1. Introduction
Tumor-induced bone disease (TIBD) is one of the major causes of morbidity in cancer patients [
1,
2]. More than half of all carcinoma patients develop bone metastases [
3,
4]. TIBD is found in 65–95% of patients with multiple myeloma and advanced breast and prostate cancers [
2,
5]. Because TIBD is associated with considerable morbidity and a median survival time of less than two years [
2], the development of more effective therapies is warranted [
5,
6]. Current TIBD therapies are palliative. Standard anti-cancer chemotherapies at their maximum tolerated doses are unable to reach effective concentrations in the bone and its microenvironment. With the bone microenvironment and metastases to this site being critical to cancer progression [
7], there is clearly a need for drugs that can attack and kill bone-associated tumor cells without prohibitive systemic toxicity.
Bisphosphonates (BPs) are bone-specific palliative treatments that reduce tumor-induced skeletal complications [
1,
8,
9,
10]. However, TIBD still progresses in BP-treated cancer patients. The development of drugs with enhanced anti-resorptive and cytotoxic characteristics to improve the treatment of patients with TIBD is greatly needed [
1].
The exploitation of the bone-seeking properties of BPs for targeted delivery of cancer therapeutics [
1,
11,
12,
13,
14] is a promising approach to target bone metastases. BPs and cytotoxic agents can be covalently linked, allowing the intact conjugate to leave the circulation and release both drugs in the bone microenvironment [
1,
13]. These conjugates could combine anti-resorptive and anti-tumor activities while localizing at the site of tumor cell-induced bone destruction. We have shown that
MBC-11 (see
Figure 1), a first-in-class conjugate of the bone-targeting BP etidronate covalently linked to the antimetabolite
cytarabine (
arabinocytidine or
araC), was well tolerated in humans and showed significant reductions in the metabolic activity of bone-associated cancer cells [
13].
In the present study, we describe the development and validation of analytical platforms to quantify a novel drug in this class, a
gemcitabine–ibandronate conjugate [
15] (
GEM-IB, see
Figure 1A), and its metabolites (
Figure 1B) and analyze its metabolism and pharmacokinetics (PK) in dogs and mice.
GEM is one of the most widely used antineoplastic agents in clinical oncology, used to treat breast, ovarian, bladder, non-small lung, and pancreatic cancer [
16,
17]. As compared with other cytotoxic chemotherapy drugs,
GEM is generally considered a tolerable compound for most cancer patients [
17]. Treatment-related adverse events are usually clinically manageable and fatal treatment-related events have been reported in only a few (<1% to 4%) patients [
17,
18,
19]. Side effects include hematological (e.g., neutropenia, anemia, thrombocytopenia, and thromboembolism) and non-hematological (e.g., vomiting, fatigue, and elevated levels of alanine aminotransferase) side effects [
17]. The earliest studies of BP use for bone metastases involved trials of clodronate [
20,
21] and pamidronate [
21,
22] in the early 1990s.
Ibandronate (
IB) is an advanced BP medication used in the prevention and treatment of osteoporosis and metastasis-associated skeletal fractures [
23].
IB is FDA-approved for the treatment and prevention of osteoporosis in post-menopausal women [
24].
GEM-IB is a novel conjugate that combines the potent antineoplastic properties of
GEM with the bone-targeting properties of
IB. No studies describing the analysis, pharmacokinetics, and stability of this bisphosphonate conjugate have been conducted before.
In a manner analogous to
MBC-11,
GEM-IB is hydrolyzed under physiological conditions to form
gemcitabine-5′-phosphate (
GEMMP) and
IB. While
IB does not undergo further degradation,
GEMMP is rapidly hydrolyzed to form
GEM or is deaminated, resulting in
dFdUMP (see
Figure 1B). Hydrolysis of
dFdUMP or deamination of
GEM will result in the formation of the essentially inert metabolite
dFdU. To investigate the absorption distribution and metabolism of
GEM-IB after administration, it is important to monitor
GEM-IB and the metabolites
GEMMP,
IB,
GEM,
dFdUMP, and
dFdU. In the present study, we describe two novel analytical platforms for the analysis of
GEM-IB and
IB (Assay A) and
GEMMP,
GEM,
dFdUMP, and
dFdU (Assay B) in mouse and dog plasma to support preclinical studies. For the first time, we provide data from two preclinical studies, one in
mice and one in
dogs, to prove the feasibility of the approach and present the first set of PK data for this novel drug.
2. Results
Various conditions were tested to achieve the acceptable chromatographic performance and separation of key components while maintaining optimal sensitivity, reproducibility, and minimal carry-over. Chromatographic performance for pyrimidine nucleosides and nucleotides (
GEM,
dFdU,
GEMMP, and
dFdU-MP) could be achieved under various common reversed-phase high-performance liquid chromatography (RP-HPLC) conditions. In contrast, achieving acceptable chromatography was challenging for
GEM-IB. Normal-phase, reversed-phase (RP), and hydrophilic interaction liquid chromatography (HILIC) resulted in poor retention, retention reproducibility, and/or robustness for
IB and
GEM-IB. Ion-pair chromatography using various mass spectrometry-compatible ion-pair reagents in combination with RP-HPLC column materials showed an improved peak shape, but lacked reproducibility and sensitivity and showed a significant carry-over effect. This changed with the use of the Hypercarb column material, which consists of 100% porous graphitic carbon (PGC). Using this material and the conditions listed in
Section 4 for Assay A, acceptable chromatographic performance was achieved (see
Figure 2A). Unfortunately, the less polar metabolites (
GEM and
dFdU) did not show sufficient separation using PGC material under these conditions. Thus, a second chromatographic platform (Assay B) using simple RP-HPLC that proved more suitable for said metabolites was used to monitor pyrimidine nucleosides and nucleotides. Complete chromatographic separation of
GEM and its monophosphate
GEMMP as well as of
dFdU from its monophosphate
dFdU-MP was required due to in-source fragmentation.
Figure 2B,C show representative extracted ion chromatograms for the analytes from extracted mouse plasma samples using RP-HPLC Assay B (please see
Section 4).
2.1. Linearity, Lower Limit of Quantitation, and Selectivity
The lower limit of quantitation (LLOQ) was defined as the lowest concentration for which the accuracies were within 20% of the nominal concentration for at least half of the samples and the imprecisions were less than 20%. The LLOQ also had a signal-to-noise (S/N) ratio greater than 8. The LLOQ was determined to be 5 ng/mL for
GEM,
GEMMP, and
dFdUMP, while dFdU and
GEM-IB had an LLOQ of 10 ng/mL and
IB had an LLOQ of 40 ng/mL in dog and mouse EDTA plasma. The upper limit of quantitation (ULOQ) used in this study was 1000 ng/mL for all compounds, except for
IB, which had a ULOQ of 4000 ng/mL. Representative extracted ion chromatograms of non-spiked blank mouse and dog plasma and spiked plasma and representative calibration curves for each compound are shown in
Supplementary Figure S1a–f. The correlation coefficients for the calibration curves were consistently r = 0.99 and better.
Figure 3 shows the chromatographic peaks for all compounds at a concentration of 2.5 ng/mL (below the lower limit), as well as the carry-over peaks for the solvent injection immediately following the injection with the highest concentration of sample (1000 ng/mL). The peak intensities of said injections indicate that the carry-over for this assay was less than 1% (See
Supplementary Figure S1a–f).
2.2. Extraction Recovery and Matrix Effects
Matrix effects and extraction recovery was evaluated using the protocol described by Matuszewski et al. [
25]. Six different individual lots of dog plasma and six different individual lots of mouse plasma were used for all recovery and matrix effect experiments. Plasma samples were enriched at three different levels within the working range of the assay and extracted (enriched before) and compared to a set of blank plasma samples that were extracted before the addition of analytes (enriched after). This was performed at three concentration levels corresponding to the high, mid, and low QC levels. The results of both sets of samples were compared and used to calculate the extraction recovery (Recovery in % = Signal area
enriched before/Signal area
enriched after × 100). The resulting mean recovery and the coefficient of variation for each compound are shown in
Table 1. The recovery of all compounds was calculated to be higher than 80%, with coefficients of variation from 4.4% to 25% (see
Table 1). Finally, the six individual lots of dog plasma and the six individual lots of mouse plasma were also used to determine any effects that unique matrices had on the MS/MS signal (ion suppression/ion enhancement). To determine the matrix effect, the blank extracted samples that were enriched after extraction (enriched after) were compared to enriched buffer samples with the same organic/aqueous buffer combination as a sample extract (absolute matrix effect = Signal area
enriched after/Signal area
buffer sample × 100, see
Table 1). A value of >100% indicates ionization enhancement, and a value of <100% indicates ionization suppression. The relative matrix effect was calculated using the analyte/internal standard area ratio. This value shows if the internal standard can compensate for individual matrix effects (
Table 1). The absolute matrix effect relative to the surrogate matrix showed the largest effect for
IB, with 26.6% ± 2.4% (SD) and 31.2% ± 1.8% (SD) for mouse and dog plasma, respectively. For this analyte, the relative matrix effect showed a much higher value (suppression effect was mitigated by the internal standard), with 76.5% ± 3.2% (SD) and 102% ± 3.2% (SD) for mouse and dog plasma, respectively. The highest standard deviation (inter-individual variation) was observed for
GEM-IB, with 137.7% ± 74.6% (SD) and 77.5% ± 34.3% (SD) for mouse and dog plasma, respectively. This variation was also compensated by the internal standard used, as shown by the relative matrix effect for
GEM-IB, with 77.8% ± 11.7% (SD) and 77.1% ± 9.9% (SD) for mouse and dog plasma, respectively.
2.3. Matrix Interference
The six individual lots of both dog and mouse plasma were used to assess matrix interferences. For each lot, three levels of quality control samples that covered the working range of the assay were analyzed and the result was compared with the nominal concentration, for a total of
n = 18 data points per compound. The calculated matrix interference of each compound is shown in
Table 1. All compounds fulfilled the predefined acceptance criteria of a mean accuracy of 80–120% and mean imprecision of less than 20% for this experiment.
2.4. Accuracy and Imprecision
The accuracy and imprecision of this assay were tested by extracting calibrators and quality control samples on three separate days (runs). For each run, two sets of calibrators and six sets of quality control samples were extracted and analyzed. Five concentration levels were chosen for the analysis of
GEMMP,
dFdU-MP, and
GEM (5, 10, 15, 200, and 800 ng/mL), while
dFdU was tested at four concentration levels (10, 15, 200, and 800 ng/mL). dFdU was not tested at 5 ng/mL since it falls below the LLOQ. Both intra-batch and inter-batch accuracies and precisions were determined (see
Supplementary Table S1). At least two-thirds of the calibration samples fell within 15% of the nominal concentration (or within 20% for the lowest concentration level). For quality control samples, at least half of the samples at each level and at least two-thirds of the samples overall fell within 15% of the expected concentration (or within 20% for the lowest concentration level). The coefficient of variation also fell within 15% (or 20% for the lowest concentration level) for all but two inter-batch quality control levels of
GEMMP, which fell between 15% and 16%.
2.5. Stability
For stability testing, QC samples were prepared by spiking plasma with QC stock solutions. These stability samples were stored for the predetermined time periods before extraction (accelerated stability and freeze–thaw stability) or after extraction (autosampler stability) and were run with freshly prepared calibrator standards. For accelerated stability, samples were stored at 4 °C and at room temperature for 4 h and 24 h prior to sample extraction. Except for
GEM-IB, all analytes were stable (±20% of nominal) for 24 h at room temperature for at least 24 h (see
Supplementary Tables S2 and S3). Assay A compounds (
GEM-IB and
IB) were also tested at 1 h and 2 h exposed to 4 °C and at room temperature to evaluate for which duration
GEM-IB is stable. While
IB was stable under all conditions,
GEM-IB was stable for up to 1 h on ice in mouse plasma with 89.2% ± 7.0% (mean ± SD) of the nominal concentration. After 2 h,
GEM-IB had degraded to 64.2% ± 11.8% (mean ± SD). In contrast,
GEM-IB in dog plasma was stable for up to 24 h on ice.
Freeze–thaw cycle stability was assessed by storing the stability samples overnight in the freezer (<−70 °C) and thawing them the next day on the bench. Once thawed, samples were placed back into the freezer. This was carried out for three freeze–thaw cycles for all compounds. Except for
GEM-IB in mouse plasma, all study compounds were within ±20% of the nominal enriched concentration (see
Supplementary Tables S5 and S6). Due to the instability of
GEM-IB, one and two freeze–thaw cycles were tested for the Assay A compounds (
GEM-IB and
IB). While
IB was stable under these conditions,
GEM-IB showed instability after one freeze–thaw cycle (53.7% ± 6.9%).
The stability of extracted dog and mouse plasma samples in the autosampler at 4 °C was established for 24 and 48 h. Except for GEM-IB, in mouse and dog plasma extracts, all analytes were within ±20% of the nominal enriched concentration for up to 48 h in the autosampler. GEM-IB was stable for up to 24 h under these conditions.
To assess the stability of study samples, PK samples were re-extracted after storage at <−70 °C for 1.5 years (see
Supplementary Table S4). Under these conditions,
GEM-IB and IB showed an accuracy of 105.5% ± 40.4% (mean ± standard deviation) and 111.4% ± 38.0% for
GEM-IB and 107.8% ± 2.5% and 113.7% ± 23.9% for
IB in dog plasma and mouse plasma, respectively. Standard deviations were higher than the anticipated ±25% for
GEM-IB but passed for IB.
GEM was shown to be stable under these conditions, with accuracies of 90.4% ± 23.9% (mean ± standard deviation) and 94.2% ± 11.8% for dog and mouse plasma, respectively. Sample stability assessment for
GEMMP,
dFdU, and
dFdU-MP failed the criteria in dog and mouse plasma at <−70 °C for 1.5 years (see
Supplementary Table S4).
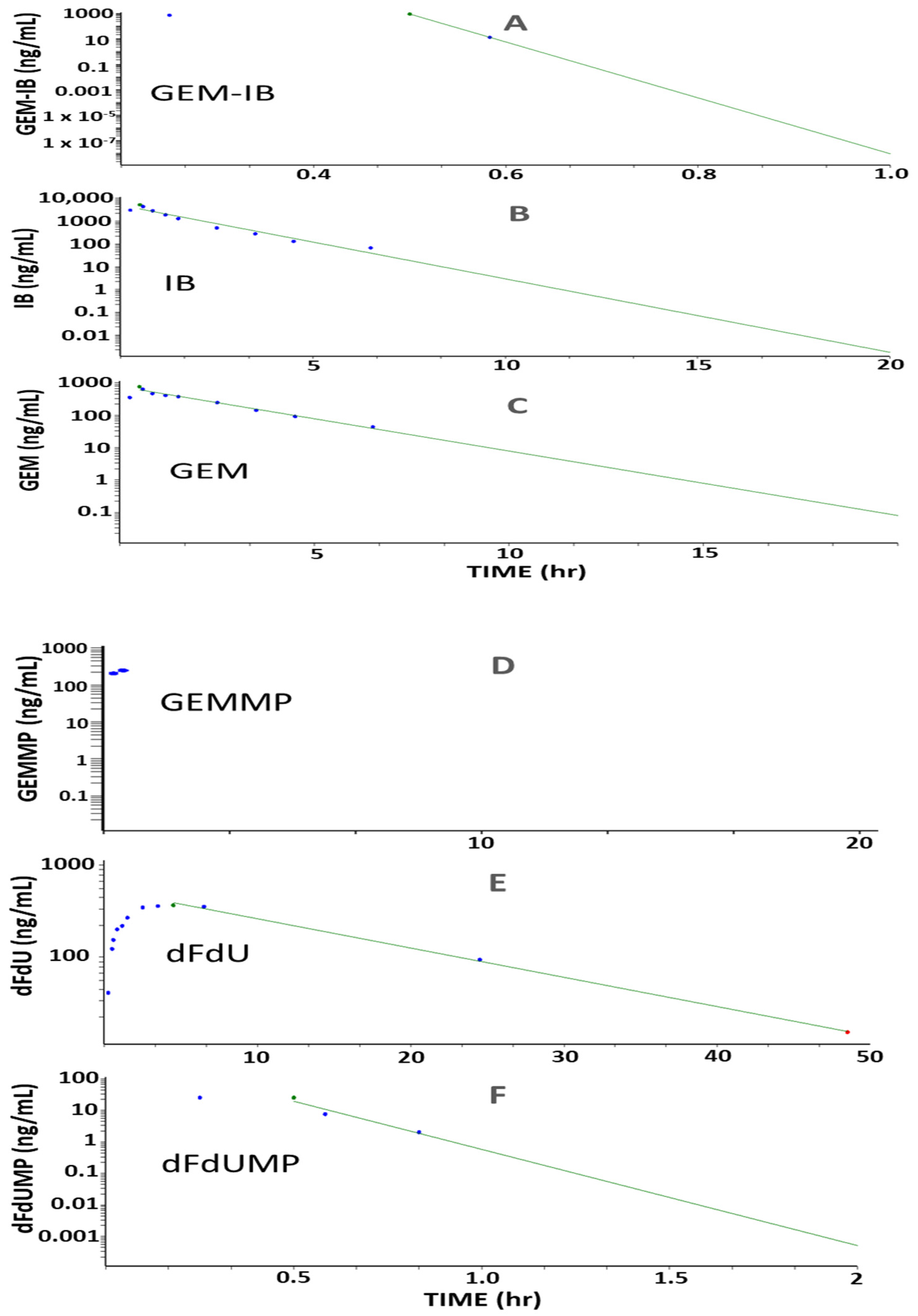
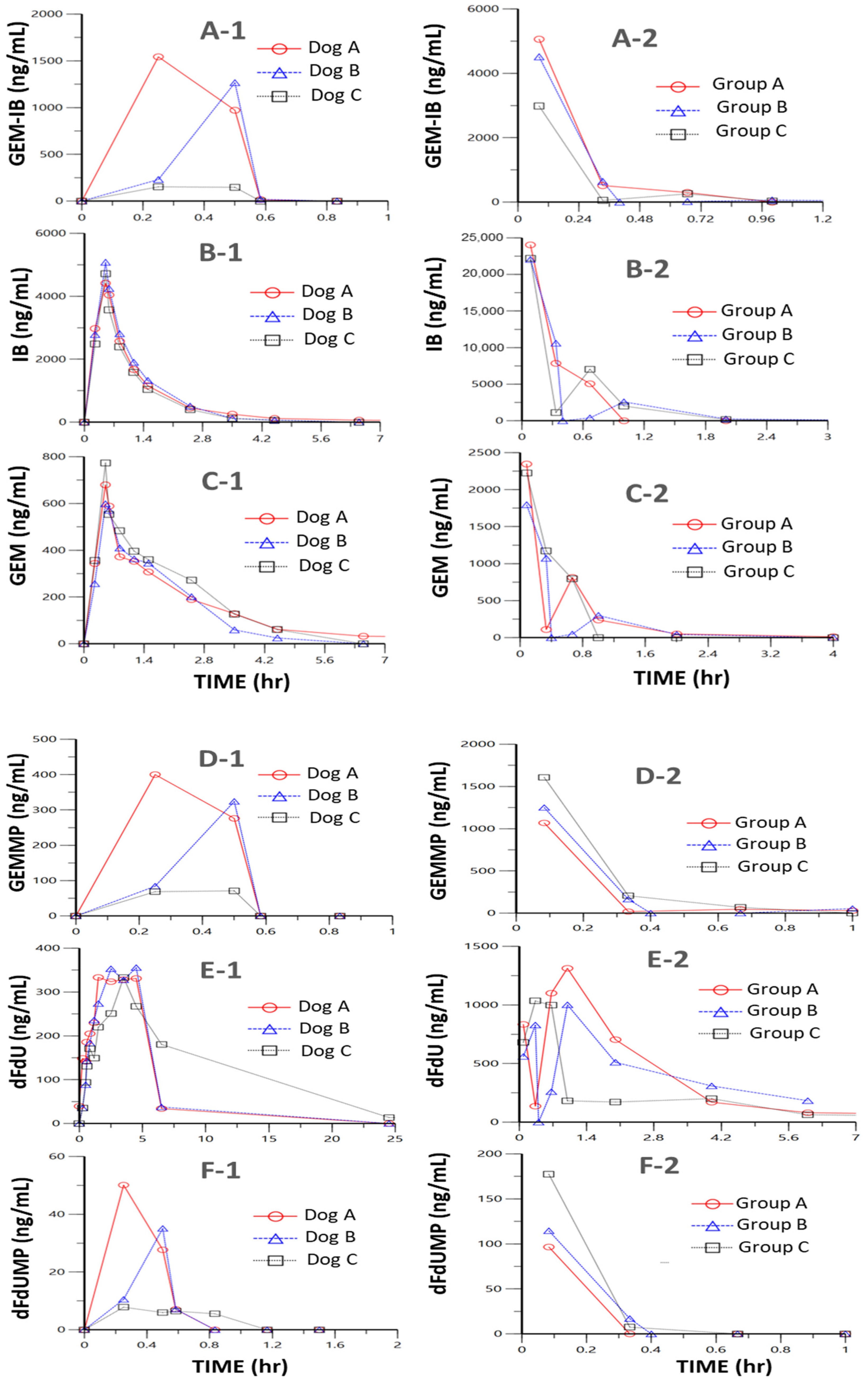
2.6. Proof-of-Concept PK Studies
Studies in mice (
n = 24, eight time points,
n = 3 per time point) and in dogs (
n = 3, twelve time points) were performed to determine key PK parameters for
GEM-IB and its metabolites in mice and dogs (
Table 2 and
Table 3 and
Figure 3,
Figure 4 and
Figure 5). After i.p. injection in mice, the maximal concentrations (C
max) were 4185, 22,777, 1309, 130, 2122, and 832 ng/mL for
GEM-IB, IB,
GEMMP,
dFdUMP,
GEM, and
dFdU, respectively. All analytes showed a time of maximal concentration (t
max) of 5 min (the first measured time point), with the exception of
dFdU, which was formed with a delay (t
max = 1 h). The areas under the time concentration curve over the observation period (AUC
0-Ͳ) in mouse plasma for
GEM-IB,
IB,
GEMMP,
GEM,
dFdU, and
dFdU-MP were 1278 h·ng/mL, 10,652 h·ng/mL, 405 h·ng/mL, 38 h·ng/mL, 1063 h·ng/mL, and 3389 h·ng/mL, respectively. In dog plasma, AUC
0-Ͳs of 295 h·ng/mL, 5725 h·ng/mL, 83 h·ng/mL, 11 h·ng/mL, 1625 h·ng/mL, and 6569 h·ng/mL were observed for
GEM-IB,
IB,
GEMMP,
dFdU-MP,
GEM, and
dFdU, respectively. The half-life of
GEM-IB was determined to be 8 min after i.p. injection in mouse plasma and less than 1 min after the end of the infusion in dog plasma.
Table 2 lists the key PK parameters determined in mice and
Table 3 lists the key PK parameters in dogs.
GEMMP could not be completely evaluated in dog plasma, since all values after the infusion period were below the LLOQ.
4. Materials and Methods
To analyze GEM-IB and its five metabolites, two analytical assays were developed. Assay A captures the polyphosphate-containing compounds GEM-IB and IB. Assay B was designed to analyze the nucleotide analog metabolites including GEMMP, dFdUMP, GEM, and dFdU.
4.1. Materials
Gemcitabine hydrochloride and
2′,2′-difluoro-2′-deoxyuridine were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
GEM-IB was synthesized according to a published procedure [
15] and
2′,2′-Difluoro-2′-deoxyuridine-monophosphate was prepared by standard deamination of
GEMMP.
GEMMP and the internal standards,
gemcitabine-13C,15N2 (
GEM-13C) and
2′,2′-difluoro-2′-deoxyuridine-13C,15N2 (
dFdU-13C), were purchased from Toronto Research Chemicals (North York, ON, Canada). Ibandronate sodium salt was purchased from Sigma Aldrich (St. Louis, MO, USA).
Adenosine-13C10,15N5 5′-triphosphate (
13C10-15N5-ATP) was purchased from Sigma Aldrich (St. Louis, MO, USA). K
2EDTA canine Beagle plasma and mouse plasma used for the assay validation were purchased from Innovative Research (Novi, MI, USA). HPLC-grade
water,
methanol,
acetonitrile,
formic acid, and
dibutylamine (
DBA) were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
4.2. Stock Solutions
Individual stocks of all compounds (GEM-IB, GEMMP, IB, GEM, dFdU, and dFdUMP) were prepared by weighing out solid material and dissolving it in HPLC-grade water to achieve a final concentration of 1 mg/mL.
4.3. Calibrators and Quality Controls
Calibration and quality control samples were prepared by spiking 180 µL of plasma with 20 µL of the corresponding calibrator or quality control stock, as listed above.
For Assay A (analysis of GEM-IB and IB), combined stock solutions with 10 µg/mL of GEM-IB and 40 µg/mL IB were prepared. These were used to prepare calibrator stock solutions used for the enrichment of mouse or dog plasma. Calibrator standards were prepared at concentrations of 2.5, 5, 10, 25, 50, 100, 250, 500, and 1000 ng/mL for GEM-IB and 10, 20, 40, 100, 200, 400, 1000, 2000, and 4000 ng/mL for IB. Quality control samples (QCLLOQ, QClow-1, QClow-2, QCmid, and QChigh) were prepared at 5, 10, 30, 200, and 800 ng/mL for GEM-IB and 20, 40, 120, 800, and 3200 ng/mL for IB.
For Assay B (analysis of GEMMP, GEM, dFdUMP, and dFdU), combined stock solutions with all four compounds were prepared at a concentration of 10 µg/mL from the individual 1 mg/mL stocks. These were used to prepare the calibrator stock solutions used for the enrichment of mouse/dog plasma. Calibrator standards were prepared at concentrations of 2.5, 5, 10, 25, 50, 100, 250, 500, and 1000 ng/mL. Quality control samples (QCLLOQ, QClow-1, QClow-2, QCmid, and QChigh) were prepared at concentrations of 5, 10, 15, 200, and 800 ng/mL. A protein precipitation solution was prepared by adding 250 ng/mL of each internal standard (GEM-13C and dFdU-13C) to 20 mM DBA in methanol. All stocks were stored at −20 °C.
4.4. Sample Extraction
Assay A (GEM-IB and IB): The extraction of calibration standards, quality control samples, and PK study samples was identical. Plasma, quality control stocks, and calibrator stocks were removed from the storage freezer and kept at room temperature until fully thawed. Aliquots of 100 μL of standards/quality control or blank sample were transferred into a 1.5 mL low-binding polypropylene vial with a conical bottom and snap-on lid. An amount of 10 µL of the of the internal standard solution (15 and 10 µg/mL of IB-D3 and ATP-IS, respectively) was added and mixed. For protein precipitation, 50 μL of methanol was added and then samples were vortexed for 10 min. This was followed by centrifugation at 25,000× g for ten minutes at 4 °C (Thermo Scientific MR 23i Centrifuge, Thermo Scientific, Waltham, MA, USA). To remove remaining protein, the supernatant was transferred to 10,000 molecular weight cut off (MWCO) centrifugal filters (800 µL volume, PES 10,000 with 2.0 mL receiver tubes, Analytical Sales and Services, Flanders, NJ, USA) and centrifuged again at 25,000× g for ten minutes at 4 °C. The filtrate was transferred into HPLC vials and placed into the HPLC autosampler maintained at 4 °C.
Assay B (GEMMP, GEM dFdUMP and dFdU): The extraction of calibration standards and quality control samples was identical to that of study samples. An amount of 200 µL of plasma sample was added to a 1.5 mL snap-top Eppendorf tube with 100 µL of protein precipitation solution. The samples were then vortexed for five minutes followed by centrifugation at 25,000× g for ten minutes at 4 °C. Hereafter, supernatants were transferred to 10K Amicon centrifugal filters (Merck-Millipore, Burlington, MA, USA) and centrifuged at the same settings for 60 min. The filtrates were then transferred into HPLC vials for analysis.
4.5. HPLC-MS/MS Analysis
Assay A (GEM-IB and IB): Quantification of the analytes was carried out using high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). Chromatography was performed using an Agilent 1260 Infinity II bio-inert HPLC system (Agilent Technologies, Santa Clara, CA, USA) on a Hypercarb Porous Graphitic Carbon HPLC column (3 µm, 2.1 × 50 mm, Thermo Scientific, Waltham, MA, USA). The HPLC system consisted of an Agilent 1260 Infinity II bio-inert quaternary pump, an Agilent 1260 Infinity II bio-inert column oven, and an Agilent 1260 Infinity II bio-inert autosampler (Agilent Technologies, Santa Clara, CA, USA). An amount of 10 µL of the sample extracts was injected. The column was maintained at 22 °C. HPLC-MS-grade water supplemented with 10 mM hexafluoroisopropanol (HFIP) and 0.05% (v/v) diethyl amine (DEA) at pH 9.4 was used for the aqueous mobile phase (Buffer A) and HPLC-grade acetonitrile was used for the organic mobile phase (Buffer B). The flow rate was set to 400 µL/min throughout the assay. The initial settings were 98% Buffer A and 2% organic Buffer B for the first two minutes. During the following 0.7 min, the organic Buffer B was increased to 30%. At minute 3.5, the gradient reached 98% Buffer B, which was kept for 0.5 min, and at minute 4.2 the column was re-equilibrated to starting conditions for 2.8 min until the next injection. The HPLC system was connected to a Sciex a 5500+ triple quadrupole mass spectrometer (SCIEX, Concord, ON, Canada) via a turbo electrospray ionization source (SCIEX) operated in the negative electrospray ionization (ESI) mode. Data were acquired in negative multiple reaction monitoring (MRM) mode and all compounds were detected as [M–H ]−. The ion transitions used during the multiple reaction monitoring mode were m/z = 318→236, 321→239, 643→342, and 521→79 for IB, IB-D3, GEM-IB, and adenosine-13C10,15N5 5′-triphosphate (13C10-15N5-ATP), respectively. The dwell time for analysis was set to 50 ms. The curtain gas was kept at 20 psi and the ion source gas at 55 psi. The ion spray voltage was −4500 volts, and the temperature of the ion source was set to 400 °C. Compounds were quantified using the analyte/internal standard area ratios based on calibration curves that were constructed with 1/x weighting and a linear regression fit. Quantification was carried out using the Sciex Analyst 1.7.1 Software (SCIEX, Foster City, CA, USA).
Assay B (GEMMP, GEM, dFdUMP and dFdU): Quantification of the analytes was carried out using HPLC-MS/MS. Chromatography was performed using an Agilent 1100 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a Kinetex XB-C18 column (150 mm × 4.60 mm, 2.6 µm particle size, Phenomenex, Torrance, CA, USA). The HPLC system consisted of an Agilent 1100 series binary pump, an Agilent 1100 degasser, an Agilent 1260 column oven, and a LEAP PAL autosampler (CTC Analytics/Archer Science, Lake Elmo, MN, USA) that was set to inject 8 µL of sample. The column was maintained at 30 °C. HPLC-grade water with 0.3% (v/v) formic acid was used for the aqueous mobile phase (Buffer A) and HPLC-grade acetonitrile (Buffer B) was used for the organic mobile phase. The flow rate was set to 600 µL/min throughout the assay. The initial settings were 99% Buffer A and 1% organic Buffer B for the first minute. During the following 1.2 min, the organic Buffer B was increased to 10% and within the following 1.3 min to 40%. At minute 4.5, the gradient reached 85% Buffer B and within the following 0.2 min the gradient was ramped up to 99% Buffer B. The 99% Buffer B was kept for 1.8 min and at minute 6.6, the column was re-equilibrated at the starting conditions for 0.9 min until the next injection. The HPLC system was connected to a Sciex a 6500 QTRAP mass spectrometer (SCIEX, Concord, ON, Canada) via a turbo electrospray ionization source (SCIEX) operated in positive electrospray ionization (ESI) mode. The MS/MS was run in the positive multiple reaction monitoring mode and all compounds were detected as [M+H]+. The ion transitions used during the multiple reaction monitoring mode were m/z = 344→112, 345→247, 264→112, 265→113, 267→115 and 268→116 for GEMMP, dFdUMP, GEM, dFdU, 13C3-GEM, and 13C3-dFdU, respectively. The dwell time was set to 20 milliseconds. The curtain gas was kept at 20 psi and the ion source gas at 40 psi. The ion spray voltage was 5500 volts, and the temperature of the ion source was set to 550 °C. The compounds were quantified using the analyte/internal standard area ratios based on calibration curves that were constructed with 1/x weighting and a linear regression fit. Quantification was carried out using the Sciex Multiquant OS Software version 1.7 or higher (SCIEX, Foster City, CA, USA).
4.6. Assay Validation
Method development and validation was conducted following applicable FDA guidelines for bioanalytical assays [
36], as considered fit for purpose.
Specificity. Endogenous interferences were excluded by analysis of blank plasma from 6 different individual lots of plasma. The lower limit of quantification (LLOQ) was determined as the lowest concentration consistently achieving an accuracy better than ±20% of the nominal concentration, with imprecision ≤20%.
Predefined acceptance criteria. The performance of the assay was considered acceptable if intra- and inter-day imprecision (coefficient of variance, %CV) at each concentration was ≤15%, except at the LLOQ (≤20%). Intra- and inter-day accuracy had to be ±15% of the nominal value, except at the LLOQ (±20%). Calibration curves had to have a correlation coefficient (r) of 0.99 or better.
Analytical recovery and imprecision. Intra- and inter-day analytical accuracy and imprecision were tested at least at four concentration levels depending on the LLOQ for each compound. Intra-day imprecision and accuracy were determined with
n = 6 per QC level. Inter-day imprecision and accuracy were measured over 3 days, with six replicates for each QC concentration (
n = 18).
Extraction efficiency and matrix effect. Extraction efficiency for
GEM-IB and metabolites was established by comparing the signals of the analytes after the extraction of QCs (
n = 6/concentrations), with the signals of the extracted blank matrix spiked with the respective concentrations of analytes after the extraction procedure [
25]. The matrix effect was determined by comparing the analyte LC-MS/MS signal after spiking of the analyte into the extracted blank matrix (matrix from 6 different individual lots, analyte concentrations as for the QC samples) with the HPLC-MS/MS signal of the same amount of analyte in neat solution [
25].
Carry-over. Carry-over was assessed by injecting blank buffer samples after analysis of the highest calibrators. Carry-over was considered insignificant if there was no signal at the relevant retention times exceeding 20% of the analyte signals at the LLOQ.
Stability. QC samples were freshly prepared. One set was extracted and was analyzed immediately to determine baseline signals. Analyte stability in plasma was evaluated at two QC concentration levels (
n = 3/concentration level) under different conditions: 1, 2, and 24 h at room temperature and at 4 °C. In addition, stability after extraction was determined after 24 h and 48 h at 4 °C in the autosampler and for up to 3 freeze (−70 °C)–thaw (ambient) cycles. Stability was assessed by comparing analyte concentrations with baseline (t
0) concentrations. Stability was assumed when the results were within ±20% of the baseline concentrations. Long-term stability estimates were performed by re-extraction of samples used during the PK study (
n = 6). Samples were initially analyzed for the PK assessment and were stored for 1.5 years (
GEM-IB and
IB) and 2.5 years (
GEM,
GEMMP,
dFdU, and
dFdU-MP) at <−70 °C prior to reanalysis. Stability was assessed by comparing analyte concentrations with the initial assessments. Stability was acceptable when the results were within ±20% of the baseline concentrations and the percent standard deviation (%-SD) was below 25%.
4.7. Proof-of-Concept Studies
Dog PK study. After completion of the validation, the assays were used to analyze plasma samples that were collected for a study investigating the PK of GEM-IB in dogs. For this proof-of-concept study, healthy beagle dogs (n = 3) received 5 mg/kg GEM-IB i.v. over the duration of 30 min. Plasma samples for PK analysis were collected before and during the infusion period (30 min) at 0, 15, and 30 min and after infusion ended at minutes 5, 20, 40, 60, 120, 180, 240, and 360. Blood samples were collected in K2EDTA containers with tetrahydrouridine 0.25 mg/mL to prevent deamination and plasma was separated. Samples were frozen and stored below −70 °C until HPLC-MS/MS analysis. GEM-IB and IB were assessed using the procedure described for Assay A and GEMMP, dFdUMP, GEM, and dFdU were analyzed using the protocol described under Assay B. Mouse PK study. Another study was performed as a proof-of-concept study to investigate the pharmacokinetics of GEM-IB in mice. Twenty-four mice were randomly assigned to three sequences. Each sequence consisted of eight mice and the plasma of each mouse was collected at one of the eight consecutive time points. Mice were injected with 5 mg/kg GEM-IB in saline i.p. Eight time points (with n = 3 mice per time point) were investigated and 0.25 mg/mL K2EDTA-tetrahydrouridine to prevent the deamination of blood samples was drawn via cardiac puncture at 5, 20, 40, 60, 120, 240, and 360 min. Plasma was separated, and samples were frozen and stored below −70 °C until HPLC-MS/MS analysis. GEM-IB and IB were assessed using the procedure described for Assay A and GEMMP, dFdUMP, GEM, and dFdU were analyzed using the protocol described under Assay B. The experimental protocols and animal care were in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Institutional Animal Care and Use Committee of Colorado State University (Ft. Collins, CO, USA). The animals were housed in an AAALAC-accredited and USDA-registered facility and the studies were carried out under the supervision of a board-certified veterinarian.
4.8. Statistics
All values are expressed as mean ± standard deviation (SD). Quantitative data were compared with analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons, using SPSS statistics version 28.0.1.0 (IBM, Armonk, NY, USA). A value of p < 0.05 was considered statistically significant. Intra- and inter-batch accuracies and precisions were calculated using Watson LIMS software version 7.6 (Thermo Fisher Scientific, Philadelphia, PA, USA). Non-compartmental PK parameters were calculated using Phoenix WinNonlin version 8.3 (Certara, Princeton, NJ, USA) or by the integrated PK module of Thermo Fisher Scientific Watson LIMS software (version 7.6, SP1).