Anticonvulsant Effects of Synthetic N-(3-Methoxybenzyl)oleamide and N-(3-Methoxybenzyl)linoleamide Macamides: An In Silico and In Vivo Study
Abstract
1. Introduction
2. Results and Discussion
2.1. In Silico Results
2.1.1. Structure and ADMET Analysis of Macamides
2.1.2. Initial Structures and Construction of Molecular Systems
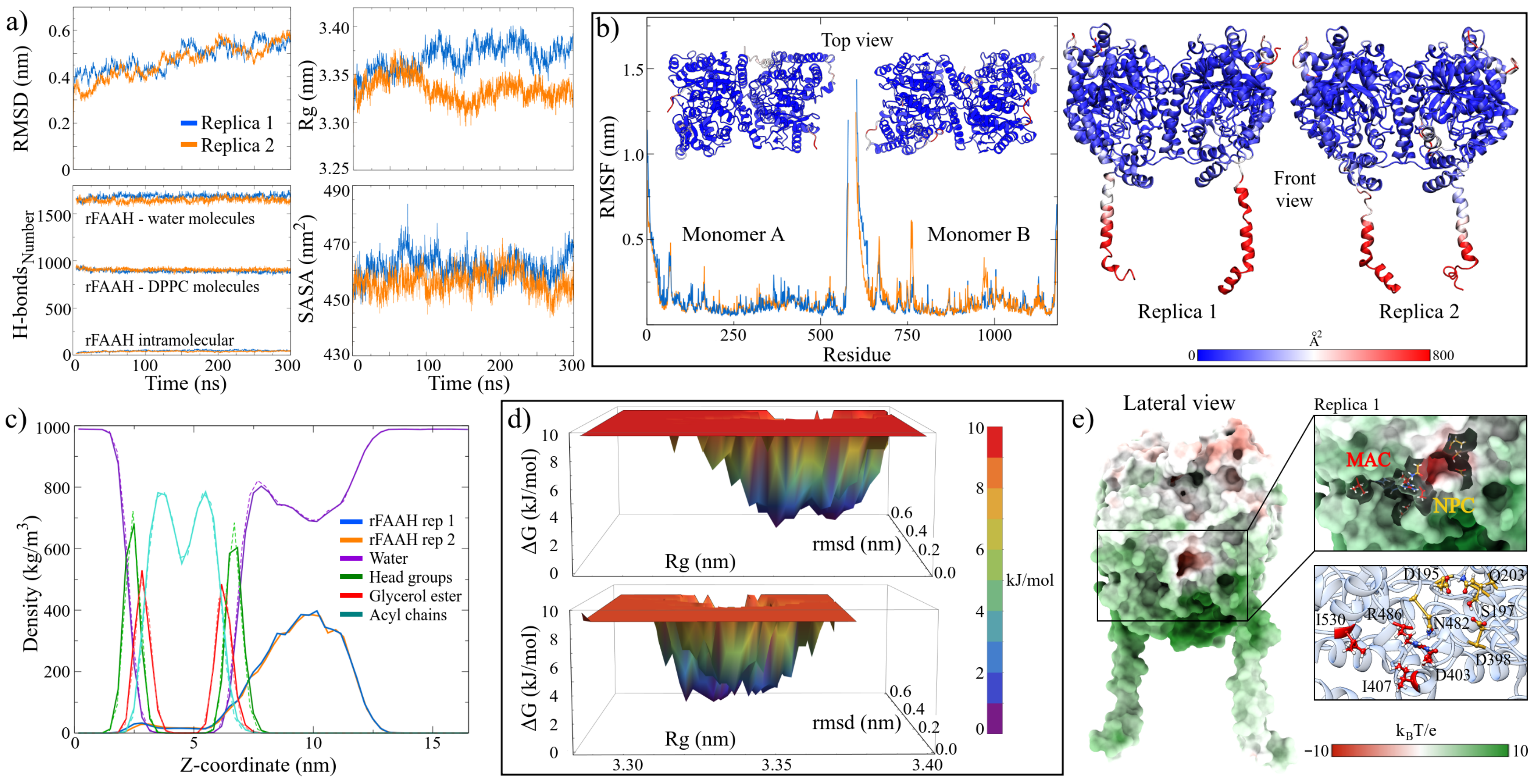
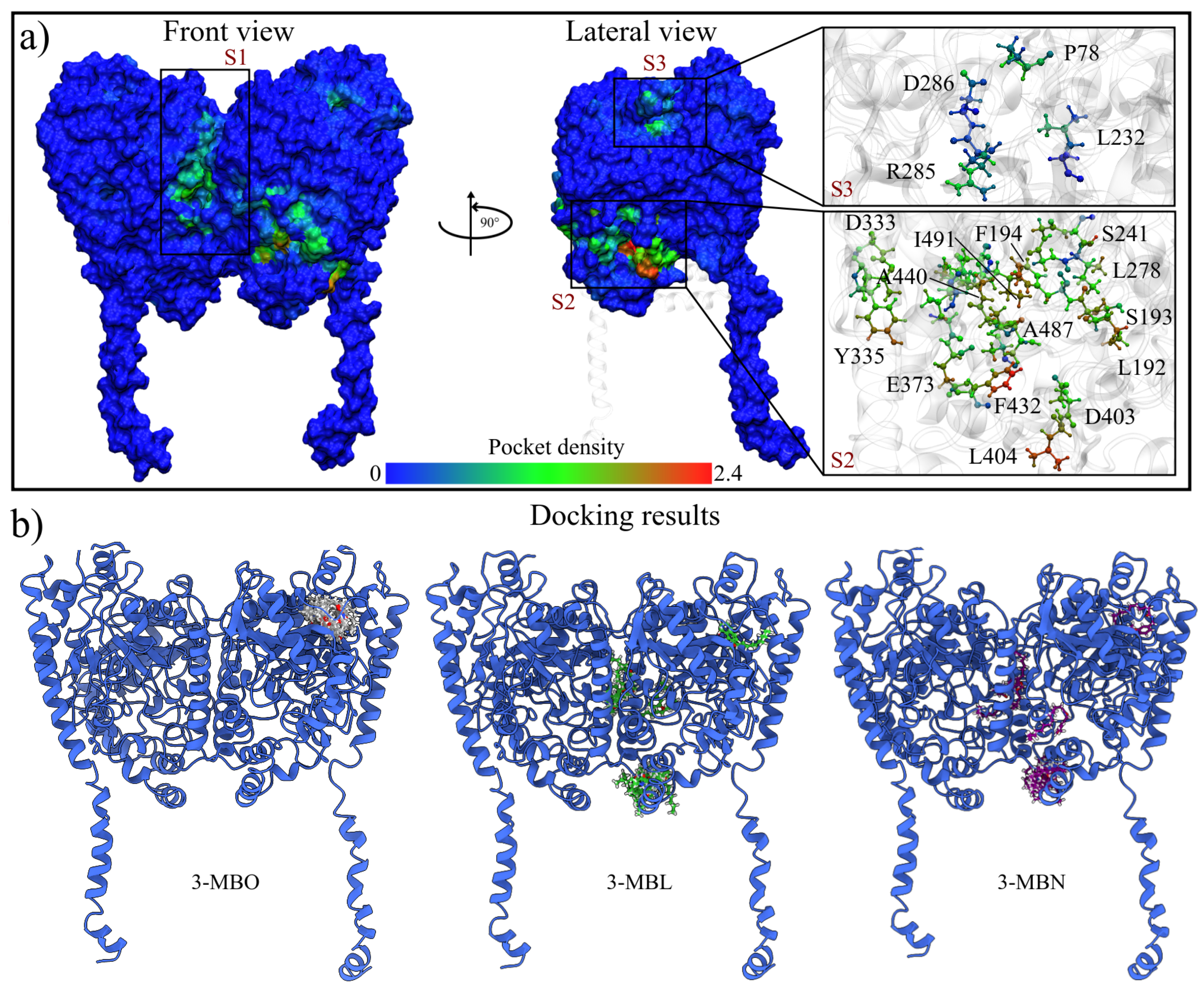
2.1.3. MD Trajectories Analysis Showed Three Regions with High Conserved Pocket Density and a New Nucleophilic Cavity on the rFAAH Structure
2.1.4. BFE Analysis Showed That 3-MBO Macamide Was Strongly Binding with rFAAH
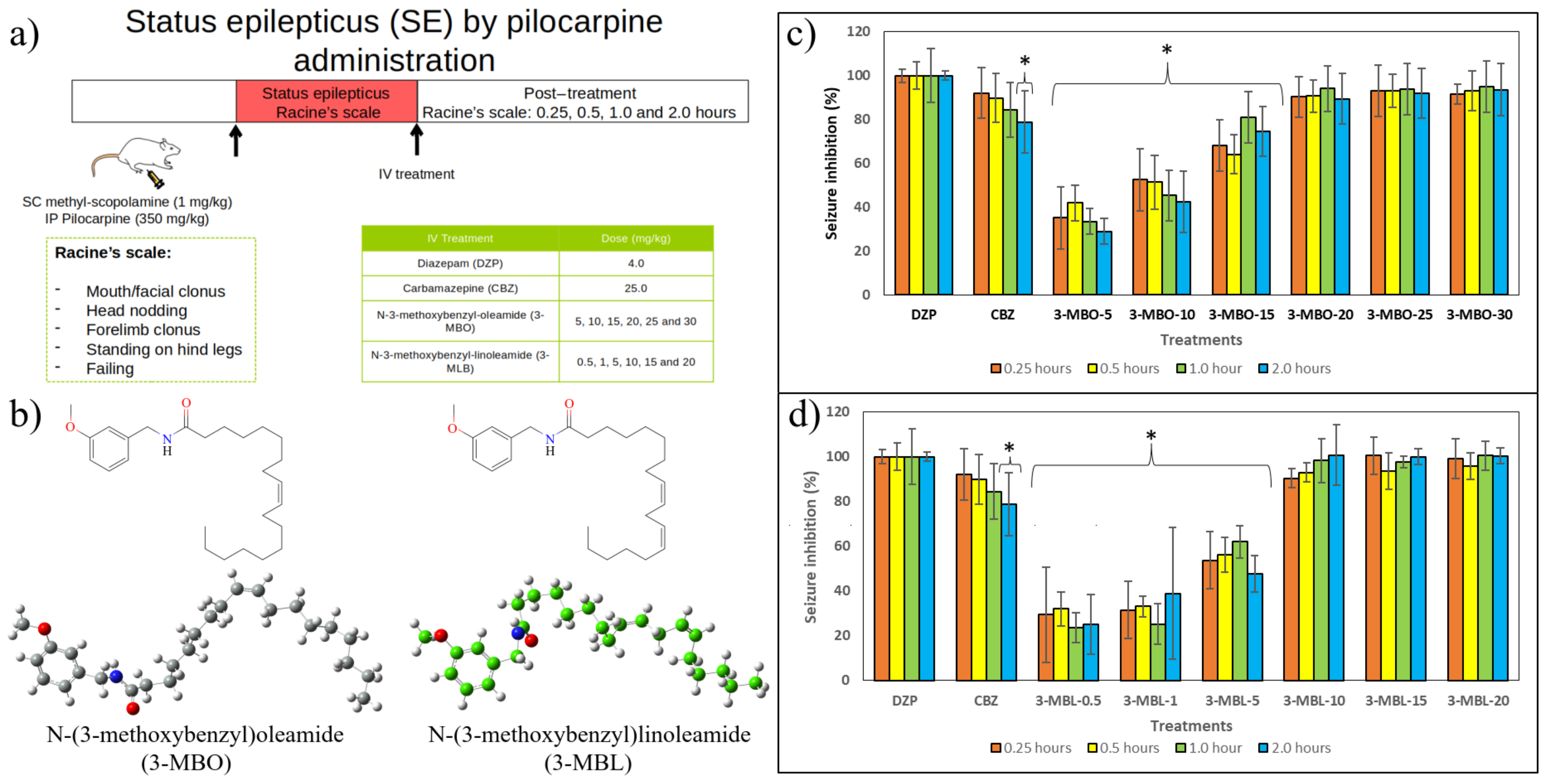
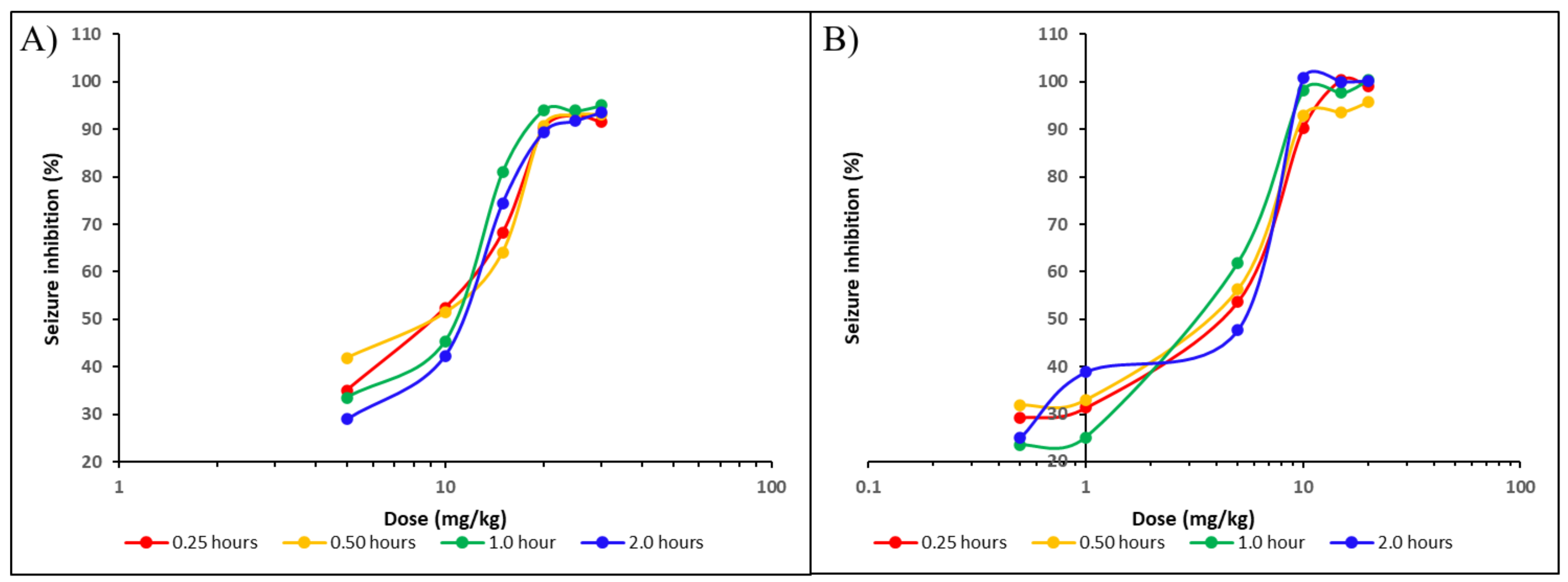
2.2. In Vivo Results
3. Materials and Methods
3.1. Computational Details
3.1.1. rFAAH and Macamide Structures
3.1.2. MD Simulations
3.1.3. Highly Conserved Pocket Search
3.1.4. Molecular Docking Calculations
3.1.5. Drug-Likeness Prediction
3.1.6. Binding Free Energy Using MM/PBSA Approximation
3.1.7. In Silico Structures and Data Analysis
3.2. In Vivo Assays
3.2.1. Reagents
3.2.2. Drugs’ Preparation
3.2.3. Animals
3.2.4. Induction of Status Epilepticus by Pilocarpine Administration
3.2.5. Experimental Procedure
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-MBO | N-(3-methoxybenzyl)oleamide |
| 3-MBL | N-(3-methoxybenzyl)linoleamide |
| 3-MBN | N-(3-methoxybenzyl)linolenamide |
| rFAAH | rat fatty acid amide hydrolase |
References
- Flores, H.E.; Walker, T.S.; Guimarães, R.L.; Bais, H.P.; Vivanco, J.M. Andean root and tuber crops: Underground rainbows. HortScience 2003, 38, 161–168. [Google Scholar] [CrossRef]
- Alasmari, M.; Bohlke, M.; Kelley, C.; Maher, T.; Pino-Figueroa, A. Inhibition of fatty acid amide hydrolase (FAAH) by macamides. Mol. Neurobiol. 2019, 56, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Cocchia, N.; Vassetti, A.; Carotenuto, D.; Esposito, L.; Maruccio, L.; Avallone, L.; Ciani, F. Lepidium meyenii (Maca) in male reproduction. Nat. Prod. Res. 2021, 35, 4550–4559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.L.; He, K.; Kim, C.H.; Rogers, L.; Shao, Y.; Huang, Z.Y.; Lu, Y.; Yan, S.J.; Qien, L.C.; Zheng, Q.Y. Effect of a lipidic extract from Lepidium meyenii on sexual behavior in mice and rats. Urology 2000, 55, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.; Piacente, S.; Plaza, A.; Sala, E.; Arletti, R.; Pizza, C. Hexanic Maca extract improves rat sexual performance more effectively than methanolic and chloroformic Maca extracts. Andrologia 2002, 34, 177–179. [Google Scholar] [CrossRef]
- Choi, E.H.; Kang, J.I.; Cho, J.Y.; Lee, S.H.; Kim, T.S.; Yeo, I.H.; Chun, H.S. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J. Funct. Foods 2012, 4, 568–573. [Google Scholar] [CrossRef]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef]
- Vera-López, K.J.; Davila-Del-Carpio, G.; Nieto-Montesinos, R. Macamides as Potential Therapeutic Agents in Neurological Disorders. Neurol. Int. 2024, 16, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F. Ethnobiology and ethnopharmacology of Lepidium meyenii (Maca), a plant from the Peruvian highlands. Evid.-Based Complement. Altern. Med. 2012, 2012, 193496. [Google Scholar] [CrossRef] [PubMed]
- Pino-Figueroa, A.; Vu, H.; Kelley, C.J.; Maher, T.J. Mechanism of action of Lepidium meyenii (Maca): An explanation for its neuroprotective activity. Am. J. Neuroprot. Neuroregen. 2011, 3, 87–92. [Google Scholar] [CrossRef]
- Gugnani, K.S.; Vu, N.; Rondón-Ortiz, A.N.; Böhlke, M.; Maher, T.J.; Pino-Figueroa, A.J. Neuroprotective activity of macamides on manganese-induced mitochondrial disruption in U-87 MG glioblastoma cells. Toxicol. Appl. Pharmacol. 2018, 340, 67–76. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, W.; Cui, Y.; Ao, M.; Liu, H.; Xu, H.; Yu, L. Protective effects of macamides from Lepidium meyenii Walp. against corticosterone-induced neurotoxicity in PC12 cells. RSC Adv. 2019, 9, 23096–23108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. The macamide relieves fatigue by acting as inhibitor of inflammatory response in exercising mice: From central to peripheral. Eur. J. Pharmacol. 2022, 917, 174758. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kelley, C.J.; Pino-Figueroa, A.; Vu, H.D.; Maher, T.J. Macamides and their synthetic analogs: Evaluation of in vitro FAAH inhibition. Bioorg. Med. Chem. 2013, 21, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Chen, J.; Deng, J.L.; Zhu, Y.Q.; Li, W.Y.; Jie, B.; Chen, T.Y. Novel macamides from maca (Lepidium meyenii Walpers) root and their cytotoxicity. Phytochem. Lett. 2018, 25, 65–69. [Google Scholar] [CrossRef]
- Thompson, A.L.; Grenald, S.; Ciccone, H.; BassiriRad, N.; Niphakis, M.; Cravatt, B.; Largent-Milnes, T.M.; Vanderah, T.W. The endocannabinoid system alleviates pain in a murine model of cancer-induced bone pain. J. Pharmacol. Exp. Ther. 2020, 373, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Piscitelli, F.; De Castro, V.; Murru, E.; Gabaglio, M.; Colucci, P.; Fanali, C.; Prini, P.; Bisogno, T.; Maccarrone, M.; et al. Lifelong imbalanced LA/ALA intake impairs emotional and cognitive behavior via changes in brain endocannabinoid system. J. Lipid Res. 2017, 58, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Alvares, L.D.O.; Genro, B.P.; Diehl, F.; Quillfeldt, J.A. Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol. Learn. Mem. 2008, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, A.; Siopi, E.; Finn, D.; Marchand-Leroux, C.; Garcia-Segura, L.; Jafarian-Tehrani, M.; Viveros, M. CB1 and CB2 Cannabinoid Receptor Antagonists Prevent Minocycline-Induced Neuroprotection Following Traumatic Brain Injury in Mice. Cereb Cortex 2013, 25, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid receptors in the central nervous system: Their signaling and roles in disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, G.C.; Oprea, T.I.; Zhao, P.; Abood, M.E.; Brailoiu, E. Intracellular cannabinoid type 1 (CB1) receptors are activated by anandamide. J. Biol. Chem. 2011, 286, 29166–29174. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, M.; Grieve, K.; Cudeiro, J.; Rivadulla, C. Anandamide activation of CB1 receptors increases spontaneous bursting and oscillatory activity in the thalamus. Neuroscience 2014, 265, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Kirkham, T.C. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology 1999, 143, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Dainese, E.; Oddi, S.; Simonetti, M.; Sabatucci, A.; Angelucci, C.B.; Ballone, A.; Dufrusine, B.; Fezza, F.; De Fabritiis, G.; Maccarrone, M. The endocannabinoid hydrolase FAAH is an allosteric enzyme. Sci. Rep. 2020, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Morena, M.; Haataja, R.; Hammar, V.; Augier, G.; Hill, M.N.; Heilig, M. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: A randomized, controlled experimental medicine trial. Biol. Psychiatry 2020, 87, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Campomanes, P.; Cavalli, A.; Rothlisberger, U.; De Vivo, M. Anandamide hydrolysis in FAAH reveals a dual strategy for efficient enzyme-assisted amide bond cleavage via nitrogen inversion. J. Phys. Chem. B 2015, 119, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Scherma, M.; Medalie, J.; Fratta, W.; Vadivel, S.K.; Makriyannis, A.; Piomelli, D.; Mikics, E.; Haller, J.; Yasar, S.; Tanda, G.; et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology 2008, 54, 129–140. [Google Scholar] [CrossRef]
- Almukadi, H.; Wu, H.; Böhlke, M.; Kelley, C.J.; Maher, T.J.; Pino-Figueroa, A. The macamide N-3-methoxybenzyl-linoleamide is a time-dependent fatty acid amide hydrolase (FAAH) inhibitor. Mol. Neurobiol. 2013, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, Z.; Nicolussi, S.; Rau, M.; Loraántfy, L.; Forgo, P.; Hohmann, J.; Csupor, D.; Gertsch, J. Identification of endocannabinoid system-modulating N-alkylamides from Heliopsis helianthoides var. scabra and Lepidium meyenii. J. Nat. Prod. 2014, 77, 1663–1669. [Google Scholar] [CrossRef]
- Wallace, M.J.; Martin, B.R.; DeLorenzo, R.J. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur. J. Pharmacol. 2002, 452, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ludányi, A.; Eross, L.; Czirják, S.; Vajda, J.; Halász, P.; Watanabe, M.; Palkovits, M.; Maglóczky, Z.; Freund, T.F.; Katona, I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 2008, 28, 2976–2990. [Google Scholar] [CrossRef]
- Di Maio, R.; Colangeli, R.; Di Giovanni, G. WIN 55,212-2 reverted pilocarpine-induced status epilepticus early changes of the interaction among 5-HT2C/NMDA/CB1 receptors in the rat hippocampus. ACS Chem. Neurosci. 2019, 10, 3296–3306. [Google Scholar] [CrossRef] [PubMed]
- Romigi, A.; Bari, M.; Placidi, F.; Marciani, M.G.; Malaponti, M.; Torelli, F.; Izzi, F.; Prosperetti, C.; Zannino, S.; Corte, F.; et al. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia 2010, 51, 768–772. [Google Scholar] [CrossRef]
- Mikheeva, I.B.; Shubina, L.; Matveeva, N.; Pavlik, L.L.; Kitchigina, V.F. Fatty acid amide hydrolase inhibitor URB597 may protect against kainic acid–induced damage to hippocampal neurons: Dependence on the degree of injury. Epilepsy Res. 2017, 137, 84–94. [Google Scholar] [CrossRef]
- Colangeli, R.; Morena, M.; Pittman, Q.J.; Hill, M.N.; Teskey, G.C. Anandamide signaling augmentation rescues amygdala synaptic function and comorbid emotional alterations in a model of epilepsy. J. Neurosci. 2020, 40, 6068–6081. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.K.P. A perspective review on fatty acid amide hydrolase (FAAH) inhibitors as potential therapeutic agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef]
- Dincheva, I.; Drysdale, A.T.; Hartley, C.A.; Johnson, D.C.; Jing, D.; King, E.C.; Ra, S.; Gray, J.M.; Yang, R.; DeGruccio, A.M.; et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 2015, 6, 6395. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.K.; Li, G.; Xia, M.; Boini, K. Anandamide and its metabolites: What are their roles in the kidney? Front. Biosci. (Sch. Ed.) 2016, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, W.; Poklis, J.L.; Lichtman, A.H.; Ritter, J.K.; Hu, G.; Xie, D.; Li, N. Inactivation of fatty acid amide hydrolase protects against ischemic reperfusion injury-induced renal fibrogenesis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166456. [Google Scholar] [CrossRef] [PubMed]
- Sathynathan, C.V.; Raman, L.S.; Vajiravelu, S.; Kumar, T.D.; Panchatcharam, T.S.; Narasimhan, G.; Doss, G.C.P.; Krishnan, M.E.G. 3-Hydroxypropane-1, 2-diyl dipalmitoleate—a natural compound with dual roles (CB1 agonist/FAAH1 blocker) in inhibiting ovarian cancer cell line. Pharmaceuticals 2021, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Gibbons, H.; Mitchell, M.; Glassa, M. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta 2003, 24, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, F.; Gandaglia, G.; Benigni, F.; Bettiga, A.; Rigatti, P.; Montorsi, F.; Gratzke, C.; Stief, C.; Colciago, G.; Hedlund, P. Expression of fatty acid amide hydrolase (FAAH) in human, mouse, and rat urinary bladder and effects of FAAH inhibition on bladder function in awake rats. Eur. Urol. 2012, 61, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Giang, D.K.; Cravatt, B.F. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. USA 1997, 94, 2238–2242. [Google Scholar] [CrossRef]
- Maleki, M.F.; Nadri, H.; Kianfar, M.; Edraki, N.; Eisvand, F.; Ghodsi, R.; Mohajeri, S.A.; Hadizadeh, F. Design and synthesis of new carbamates as inhibitors for fatty acid amide hydrolase and cholinesterases: Molecular dynamic, in vitro and in vivo studies. Bioorg. Chem. 2021, 109, 104684. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, C.M.; Lupia, A.; Deplano, A.; Pirone, L.; Fiorillo, B.; Pedone, E.; Luque, F.J.; Onnis, V.; Moraca, F.; Catalanotti, B. Molecular Basis for Non-Covalent, Non-Competitive FAAH Inhibition. Int. J. Mol. Sci. 2022, 23, 15502. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, gkae236. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.W.; Gu, C. The molecular constituents of the blood–brain barrier. Trends Neurosci. 2015, 38, 598–608. [Google Scholar] [CrossRef]
- Benz, F.; Liebner, S. Structure and function of the blood–brain barrier (BBB). In Physiology, Pharmacology and Pathology of the Blood-Brain Barrier; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–31. [Google Scholar]
- Lacoste, B.; Prat, A.; Freitas-Andrade, M.; Gu, C. The Blood–Brain Barrier: Composition, Properties, and Roles in Brain Health. Cold Spring Harb. Perspect. Biol. 2024, a041422. [Google Scholar] [CrossRef]
- Williams, J.A.; Hyland, R.; Jones, B.C.; Smith, D.A.; Hurst, S.; Goosen, T.C.; Peterkin, V.; Koup, J.R.; Ball, S.E. Drug-drug interactions for UDP-glucuronosyltransferase substrates: A pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab. Dispos. 2004, 32, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Kacevska, M.; Robertson, G.R.; Clarke, S.J.; Liddle, C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: Impact and implications for chemotherapeutic drug dosing. Expert Opin. Drug Metab. Toxicol. 2008, 4, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. Fatty-Acid Amide Hydrolase 1 - P97612, FAAH1-RAT. 2024. Available online: https://www.uniprot.org/uniprotkb/P97612/entry (accessed on 29 May 2024).
- Leonenko, Z.; Finot, E.; Ma, H.; Dahms, T.; Cramb, D. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef]
- Paco-Chipana, M.; Febres-Molina, C.; Aguilar-Pineda, J.A.; Gómez, B. Novel in silico insights into Rv1417 and Rv2617c as potential protein targets: The importance of the medium on the structural interactions with exported repetitive protein (Erp) of Mycobacterium tuberculosis. Polymers 2022, 14, 2577. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pineda, J.A.; Febres-Molina, C.; Cordova-Barrios, C.C.; Campos-Olazával, L.M.; Del-Carpio-Martinez, B.A.; Ayqui-Cueva, F.; Gamero-Begazo, P.L.; Gómez, B. Study of the Rv1417 and Rv2617c membrane proteins and their interactions with nicotine derivatives as potential inhibitors of Erp virulence-associated factor in Mycobacterium tuberculosis: An in silico approach. Biomolecules 2023, 13, 248. [Google Scholar] [CrossRef]
- Aguilar-Pineda, J.A.; González-Melchor, M. Influence of the Water Model on the Structure and Interactions of the GPR40 Protein with the Lipid Membrane and the Solvent: Rigid versus Flexible Water Models. J. Chem. Theory Comput. 2024, 20, 6369–6387. [Google Scholar] [CrossRef]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef]
- Schmidtke, P.; Le Guilloux, V.; Maupetit, J.; Tufféry, P. Fpocket: Online tools for protein ensemble pocket detection and tracking. Nucleic Acids Res. 2010, 38, W582–W589. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, R.; Consortium, O.S.D.D.; Lynn, A. g_mmpbsa A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Biagini, G.; de Curtis, M.; Gnatkovsky, V.; Pitsch, J.; Wang, S.; Avoli, M. The pilocarpine model of mesial temporal lobe epilepsy: Over one decade later, with more rodent species and new investigative approaches. Neurosci. Biobehav. Rev. 2021, 130, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, H.; Matagne, A.; Vanneste-Goemaere, J.; Margineanu, D.G. Pilocarpine-induced epileptogenesis in the rat:: Impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res. 2002, 51, 93–107. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Price, G.; Patel, D.A. Drug Bioavailability; StatPearls: St. Petersburg, FL, USA, 2020. [Google Scholar]
- Cyriac, J.M.; James, E. Switch over from intravenous to oral therapy: A concise overview. J. Pharmacol. Pharmacother. 2014, 5, 83–87. [Google Scholar] [CrossRef] [PubMed]
- AlphaFold Protein Structure Database. Fatty-Acid Amide Hydrolase 1. 2022. Available online: https://alphafold.ebi.ac.uk/entry/P97612 (accessed on 3 June 2024).
- Kono, M.; Matsumoto, T.; Kawamura, T.; Nishimura, A.; Kiyota, Y.; Oki, H.; Miyazaki, J.; Igaki, S.; Behnke, C.A.; Shimojo, M.; et al. Synthesis, SAR study, and biological evaluation of a series of piperazine ureas as fatty acid amide hydrolase (FAAH) inhibitors. Bioorg. Med. Chem. 2013, 21, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Lemkul, J.A. From proteins to perturbed Hamiltonians: A suite of tutorials for the GROMACS-2018 molecular simulation package [article v1.0]. Living J. Comp. Mol. Sci. 2019, 1, 33011. [Google Scholar] [CrossRef]
- Kandt, C.; Ash, W.L.; Tieleman, D.P. Setting up and running molecular dynamics simulations of membrane proteins. Methods 2007, 41, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Florová, P.; Sklenovsky, P.; Banas, P.; Otyepka, M. Explicit water models affect the specific solvation and dynamics of unfolded peptides while the conformational behavior and flexibility of folded peptides remain intact. J. Chem. Theory Comput. 2010, 6, 3569–3579. [Google Scholar] [CrossRef]
- Dainese, E.; De Fabritiis, G.; Sabatucci, A.; Oddi, S.; Angelucci, C.B.; Di Pancrazio, C.; Giorgino, T.; Stanley, N.; Del Carlo, M.; Cravatt, B.F.; et al. Membrane lipids are key modulators of the endocannabinoid-hydrolase FAAH. Biochem. J. 2014, 457, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Silvestri, C.; Vitale, R.M.; Martos, J.L.; Piscitelli, F.; Wang, J.W.; Allarà, M.; Carling, R.W.; Luongo, L.; Guida, F.; et al. FAAH-Catalyzed C–C Bond Cleavage of a New Multitarget Analgesic Drug. ACS Chem. Neurosci. 2018, 10, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.M.; Elmasry, G.F.; Refaey, R.H.; El-Shiekh, R.A. Lepidium meyenii (maca) roots: UPLC-HRMS, molecular docking, and molecular dynamics. ACS Omega 2022, 7, 17339–17357. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView 6.0. 16, Semichem Inc.: Shawnee Mission, KS, USA, 2016. Available online: https://gaussian.com/gaussview6/ (accessed on 30 November 2023).
- Dewar, M.J.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J. Development and use of quantum mechanical molecular models. 76. AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 16, Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Ritchie, J.P.; Bachrach, S.M. Some methods and applications of electron density distribution analysis. J. Comput. Chem. 1987, 8, 499–509. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef] [PubMed]
- Dodda, L.S.; Vilseck, J.Z.; Tirado-Rives, J.; Jorgensen, W.L. 1.14* CM1A-LBCC: Localized bond-charge corrected CM1A charges for condensed-phase simulations. J. Phys. Chem. B 2017, 121, 3864–3870. [Google Scholar] [CrossRef] [PubMed]
- Dodda, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Kutzner, C.; Páll, S.; Fechner, M.; Esztermann, A.; de Groot, B.L.; Grubmüller, H. More bang for your buck: Improved use of GPU nodes for GROMACS 2018. J. Comput. Chem. 2019, 40, 2418–2431. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Tieleman, D.P.; Berendsen, H.J.C. Molecular dynamics simulations of a fully hydrated dipalmitoylphosphatidylcholine bilayer with different macroscopic boundary conditions and parameters. J. Chem. Phys. 1996, 105, 4871–4880. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Refinetti, R.; Ma, H.; Satinoff, E. Body temperature rhythms, cold tolerance, and fever in young and old rats of both genders. Exp. Gerontol. 1990, 25, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.; Fraaije, J.G. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Hess, B. P-LINCS: A parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef]
- Mashiach, E.; Schneidman-Duhovny, D.; Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: A web server for fast interaction refinement in molecular docking. Nucleic Acids Res. 2008, 36, W229–W232. [Google Scholar] [CrossRef] [PubMed]
- Homeyer, N.; Gohlke, H. Free energy calculations by the molecular mechanics Poisson- Boltzmann surface area method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Honig, B.; Nicholls, A. Classical electrostatics in biology and chemistry. Science 1995, 268, 1144–1149. [Google Scholar] [CrossRef]
- Levy, R.M.; Zhang, L.Y.; Gallicchio, E.; Felts, A.K. On the nonpolar hydration free energy of proteins: Surface area and continuum solvent models for the solute- solvent interaction energy. J. Am. Chem. Soc. 2003, 125, 9523–9530. [Google Scholar] [CrossRef]
- Tan, C.; Tan, Y.H.; Luo, R. Implicit nonpolar solvent models. J. Phys. Chem. B 2007, 111, 12263–12274. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Grace, X. XMGrace Software, Version 5.1. 19, Center for Coastal and Land-Margin Research, Oregon Graduate Institute of Science and Technology: Beaverton, OR, USA, 2005.
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- Wolfram Research, Inc. Mathematica, Version 12.1, Wolfram Research, Inc.: Champaign, IL, USA, 2020.
- Turski, W.A.; Cavalheiro, E.A.; Schwarz, M.; Czuczwar, S.J.; Kleinrok, Z.; Turski, L. Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983, 9, 315–335. [Google Scholar] [CrossRef]
- Brophy, G.M.; Bell, R.; Claassen, J.; Alldredge, B.; Bleck, T.P.; Glauser, T.; LaRoche, S.M.; Riviello, J.J.; Shutter, L.; Sperling, M.R.; et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit. Care 2012, 17, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Moussally, J.; Cash, S.S.; Karnam, H.B.; Cole, A.J. Intravenous levetiracetam in the rat pilocarpine-induced status epilepticus model: Behavioral, physiological and histological studies. Neuropharmacology 2010, 58, 793–798. [Google Scholar] [CrossRef] [PubMed]


| Property | Parameter | Predicted Value a | Property | Parameter | Predicted Value a | ||||
|---|---|---|---|---|---|---|---|---|---|
| 3-MBO | 3-MBL | 3-MBN | 3-MBO | 3-MBL | 3-MBN | ||||
| Physicochemical | logS | −6.585 | −6.623 | −6.216 | Metabolism | CYP1A2 inhibitor | +++ | +++ | +++ |
| logP | 7.818 | 7.244 | 6.395 | CYP1A2 substrate | - - - | - - - | - - - | ||
| logD7.4 | 4.621 | 4.465 | 4.305 | CYP2C19 inhibitor | +++ | +++ | +++ | ||
| CYP2C19 substrate | - - - | +++ | ++ | ||||||
| Medicinal–Chemical | QED (>0.67) | 0.206 | 0.224 | 0.243 | CYP2C9 inhibitor | + | +++ | ++ | |
| SAscore | Easy | Easy | Easy | CYP2C9 substrate | +++ | +++ | +++ | ||
| NPscore | −0.115 | 0.104 | 0.222 | CYP2D6 inhibitor | +++ | - | - - - | ||
| CYP2D6 substrate | +++ | +++ | +++ | ||||||
| Absorption | Caco-2 Perm. (>−5.15) | −5.016 | −5.009 | −4.982 | CYP3A4 inhibitor | +++ | +++ | +++ | |
| MDCK Perm. | 0.0 | 0.0 | 0.0 | CYP3A4 substrate | - - - | - - - | - - - | ||
| PAMPA | - - - | - - - | - - - | ||||||
| Pgp-inhibitor | + | ++ | +++ | ||||||
| Pgp-substrate | - - - | - - - | - - - | Toxicity | hERG Blockers | 0.825 | 0.849 | 0.864 | |
| HIA | - - - | - - - | - - - | DILI | 0.04 | 0.002 | 0.0 | ||
| AMES Tox. | 0.384 | 0.435 | 0.888 | ||||||
| Distribution | PPB (<0.90) | 0.987 | 0.984 | 0.983 | Rat Oral Acute Tox. | 0.071 | 0.028 | 0.032 | |
| VDss (0.04–20) | 0.595 | 0.792 | 0.707 | FDAMDD | 0.292 | 0.052 | 0.001 | ||
| BBB | 0.081 | 0.067 | 0.174 | Skin Sensitization | 1.0 | 1.0 | 1.0 | ||
| Fu (%) | 0.50 | 1.00 | 1.30 | Carcinogencity | 0.13 | 0.021 | 0.001 | ||
| Eye Corrosion | 0.141 | 0.45 | 1.0 | ||||||
| Excretion | CL | 5.562 | 6.172 | 6.536 | Eye Irritation | 0.912 | 0.969 | 1.0 | |
| 0.386 | 0.191 | 0.158 | Respiratory Tox. | 0.545 | 0.56 | 0.944 | |||
| System | a RMSD | a Radius of Gyration | a RMSF | b SASA | c H-Bonds | |||
|---|---|---|---|---|---|---|---|---|
| Intra | Prot-Solv | Prot-Mem | Mem-Solv | |||||
| FAAH-r1 | 0.54 ± 0.03 | 3.38 ± 0.01 | 0.12 ± 0.10 | 461.30 ± 4.31 | 886 ± 14 | 1686 ± 24 | 50 ± 6 | 1497 ± 27 |
| FAAH-r2 | 0.53 ± 0.03 | 3.33 ± 0.01 | 0.12 ± 0.11 | 456.15 ± 4.98 | 905 ± 14 | 1653 ± 24 | 41 ± 5 | 1525 ± 25 |
| FAAH–macamide complex | Intra | Prot-maca | Solv-maca | |||||
| 3-MBO-s1 | 0.22 ± 0.01 | 2.29 ± 0.01 | 0.10 ± 0.05 | 211.93 ± 3.02 | 402 ± 10 | 0.17 ± 0.38 | 0.96 ± 0.81 | |
| 3-MBL-s1 | 0.18 ± 0.01 | 2.28 ± 0.01 | 0.10 ± 0.05 | 212.83 ± 3.53 | 408 ± 10 | 0.13 ± 0.35 | 2.56 ± 1.18 | |
| 3-MBN-s1 | 0.21 ± 0.01 | 2.29 ± 0.00 | 0.10 ± 0.04 | 212.92 ± 2.74 | 415 ± 10 | 0.21 ± 0.42 | 3.37 ± 1.26 | |
| 3-MBO-s2 | 0.21 ± 0.01 | 2.29 ± 0.01 | 0.10 ± 0.05 | 213.56 ± 3.38 | 415 ± 10 | 1.02 ± 0.38 | 1.06 ± 0.82 | |
| 3-MBL-s2 | 0.23 ± 0.01 | 2.29 ± 0.00 | 0.11 ± 0.06 | 218.88 ± 2.95 | 412 ± 10 | 0.17 ± 0.38 | 0.68 ± 0.66 | |
| 3-MBN-s2 | 0.20 ± 0.02 | 2.29 ± 0.00 | 0.11 ± 0.06 | 215.71 ± 2.93 | 412 ± 9 | 0.09 ± 0.29 | 0.94 ± 0.77 | |
| System | Van der Waals | Electrostatic | Polar Solvation | SASA | BFE |
|---|---|---|---|---|---|
| 3-MBO-s2 | −214.10 ± 0.90 | −104.52 ± 1.38 | 126.82 ± 0.78 | −26.98 ± 0.07 | −218.77 ± 2.72 |
| 3-MBL-s2 | −225.38 ± 0.93 | −19.34 ± 1.23 | 62.30 ± 0.88 | −26.24 ± 0.09 | −208.66 ± 2.55 |
| 3-MBN-s2 | −177.85 ± 1.24 | −18.24 ± 1.34 | 46.68 ± 0.92 | −23.44 ± 0.11 | −172.85 ± 3.23 |
| 3-MBO-s3 | −173.36 ± 0.93 | −4.98 ± 0.64 | 68.21 ± 0.89 | −22.14 ± 0.10 | −132.27 ± 2.48 |
| 3-MBL-s3 | −188.88 ± 1.19 | −17.37 ± 1.31 | 109.81 ± 1.10 | −22.20 ± 0.10 | −118.64 ± 2.33 |
| 3-MBN-s3 | −90.17 ± 1.95 | −10.50 ± 1.01 | 38.27 ± 1.56 | −13.46 ± 0.27 | −75.85 ± 4.48 |
| Residue | FAAH | Site 2 | Site 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1-chA | R1-chB | R2-chA | R2-chB | 3-MBO | 3-MBL | 3-MBN | 3-MBO | 3-MBL | 3-MBN | ||
| MAC | Asp403 | 0.21 | 0.60 | 0.36 | 0.61 | 0.11 | 0.05 | 0.10 | 0.38 | 0.19 | 0.15 |
| Ile407 | 0.35 | 0.65 | 0.46 | 0.78 | 0.11 | 0.06 | 0.22 | 0.05 | 0.17 | 0.05 | |
| Arg486 | 0.13 | 0.37 | 0.25 | 0.27 | 0.39 | 0.34 | 0.46 | 0.34 | 0.32 | 0.32 | |
| Ile530 | 0.15 | 0.33 | 0.21 | 0.37 | 0.03 | 0.06 | 0.02 | 0.02 | 0.08 | 0.01 | |
| ACB | Tyr335 | 0.31 | 0.81 | 0.25 | 0.31 | 0.54 | 0.57 | 0.69 | 0.13 | 0.20 | 0.53 |
| Glu373 | 0.20 | 0.77 | 0.26 | 0.34 | 0.09 | 0.21 | 0.24 | 0.07 | 0.03 | 0.07 | |
| Arg428 | 0.30 | 0.46 | 0.13 | 0.21 | 0.55 | 0.45 | 0.76 | 0.16 | 0.38 | 0.74 | |
| Phe527 | 0.18 | 0.45 | 0.05 | 0.02 | 0.17 | 0.18 | 0.33 | 0.04 | 0.09 | 0.23 | |
| MAC/ACB transition region | Phe381 | 0.70 | 0.61 | 0.66 | 0.90 | 0.86 | 0.49 | 0.81 | 0.57 | 0.69 | 0.89 |
| Phe432 | 0.35 | 0.98 | 0.63 | 0.60 | 0.73 | 0.65 | 0.68 | 0.63 | 0.79 | 0.61 | |
| Trp531 | 0.22 | 0.66 | 0.24 | 0.29 | 0.58 | 0.37 | 0.77 | 0.16 | 0.34 | 0.60 | |
| Catalytic triad | Lys142 | 0.34 | 0.26 | 0.53 | 0.45 | 0.02 | 0.01 | 0.29 | 0.06 | 0.03 | 0.20 |
| Ser217 | 0.38 | 0.54 | 0.68 | 0.47 | 0.44 | 0.24 | 0.39 | 0.39 | 0.50 | 0.55 | |
| Ser241 | 0.75 | 0.89 | 0.65 | 0.82 | 0.68 | 0.53 | 0.62 | 0.68 | 0.82 | 1.00 | |
| Oxyanion hole | Ile238 | 0.50 | 0.72 | 0.54 | 0.75 | 0.61 | 0.53 | 0.74 | 0.68 | 0.80 | 0.81 |
| Gly239 | 0.73 | 0.60 | 0.47 | 0.68 | 0.74 | 0.66 | 0.65 | 0.71 | 0.62 | 0.81 | |
| Gly240 | 0.47 | 0.68 | 0.13 | 0.51 | 0.44 | 0.41 | 0.43 | 0.34 | 0.50 | 0.49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-López, K.J.; Aguilar-Pineda, J.A.; Moscoso-Palacios, R.M.; Davila-Del-Carpio, G.; Manrique-Murillo, J.L.; Gómez, B.; González-Melchor, M.; Nieto-Montesinos, R. Anticonvulsant Effects of Synthetic N-(3-Methoxybenzyl)oleamide and N-(3-Methoxybenzyl)linoleamide Macamides: An In Silico and In Vivo Study. Molecules 2025, 30, 333. https://doi.org/10.3390/molecules30020333
Vera-López KJ, Aguilar-Pineda JA, Moscoso-Palacios RM, Davila-Del-Carpio G, Manrique-Murillo JL, Gómez B, González-Melchor M, Nieto-Montesinos R. Anticonvulsant Effects of Synthetic N-(3-Methoxybenzyl)oleamide and N-(3-Methoxybenzyl)linoleamide Macamides: An In Silico and In Vivo Study. Molecules. 2025; 30(2):333. https://doi.org/10.3390/molecules30020333
Chicago/Turabian StyleVera-López, Karin Jannet, Jorge Alberto Aguilar-Pineda, Rodrigo Martín Moscoso-Palacios, Gonzalo Davila-Del-Carpio, José Luis Manrique-Murillo, Badhin Gómez, Minerva González-Melchor, and Rita Nieto-Montesinos. 2025. "Anticonvulsant Effects of Synthetic N-(3-Methoxybenzyl)oleamide and N-(3-Methoxybenzyl)linoleamide Macamides: An In Silico and In Vivo Study" Molecules 30, no. 2: 333. https://doi.org/10.3390/molecules30020333
APA StyleVera-López, K. J., Aguilar-Pineda, J. A., Moscoso-Palacios, R. M., Davila-Del-Carpio, G., Manrique-Murillo, J. L., Gómez, B., González-Melchor, M., & Nieto-Montesinos, R. (2025). Anticonvulsant Effects of Synthetic N-(3-Methoxybenzyl)oleamide and N-(3-Methoxybenzyl)linoleamide Macamides: An In Silico and In Vivo Study. Molecules, 30(2), 333. https://doi.org/10.3390/molecules30020333







