Role of Copper and Zinc Ions in the Hydrolytic Degradation of Neurodegeneration-Related Peptides
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Chen, T.; Cai, J.; Ren, R. Protein aggregation and its affecting mechanisms in neurodegenerative diseases. Neurochem. Int. 2024, 180, 105880. [Google Scholar] [CrossRef] [PubMed]
- Tyczyńska, M.; Gędek, M.; Brachet, A.; Stręk, W.; Flieger, J.; Teresiński, G.; Baj, J. Trace Elements in Alzheimer’s Disease and Dementia: The Current State of Knowledge. J. Clin. Med. 2024, 13, 2381. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, B.; Ding, Y.; Zhang, Y.; Yu, P.; Chang, Y.Z.; Gao, G. The role of iron transporters and regulators in Alzheimer’s disease and Parkinson’s disease: Pathophysiological insights and therapeutic prospects. Biomed. Pharmacother. 2024, 179, 117419. [Google Scholar] [CrossRef]
- Sulatsky, M.I.; Stepanenko, O.V.; Povarova, O.I.; Kuznetsova, I.M.; Turoverov, K.K.; Sulatskaya, A.I. Broken but not beaten: Challenge of reducing the amyloids pathogenicity by degradation. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Rinauro, D.J.; Chiti, F.; Vendruscolo, M.; Limbocker, R. Misfolded protein oligomers: Mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases. Mol. Neurodegener. 2024, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Aiman, A.; Shahid, M.; Chaudhary, A.A.; Sami, N.; Basir, S.F.; Hassan, I.; Islam, A. Amyloid-induced neurodegeneration: A comprehensive review through aggregomics perception of proteins in health and pathology. Ageing Res. Rev. 2024, 96, 102276. [Google Scholar] [CrossRef] [PubMed]
- Siman, R.; Mistretta, S.; Durkin, J.T.; Savage, M.J.; Loh, T.; Trusko, S.; Scott, R.W. Processing of the beta-amyloid precursor. Multiple proteases generate and degrade potentially amyloidogenic fragments. J. Biol. Chem. 1993, 268, 16602–16609. [Google Scholar] [CrossRef]
- Abedin, F.; Kandel, N.; Tatulian, S.A. Effects of Aβ-derived peptide fragments on fibrillogenesis of Aβ. Sci. Rep. 2021, 11, 19262. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Singh, R.; Panghal, A.; Jadhav, K.; Thakur, A.; Verma, R.K.; Singh, C.; Goyal, M.; Kumar, J.; Namdeo, A.G. Recent Advances in Targeting Transition Metals (Copper, Iron, and Zinc) in Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 10916–10940. [Google Scholar] [CrossRef]
- Stewart, K.L.; Radford, S.E. Amyloid plaques beyond Aβ: A survey of the diverse modulators of amyloid aggregation. Biophys. Rev. 2017, 9, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Dodani, S.C.; Firl, A.; Chan, J.; Nam, C.I.; Aron, A.T.; Onak, C.S.; Ramos-Torres, K.M.; Paek, J.; Webster, C.M.; Feller, M.B.; et al. Copper is an endogenous modulator of neural circuit spontaneous activity. Proc. Natl. Acad. Sci. USA 2014, 111, 16280–16285. [Google Scholar] [CrossRef]
- Krall, R.F.; Moutal, A.; Phillips, M.B.; Asraf, H.; Johnson, J.W.; Khanna, R.; Hershfinkel, M.; Aizenman, E.; Tzounopoulos, T. Synaptic zinc inhibition of NMDA receptors depends on the association of GluN2A with the zinc transporter ZnT1. Sci. Adv. 2020, 6, eabb1515. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Harris, P.L.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Cappai, R.; Barnham, K.J. The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim. Biophys. Acta 2007, 1768, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Bacchella, C.; Dell’Acqua, S.; Nicolis, S.; Monzani, E.; Casella, L. The reactivity of copper complexes with neuronal peptides promoted by catecholamines and its impact on neurodegeneration. Coord. Chem. Rev. 2022, 471, 214756. [Google Scholar] [CrossRef]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, Oxidative Stress and Protein-Quinone Modifications in Parkinson’s and Other Neurodegenerative Diseases. Angew. Chem. Int. Ed. Engl. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Bacchella, C.; Brewster, J.T.; Bähring, S.; Dell’Acqua, S.; Root, H.D.; Thiabaud, G.D.; Reuther, J.F.; Monzani, E.; Sessler, J.L.; Casella, L. Condition-Dependent Coordination and Peroxidase Activity of Hemin-Aβ Complexes. Molecules 2020, 25, 5044. [Google Scholar] [CrossRef]
- Pirota, V.; Dell’Acqua, S.; Monzani, E.; Nicolis, S.; Casella, L. Copper-Aβ Peptides and Oxidation of Catecholic Substrates: Reactivity and Endogenous Peptide Damage. Chemistry 2016, 22, 16964–16973. [Google Scholar] [CrossRef]
- Wezynfeld, N.E.; Frączyk, T.; Bal, W. Metal assisted peptide bond hydrolysis: Chemistry, biotechnology and toxicological implications. Coord. Chem. Rev. 2016, 327–328, 166–187. [Google Scholar] [CrossRef]
- Turner, A.J.; Fisk, L.; Nalivaeva, N.N. Targeting amyloid-degrading enzymes as therapeutic strategies in neurodegeneration. Ann. N. Y Acad. Sci. 2004, 1035, 1–20. [Google Scholar] [CrossRef]
- Mukherjee, A.; Song, E.; Kihiko-Ehmann, M.; Goodman, J.P.; Pyrek, J.S.; Estus, S.; Hersh, L.B. Insulysin hydrolyzes amyloid beta peptides to products that are neither neurotoxic nor deposit on amyloid plaques. J. Neurosci. 2000, 20, 8745–8749. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Jo, S.A. Mechanisms of Amyloid-β Peptide Clearance: Potential Therapeutic Targets for Alzheimer’s Disease. Biomol. Ther. 2012, 20, 245–255. [Google Scholar] [CrossRef]
- Taguchi, H.; Planque, S.; Sapparapu, G.; Boivin, S.; Hara, M.; Nishiyama, Y.; Paul, S. Exceptional amyloid beta peptide hydrolyzing activity of nonphysiological immunoglobulin variable domain scaffolds. J. Biol. Chem. 2008, 283, 36724–36733. [Google Scholar] [CrossRef]
- Pike, C.J.; Walencewicz-Wasserman, A.J.; Kosmoski, J.; Cribbs, D.H.; Glabe, C.G.; Cotman, C.W. Structure-activity analyses of beta-amyloid peptides: Contributions of the beta 25-35 region to aggregation and neurotoxicity. J. Neurochem. 1995, 64, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Brion, J.P. Immunological demonstration of tau protein in neurofibrillary tangles of Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 177–185. [Google Scholar] [CrossRef]
- Estaun-Panzano, J.; Arotcarena, M.L.; Bezard, E. Monitoring α-synuclein aggregation. Neurobiol. Dis. 2023, 176, 105966. [Google Scholar] [CrossRef] [PubMed]
- Moons, R.; Konijnenberg, A.; Mensch, C.; Van Elzen, R.; Johannessen, C.; Maudsley, S.; Lambeir, A.M.; Sobott, F. Metal ions shape α-synuclein. Sci. Rep. 2020, 10, 16293. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, J.S.; Santos, R.; Gomes, C.M. Metals and Neuronal Metal Binding Proteins Implicated in Alzheimer’s Disease. Oxid. Med. Cell Longev. 2016, 2016, 9812178. [Google Scholar] [CrossRef] [PubMed]
- von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.M.; Mandelkow, E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129–5134. [Google Scholar] [CrossRef]
- Matsumoto, S.E.; Motoi, Y.; Ishiguro, K.; Tabira, T.; Kametani, F.; Hasegawa, M.; Hattori, N. The twenty-four KDa C-terminal tau fragment increases with aging in tauopathy mice: Implications of prion-like properties. Hum. Mol. Genet. 2015, 24, 6403–6416. [Google Scholar] [CrossRef]
- Wang, Y.P.; Biernat, J.; Pickhardt, M.; Mandelkow, E.; Mandelkow, E.M. Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. Proc. Natl. Acad. Sci. USA 2007, 104, 10252–10257. [Google Scholar] [CrossRef]
- Chen, H.H.; Liu, P.; Auger, P.; Lee, S.H.; Adolfsson, O.; Rey-Bellet, L.; Lafrance-Vanasse, J.; Friedman, B.A.; Pihlgren, M.; Muhs, A.; et al. Calpain-mediated tau fragmentation is altered in Alzheimer’s disease progression. Sci. Rep. 2018, 8, 16725. [Google Scholar] [CrossRef] [PubMed]
- Chesser, A.S.; Pritchard, S.M.; Johnson, G.V. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front. Neurol. 2013, 4, 122. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L.; Emmanouilidou, E.; Pantazopoulou, M.; Kirik, D.; Vekrellis, K.; Tofaris, G.K. How is alpha-synuclein cleared from the cell? J. Neurochem. 2019, 150, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wan, F.; Liu, C.; Zhang, X.; Liu, Z.; Zhang, J.; Xiong, J.; Wang, T.; Zhang, Z. Asparagine endopeptidase inhibitor protects against fenpropathrin-induced neurodegeneration via suppressing α-synuclein aggregation and neuroinflammation. Eur. J. Pharmacol. 2020, 888, 173586. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, S.S.; Liu, X.; Ahn, E.H.; He, L.; Iuvone, P.M.; Duong, D.M.; Seyfried, N.T.; Benskey, M.J.; Manfredsson, F.P.; et al. Asparagine endopeptidase cleaves α-synuclein and mediates pathologic activities in Parkinson’s disease. Nat. Struct. Mol. Biol. 2017, 24, 632–642. [Google Scholar] [CrossRef]
- Nugrahadi, P.P.; Hinrichs, W.L.J.; Frijlink, H.W.; Schöneich, C.; Avanti, C. Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review. Pharmaceutics 2023, 15, 935. [Google Scholar] [CrossRef] [PubMed]
- Alies, B.; Conte-Daban, A.; Sayen, S.; Collin, F.; Kieffer, I.; Guillon, E.; Faller, P.; Hureau, C. Zinc(II) Binding Site to the Amyloid-β Peptide: Insights from Spectroscopic Studies with a Wide Series of Modified Peptides. Inorg. Chem. 2016, 55, 10499–10509. [Google Scholar] [CrossRef]
- El Khoury, Y.; Dorlet, P.; Faller, P.; Hellwig, P. New insights into the coordination of Cu(II) by the amyloid-B 16 peptide from Fourier transform IR spectroscopy and isotopic labeling. J. Phys. Chem. B 2011, 115, 14812–14821. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, G.; Biasotto, F.; Hecel, A.; Luczkowski, M.; Kozlowski, H.; Valensin, D. Structural analysis of copper(I) interaction with amyloid β peptide. J. Inorg. Biochem. 2019, 195, 31–38. [Google Scholar] [CrossRef]
- Minicozzi, V.; Stellato, F.; Comai, M.; Dalla Serra, M.; Potrich, C.; Meyer-Klaucke, W.; Morante, S. Identifying the minimal copper- and zinc-binding site sequence in amyloid-beta peptides. J. Biol. Chem. 2008, 283, 10784–10792. [Google Scholar] [CrossRef]
- Reubsaet, J.L.; Beijnen, J.H.; Bult, A.; van Maanen, R.J.; Marchal, J.A.; Underberg, W.J. Analytical techniques used to study the degradation of proteins and peptides: Chemical instability. J. Pharm. Biomed. Anal. 1998, 17, 955–978. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef]
- Butré, C.I.; Buhler, S.; Sforza, S.; Gruppen, H.; Wierenga, P.A. Spontaneous, non-enzymatic breakdown of peptides during enzymatic protein hydrolysis. Biochim. Biophys. Acta 2015, 1854, 987–994. [Google Scholar] [CrossRef]
- Gursky, O.; Aleshkov, S. Temperature-dependent beta-sheet formation in beta-amyloid Abeta(1-40) peptide in water: Uncoupling beta-structure folding from aggregation. Biochim. Biophys. Acta 2000, 1476, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, Y.; Lomakin, A.; Teplow, D.B.; Benedek, G.B. Temperature dependence of amyloid beta-protein fibrillization. Proc. Natl. Acad. Sci. USA 1998, 95, 12277–12282. [Google Scholar] [CrossRef] [PubMed]
- LeVine, H. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef]
- Tjernberg, L.O.; Näslund, J.; Lindqvist, F.; Johansson, J.; Karlström, A.R.; Thyberg, J.; Terenius, L.; Nordstedt, C. Arrest of beta-amyloid fibril formation by a pentapeptide ligand. J. Biol. Chem. 1996, 271, 8545–8548. [Google Scholar] [CrossRef] [PubMed]
- Tjernberg, L.O.; Lilliehöök, C.; Callaway, D.J.; Näslund, J.; Hahne, S.; Thyberg, J.; Terenius, L.; Nordstedt, C. Controlling amyloid beta-peptide fibril formation with protease-stable ligands. J. Biol. Chem. 1997, 272, 12601–12605. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, M.; Tiwari, M.K.; Hassenkam, T.; Li, M.; Bjerrum, M.J.; Meldal, M. Cascade autohydrolysis of Alzheimer’s Aβ peptides. Chem. Sci. 2023, 14, 4986–4996. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Jankowska, T.; Ruta, M.; Wiśniewska, K.; Łankiewicz, L.; Dyba, M. Products of Cu(II)-catalyzed oxidation in the presence of hydrogen peroxide of the 1-10, 1-16 fragments of human and mouse beta-amyloid peptide. J. Inorg. Biochem. 2004, 98, 940–950. [Google Scholar] [CrossRef]
- Bacchella, C.; Nicolis, S.; Dell’Acqua, S.; Rizzarelli, E.; Monzani, E.; Casella, L. Membrane Binding Strongly Affecting the Dopamine Reactivity Induced by Copper Prion and Copper/Amyloid-β (Aβ) Peptides. A Ternary Copper/Aβ/Prion Peptide Complex Stabilized and Solubilized in Sodium Dodecyl Sulfate Micelles. Inorg. Chem. 2020, 59, 900–912. [Google Scholar] [CrossRef]
- Kanchi, P.K.; Dasmahapatra, A.K. Destabilization of the Alzheimer’s amyloid-β peptide by a proline-rich β-sheet breaker peptide: A molecular dynamics simulation study. J. Mol. Model. 2021, 27, 356. [Google Scholar] [CrossRef] [PubMed]
- Soragni, A.; Zambelli, B.; Mukrasch, M.D.; Biernat, J.; Jeganathan, S.; Griesinger, C.; Ciurli, S.; Mandelkow, E.; Zweckstetter, M. Structural characterization of binding of Cu(II) to tau protein. Biochemistry 2008, 47, 10841–10851. [Google Scholar] [CrossRef]
- Zhou, L.X.; Du, J.T.; Zeng, Z.Y.; Wu, W.H.; Zhao, Y.F.; Kanazawa, K.; Ishizuka, Y.; Nemoto, T.; Nakanishi, H.; Li, Y.M. Copper (II) modulates in vitro aggregation of a tau peptide. Peptides 2007, 28, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.F.; Li, Y.M.; Du, J.T.; Kanazawa, K.; Nemoto, T.; Nakanishi, H.; Zhao, Y.F. Binding of copper (II) ion to an Alzheimer’s tau peptide as revealed by MALDI-TOF MS, CD, and NMR. Biopolymers 2005, 79, 74–85. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Du, J.; Liu, H.; Kanazawa, K.; Nemoto, T.; Nakanishi, H.; Zhao, Y. Copper binding properties of a tau peptide associated with Alzheimer’s disease studied by CD, NMR, and MALDI-TOF MS. Peptides 2006, 27, 841–849. [Google Scholar] [CrossRef]
- Shin, B.K.; Saxena, S. Insight into potential Cu(II)-binding motifs in the four pseudorepeats of tau protein. J. Phys. Chem. B 2011, 115, 15067–15078. [Google Scholar] [CrossRef] [PubMed]
- Valensin, D.; Dell’Acqua, S.; Kozlowski, H.; Casella, L. Coordination and redox properties of copper interaction with α-synuclein. J. Inorg. Biochem. 2016, 163, 292–300. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Heitger, D.R.; Fernández, R.D.; Forney, A.K.; Lucas, H.R. C-Terminal Cu. ACS Chem. Neurosci. 2019, 10, 1402–1410. [Google Scholar] [CrossRef]
- Rasia, R.M.; Bertoncini, C.W.; Marsh, D.; Hoyer, W.; Cherny, D.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernández, C.O. Structural characterization of copper(II) binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 4294–4299. [Google Scholar] [CrossRef]
- Moriarty, G.M.; Minetti, C.A.; Remeta, D.P.; Baum, J. A revised picture of the Cu(II)-α-synuclein complex: The role of N-terminal acetylation. Biochemistry 2014, 53, 2815–2817. [Google Scholar] [CrossRef] [PubMed]
- Dell’Acqua, S.; Pirota, V.; Anzani, C.; Rocco, M.M.; Nicolis, S.; Valensin, D.; Monzani, E.; Casella, L. Reactivity of copper-α-synuclein peptide complexes relevant to Parkinson’s disease. Metallomics 2015, 7, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Bacchella, C.; Camponeschi, F.; Kolkowska, P.; Kola, A.; Tessari, I.; Baratto, M.C.; Bisaglia, M.; Monzani, E.; Bubacco, L.; Mangani, S.; et al. Copper Binding and Redox Activity of α-Synuclein in Membrane-Like Environment. Biomolecules 2023, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Bacchella, C.; Gentili, S.; Bellotti, D.; Quartieri, E.; Draghi, S.; Baratto, M.C.; Remelli, M.; Valensin, D.; Monzani, E.; Nicolis, S.; et al. Binding and Reactivity of Copper to R. Inorg. Chem. 2020, 59, 274–286. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wu, B.; Song, R.; Zhu, S.; Simpson, A.; Wilson, D.J.; Kraatz, H.B. Exploring the interactions of iron and zinc with the microtubule binding repeats R1 and R4. J. Inorg. Biochem. 2020, 205, 110987. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. J. Chem. Soc. (Resumed) 1953, 3192–3210. [Google Scholar] [CrossRef]
- Suh, J.-M.; Kim, M.; Yoo, J.; Han, J.; Paulina, C.; Lim, M.H. Intercommunication between metal ions and amyloidogenic peptides or proteins in protein misfolding disorders. Coord. Chem. Rev. 2023, 478, 214978. [Google Scholar] [CrossRef]
- Chakrabartty, A.; Doig, A.J.; Baldwin, R.L. Helix capping propensities in peptides parallel those in proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 11332–11336. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, A.S.; Ying, J.; Bax, A. Impact of N-terminal acetylation of α-synuclein on its random coil and lipid binding properties. Biochemistry 2012, 51, 5004–5013. [Google Scholar] [CrossRef]
- Kang, L.; Moriarty, G.M.; Woods, L.A.; Ashcroft, A.E.; Radford, S.E.; Baum, J. N-terminal acetylation of α-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci. 2012, 21, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, M.; Bal, W. Cu(II) complexation by "non-coordinating" N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES buffer). J. Inorg. Biochem. 2005, 99, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Conte-Daban, A.; Borghesani, V.; Sayen, S.; Guillon, E.; Journaux, Y.; Gontard, G.; Lisnard, L.; Hureau, C. Link between Affinity and Cu(II) Binding Sites to Amyloid-β Peptides Evaluated by a New Water-Soluble UV-Visible Ratiometric Dye with a Moderate Cu(II) Affinity. Anal. Chem. 2017, 89, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, C.G.; Walter, E.D.; Millhauser, G.L. Coordination features and affinity of the Cu2+ site in the α-synuclein protein of Parkinson’s disease. Biochemistry 2011, 50, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Faller, P.; Hasler, D.W.; Zerbe, O.; Klauser, S.; Winge, D.R.; Vasák, M. Evidence for a dynamic structure of human neuronal growth inhibitory factor and for major rearrangements of its metal-thiolate clusters. Biochemistry 1999, 38, 10158–10167. [Google Scholar] [CrossRef]
- Peana, M.; Frączyk, T.; Zoroddu, M.A. Interplay of Metal Ions and Posttranslational Modifications in Proteins. Eur. J. Inorg. Chem. 2024, 27, e202400175. [Google Scholar] [CrossRef]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid. Redox Signal 2016, 24, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Tabner, B.J.; El-Agnaf, O.M.; German, M.J.; Fullwood, N.J.; Allsop, D. Protein aggregation, metals and oxidative stress in neurodegenerative diseases. Biochem. Soc. Trans. 2005, 33, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C.; La Penna, G. Metal ions and intrinsically disordered proteins and peptides: From Cu/Zn amyloid-β to general principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowich-Knipp, S.J.; Chakrabarti, S.; Williams, T.D.; Dillman, R.K.; Siahaan, T.J. Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. 1999, 53, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Brange, J.; Langkjaer, L.; Havelund, S.; Vølund, A. Chemical stability of insulin. 1. Hydrolytic degradation during storage of pharmaceutical preparations. Pharm. Res. 1992, 9, 715–726. [Google Scholar] [CrossRef] [PubMed]
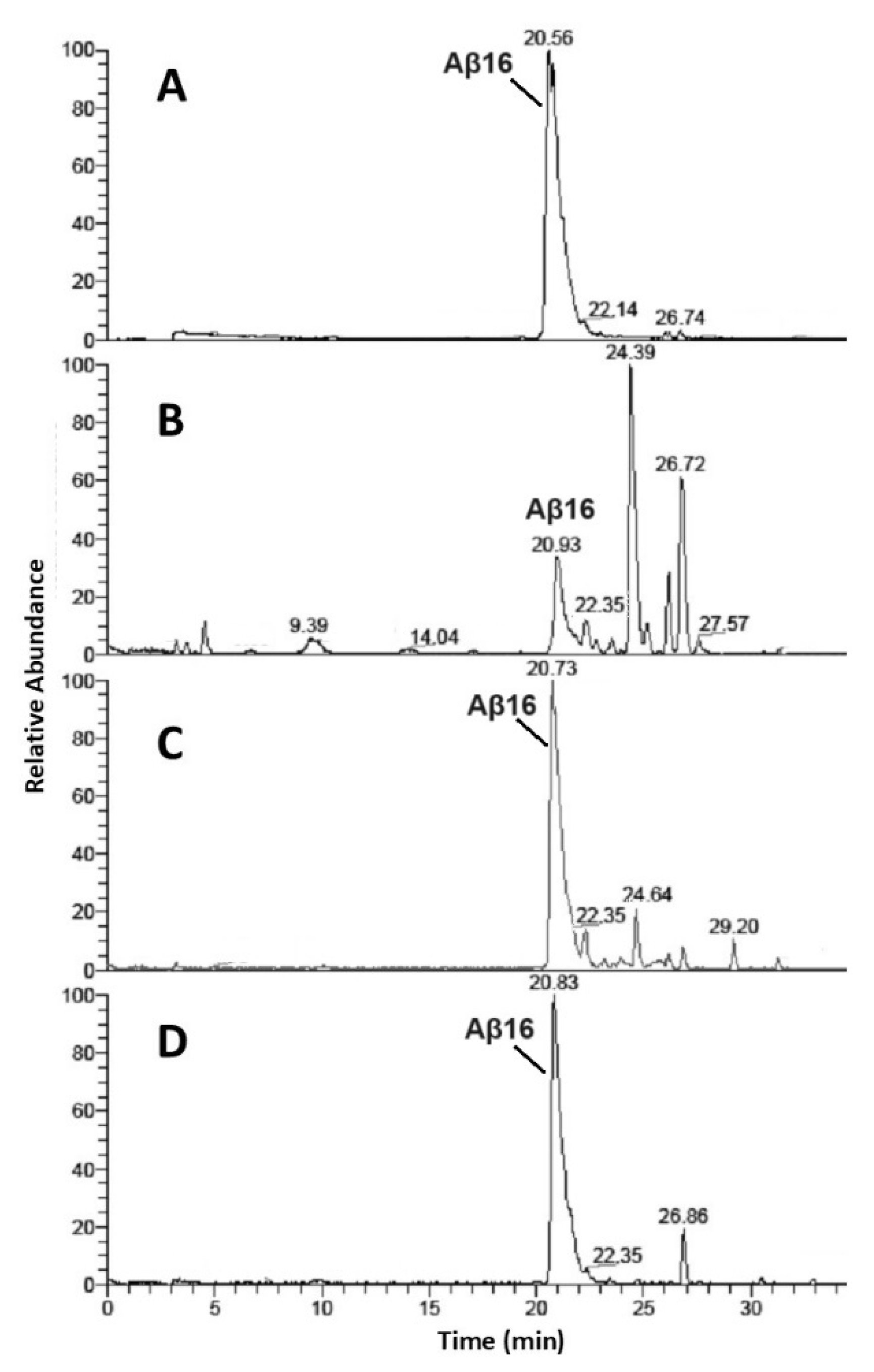
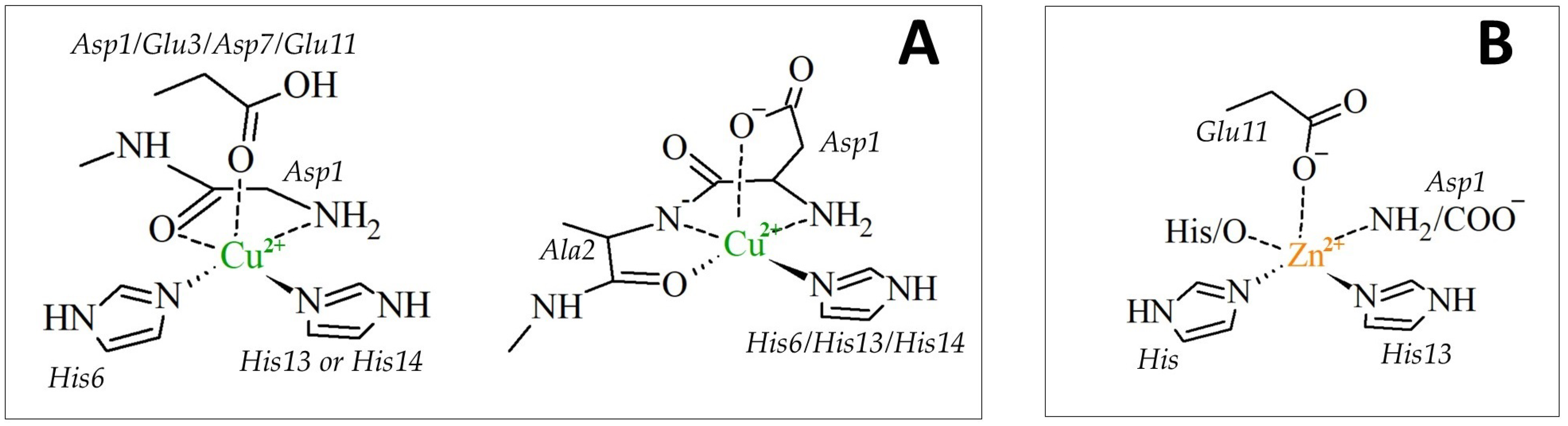
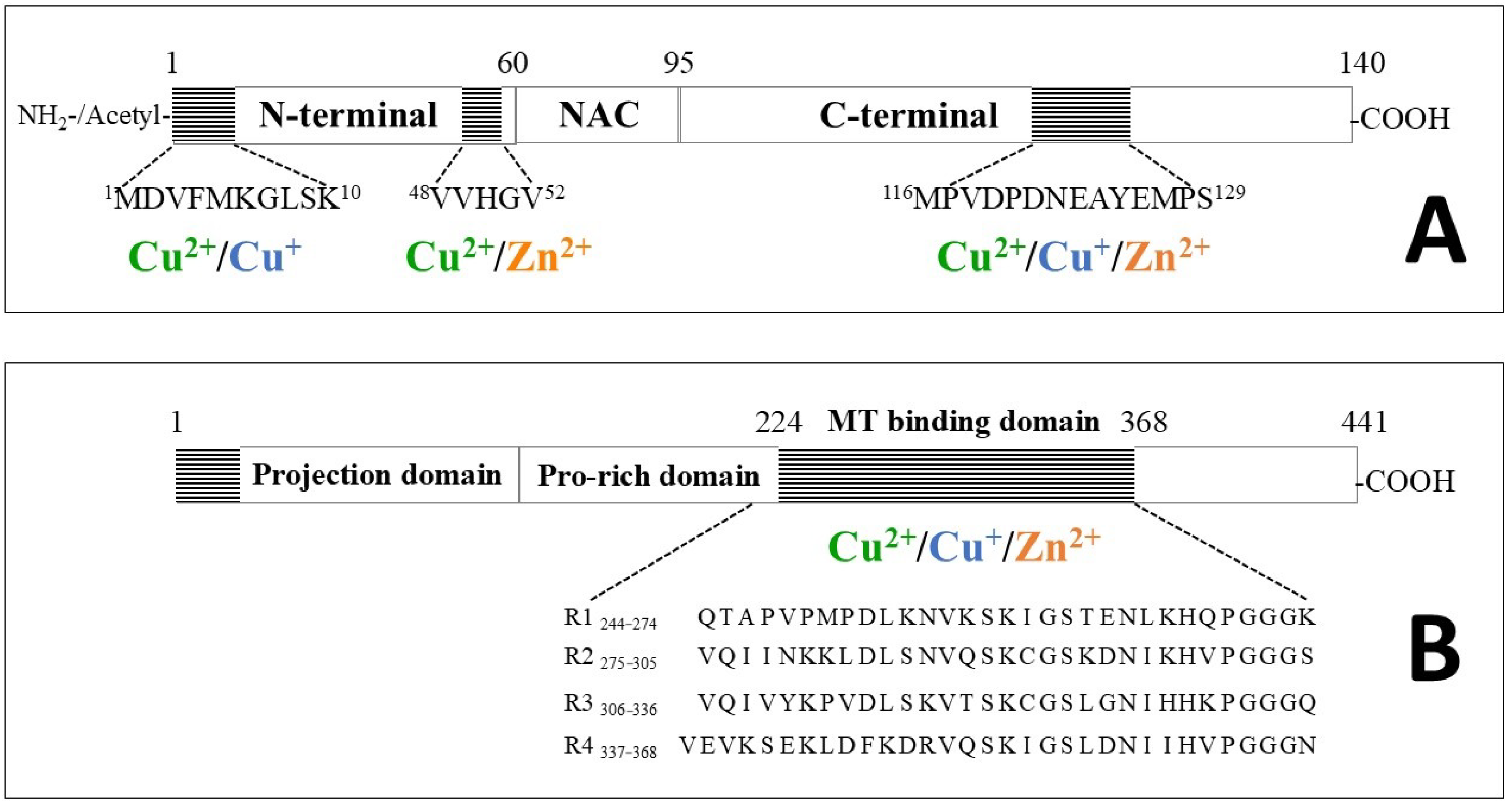
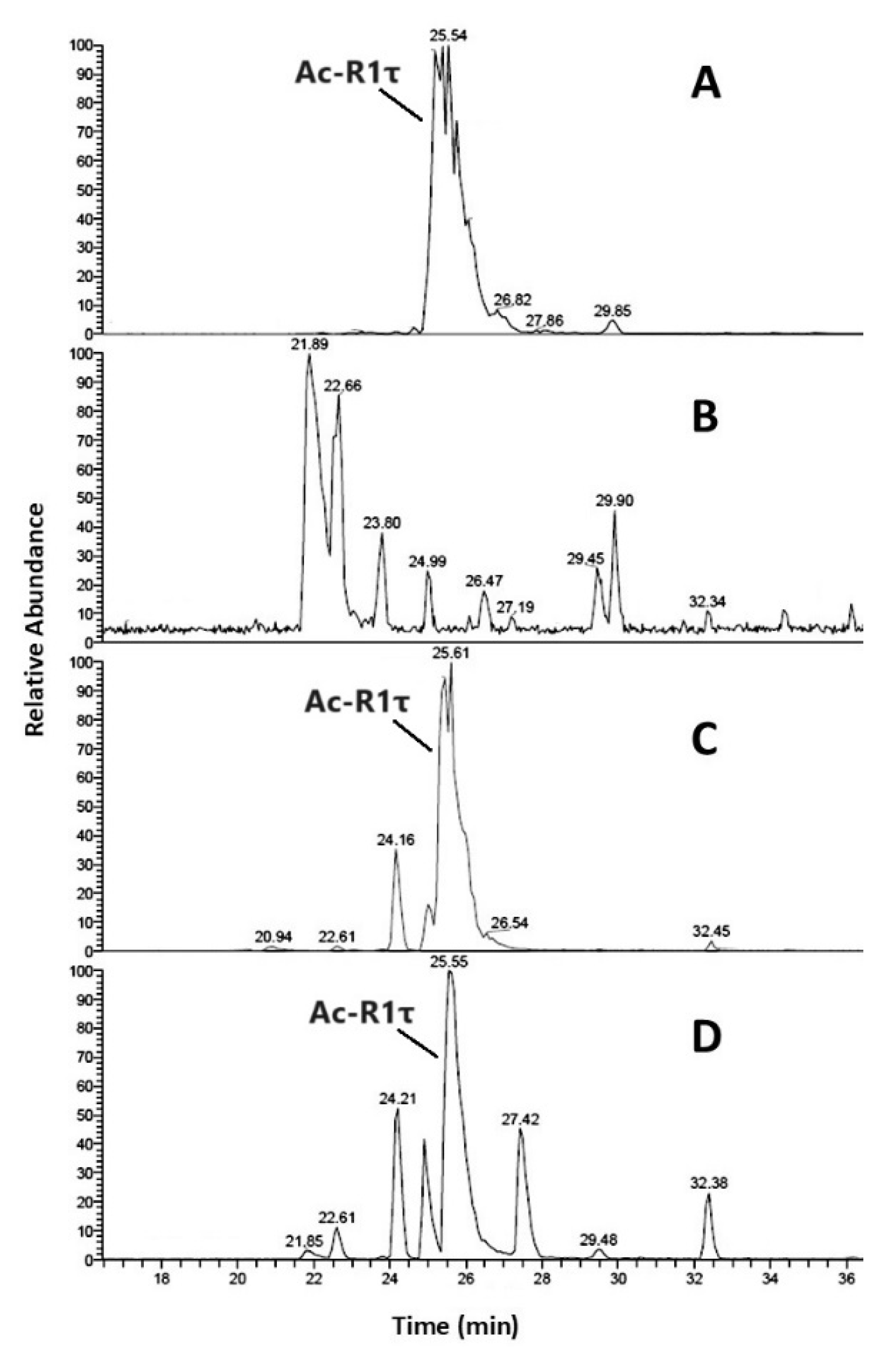
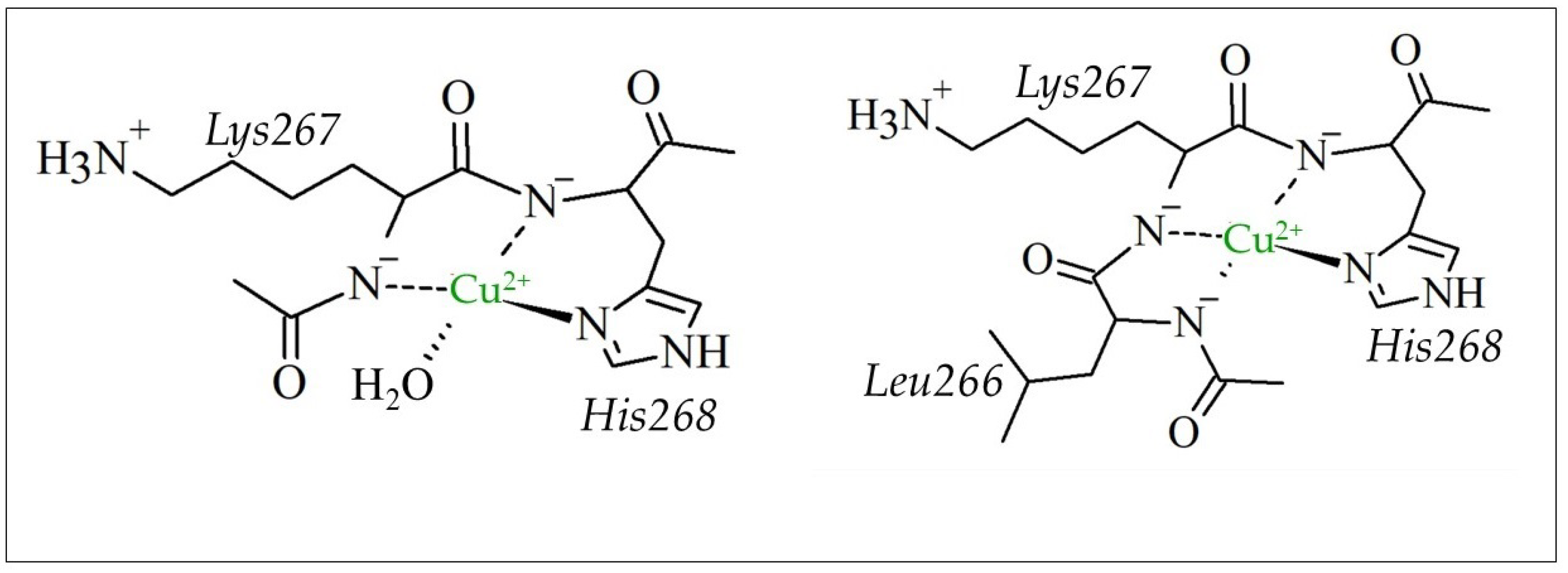
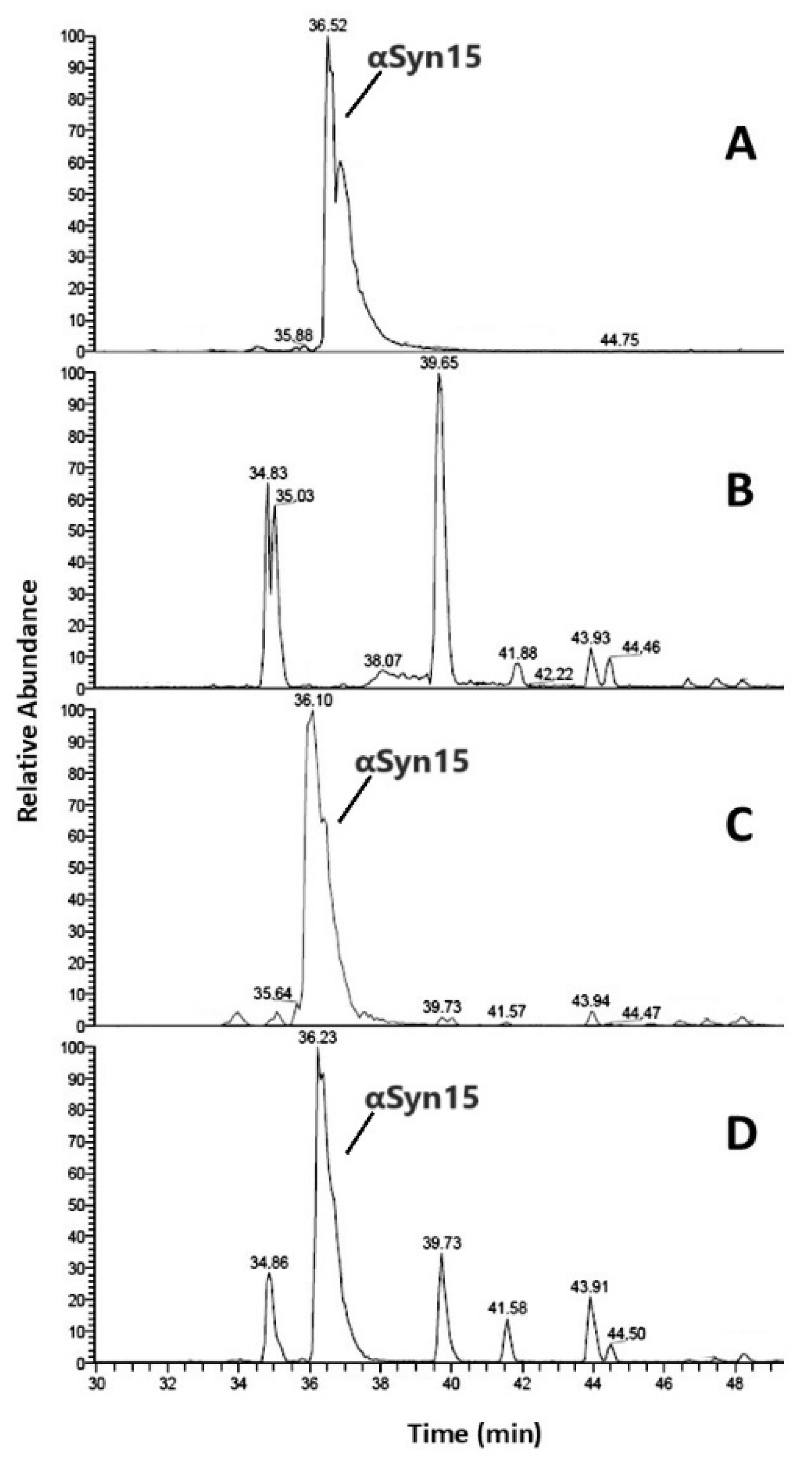
| Aβ16 | Cu2+-Aβ16 | Zn2+-Aβ16 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r.t. (min) | Species | a.a. | 7 Days | 14 Days | 7 Days | 14 Days | 21 Days | 7 Days | 14 Days | 21 Days |
| 9.4 | DAEFRHD | 1–7 | 4% | 12% | -- | -- | -- | -- | -- | -- |
| 14 | SGYEVH | 8–13 | 2% | 6% | -- | -- | -- | -- | 6% | 19% |
| 20.9 | Aβ16 | 1–16 | 26% | -- | 83% | 63% | 28% | 90% | 78% | 20% |
| 22.3 | FRHDSGYEVH | 4–13 | 4% | 6% | 5% | 8% | 14% | 3% | 3% | -- |
| 23.5 | AEFRHDSGYE | 2–11 | 2% | 3% | -- | -- | -- | -- | -- | -- |
| 24.4 | DAEFRHDSGYEVHHQ | 1–15 | 35% | 28% | 6% | 10% | 18% | -- | -- | -- |
| 25.2 | DAEFRHDSGYEVHH | 1–14 | 3% | 8% | 2% | -- | 2% | -- | -- | -- |
| 26.2 | DAEFRHDSGYE | 1–11 | 5% | 14% | -- | 4% | 12% | -- | -- | 5% |
| 26.7 | DAEFRHDSGYEVH | 1–13 | 17% | 19% | 2% | 8% | 13% | 7% | 13% | 51% |
| 27.6 | AEFRHDSGYEVHH | 2–14 | 2% | 2% | -- | -- | -- | -- | -- | -- |
| 29.2 | DAEFRHDSGYEV | 1–12 | -- | 2% | 2% | 7% | 13% | -- | -- | 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirota, V.; Monzani, E.; Dell’Acqua, S.; Bacchella, C. Role of Copper and Zinc Ions in the Hydrolytic Degradation of Neurodegeneration-Related Peptides. Molecules 2025, 30, 363. https://doi.org/10.3390/molecules30020363
Pirota V, Monzani E, Dell’Acqua S, Bacchella C. Role of Copper and Zinc Ions in the Hydrolytic Degradation of Neurodegeneration-Related Peptides. Molecules. 2025; 30(2):363. https://doi.org/10.3390/molecules30020363
Chicago/Turabian StylePirota, Valentina, Enrico Monzani, Simone Dell’Acqua, and Chiara Bacchella. 2025. "Role of Copper and Zinc Ions in the Hydrolytic Degradation of Neurodegeneration-Related Peptides" Molecules 30, no. 2: 363. https://doi.org/10.3390/molecules30020363
APA StylePirota, V., Monzani, E., Dell’Acqua, S., & Bacchella, C. (2025). Role of Copper and Zinc Ions in the Hydrolytic Degradation of Neurodegeneration-Related Peptides. Molecules, 30(2), 363. https://doi.org/10.3390/molecules30020363







