, R.; Howarter, J.A.; Erk, K.A.; Szczepanski, C.R.; Youngblood, J.P. Structure–property relationship of cellulose nanocrystal–polyvinyl alcohol thin films for high barrier coating applications. ACS Appl. Mater. Interfaces 2021, 13, 12472–12482. [Google Scholar] [CrossRef]
- Sirvio, J.A.; Honkaniemi, S.; Visanko, M.; Liimatainen, H. Composite films of poly (vinyl alcohol) and bifunctional cross-linking cellulose nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 19691–19699. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Guo, H. Research progress of polyvinyl alcohol water-resistant film materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Development of polyvinyl alcohol/chitosan bio-nanocomposite films reinforced with cellulose nanocrystals isolated from rice straw. Appl. Surf. Sci. 2018, 449, 591–602. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Sadiku, E.R.; Li, X.; He, Y. Extraction of cellulose nanocrystals from areca waste and its application in eco-friendly biocomposite film. Chemosphere 2022, 287, 132084. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, T.; Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos. Part A Appl. Sci. Manuf. 2008, 39, 738–746. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Fardis, D.; Ntampou, X.; Tsivintzelis, I.; Panayiotou, C. Thermal behavior of poly (vinyl alcohol) in the form of physically crosslinked film. Polym. J. 2023, 15, 1843. [Google Scholar] [CrossRef]
- Liu, R.; Morrell, J.J.; Yan, L. Thermogravimetric analysis studies of thermally treated glycerol impregnated poplar wood. BioResources 2018, 13, 1563–1575. [Google Scholar] [CrossRef]
- Ramezani Kakroodi, A.; Cheng, S.; Sain, M.; Asiri, A. Mechanical, thermal, and morphological properties of nanocomposites based on polyvinyl alcohol and cellulose nanofiber from Aloe vera rind. J. Nanomater. 2014, 2014, 903498. [Google Scholar] [CrossRef]
- Ramezani Kakroodi, A.; Leduc, S.; Rodrigue, D. Effect of hybridization and compatibilization on the mechanical properties of recycled polypropylene-hemp composites. J. Appl. Polym. Sci. 2012, 124, 2494–2500. [Google Scholar] [CrossRef]
- D882–12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing Materials: West Conshohocken, PA, USA, 2012.
- E96/E96M; Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing Materials: West Conshohocken, PA, USA, 2005.
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- D3985-025; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using s Coulometric Sensor. American Society for Testing Materials: West Conshohocken, PA, USA, 2010; pp. 1–7.
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef] [PubMed]
| Fibre | Cellulose | Hemicellulose | Lignin | Ashes | WI |
|---|---|---|---|---|---|
| AS | 26.8 ± 1.3 | 23.6 ± 0.2 | 21.2 ± 2.0 | 2.0 ± 0.2 | - |
| 160-P | 70.5 ± 0.9 b | 20.3 ± 1.0 a | 8.5 ± 2.0 a | 4.3 ± 0.3 a | 61.7 ± 0.0 d |
| 180-P | 78.4 ± 0.2 a | 12.2 ± 1.2 b | 4.9 ± 1.2 ab | 4.1 ± 0.3 a | 68.5 ± 0.1 c |
| 160-C | 77.0 ± 4.0 a | 20.0 ± 2.0 a | 3.5 ± 0.5 b | 1.7 ± 0.3 c | 72.4 ± 0.0 b |
| 180-C | 83.7 ± 2.4 a | 12.5 ± 0.7 b | 2.7 ± 0.6 b | 1.1 ± 0.1 c | 79.3 ± 0.4 a |
| Sample | L | Cab* | hab* | ΔE* |
|---|---|---|---|---|
PVA  | 73.2 ± 0.9 a | 16.0 ± 3.0 d | 46.0 ± 0.1 b | - |
160-P  | 55.2 ± 0.3 d | 52.9 ± 0.0 a | 73.3 ± 0.0 a | 24.0 ± 0.9 a |
180-P  | 53.7 ± 0.9 d | 51.0 ± 1.0 a | 73.4 ± 0.3 a | 24.9 ± 2.2 a |
160-C  | 62.9 ± 0.5 b | 39.1 ± 0.8 c | 74.0 ± 0.4 a | 17.5 ± 0.7 b |
180-C  | 60.1 ± 0.1 c | 43.9 ± 1.2 b | 73.6 ± 0.3 a | 20.0 ± 0.7 b |
| Sample | Moisture (g/100 g Solids) | Contact Angle (o) | OP × 1014 (cm3/m s Pa) | WVP × 1011 (g/Pa s m) |
|---|---|---|---|---|
| PVA | 12.1 ± 1.1 a | 44 ± 12 a | 11.5 ± 0.8 a | 219 ± 5 b |
| 160-P | 7.0 ± 0.8 b | 52 ± 13 a | 3.0 ± 0.6 c | 162 ± 3 d |
| 180-P | 10.8± 1.6 ab | 48 ± 16 a | 4.3 ± 0.2 c | 182 ± 6 c |
| 160-C | 10.0 ± 4.0 ab | 56 ± 11 a | 2.8 ± 0.1 c | 267 ± 14 a |
| 180-C | 10.0 ± 3.0 ab | 42 ± 10 a | 6.9 ± 1.0 b | 211 ± 12 b |
| Sample | EM (MPa) | TS (MPa) | E (%) |
|---|---|---|---|
| PVA | 123 ± 7 c | 44.0 ± 7.0 a | 85.1 ± 0.6 a |
| 160-P | 150 ± 20 ab | 17.1 ± 1.6 b | 51.3 ± 0.3 b |
| 180-P | 160 ± 8 ab | 18.7 ± 0.9 b | 44.0 ± 14 b |
| 160-C | 185 ± 9 a | 18.7 ± 2.9 b | 40.0 ± 12 b |
| 180-C | 191 ± 18 a | 19.4 ± 2.5 b | 49.0 ± 5.0 b |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|
| 0% RH | ||||
| PVA | 54.5 ± 0.4 a | 129.2 ± 0.5 c | 172.0 ± 1.0 b | 33.0 ± 3.0 a |
| 160-P | 55.0 ± 4.0 a | 139.0 ± 5.0 ab | 178.0 ± 1.0 a | 34.0 ± 4.0 a |
| 180-P | 57.0 ± 2.0 a | 131.6 ± 0.5 bc | 174.0 ± 2.0 b | 31.2 ± 1.2 a |
| 160-C | 54.0 ± 3.0 a | 146.0 ± 2.0 a | 180.0 ± 1.3 a | 37.7 ± 1.2 a |
| 180-C | 54.3 ± 1.7 a | 145.0 ± 2.0 a | 180.0 ± 1.2 a | 33.0 ± 3.0 a |
| 53% RH | ||||
| PVA | 11.8 ± 1.7 a | 136.0 ± 4.0 a | 169.0 ± 2.0 a | 60.0 ± 6.0 a |
| 160-P | 10.7 ± 0.7 a | 126.1 ± 0.8 a | 164.0 ± 5.0 a | 46.0 ± 3.0 b |
| 180-P | 12.2 ± 1.8 a | 131.0 ± 5.0 a | 164.5 ± 1.7 a | 42.0 ± 4.0 b |
| 160-C | 14.5 ± 0.2 a | 131.0 ± 3.0 a | 165.1 ± 1.4 a | 39.4 ± 0.7 b |
| 180-C | 13.5 ±3.3 a | 137.0 ± 1.0 a | 166.3± 0.2 a | 40.4 ± 1.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Guillén, I.; González-Martínez, C.; Chiralt, A. Influence of the Cellulose Purification Method on the Properties of PVA Composites with Almond Shell Fibres. Molecules 2025, 30, 372. https://doi.org/10.3390/molecules30020372
Gil-Guillén I, González-Martínez C, Chiralt A. Influence of the Cellulose Purification Method on the Properties of PVA Composites with Almond Shell Fibres. Molecules. 2025; 30(2):372. https://doi.org/10.3390/molecules30020372
Chicago/Turabian StyleGil-Guillén, Irene, Chelo González-Martínez, and Amparo Chiralt. 2025. "Influence of the Cellulose Purification Method on the Properties of PVA Composites with Almond Shell Fibres" Molecules 30, no. 2: 372. https://doi.org/10.3390/molecules30020372
APA StyleGil-Guillén, I., González-Martínez, C., & Chiralt, A. (2025). Influence of the Cellulose Purification Method on the Properties of PVA Composites with Almond Shell Fibres. Molecules, 30(2), 372. https://doi.org/10.3390/molecules30020372





