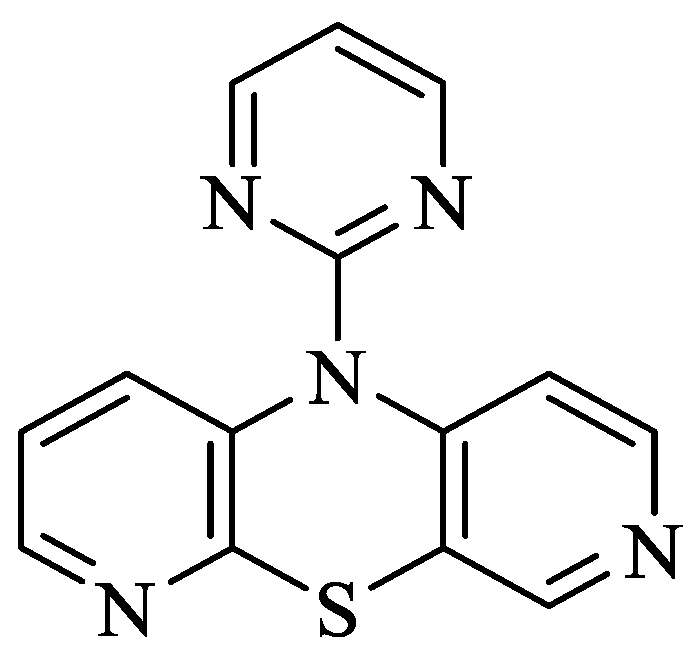
Human Serum Albumin and Human Serum Albumin Nanoparticles as Carriers of 10-(2′-Pyrimidyl)-3,6-diazaphenothiazine: In Vitro Spectroscopic Studies
Abstract
1. Introduction
2. Results
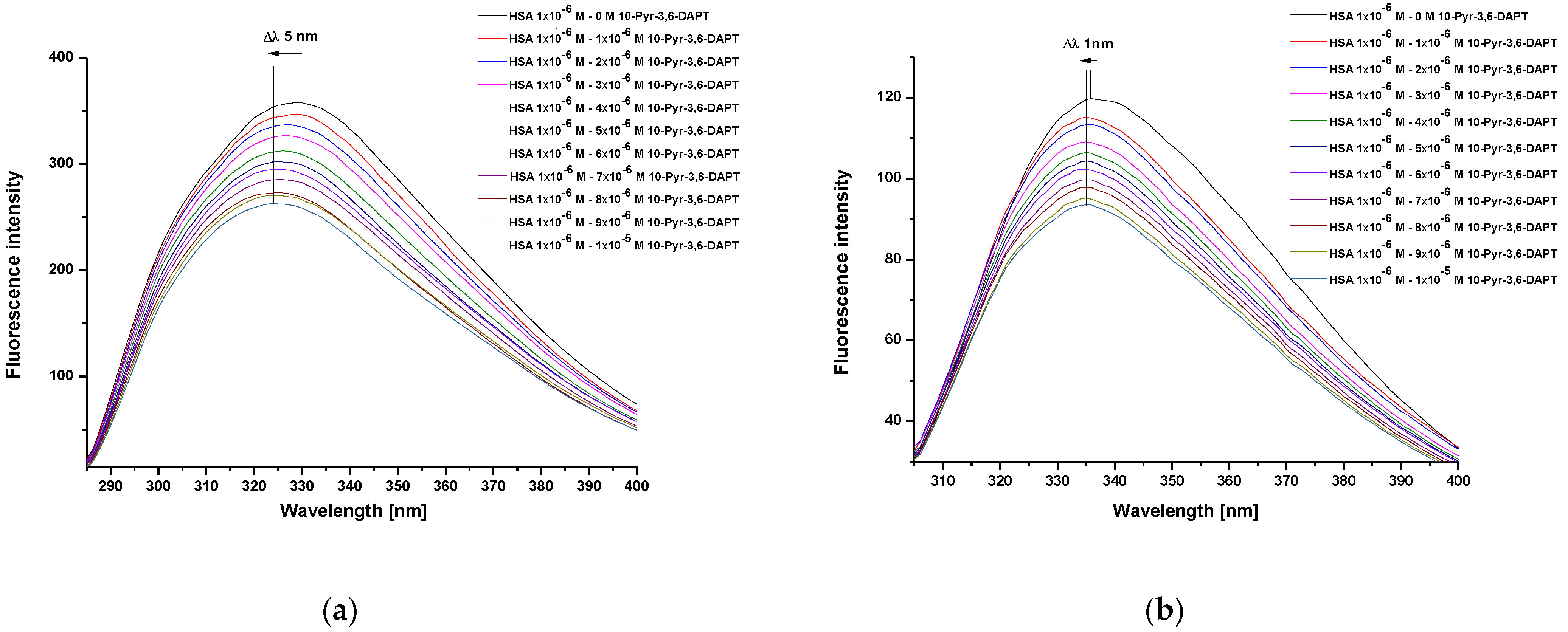
2.1. Study of HSA as a Carrier of 10-Pyr-3,6-DAPT
2.2. Study of 10-Pyr-3,6-DAPT Encapsulation into HSA-NPs
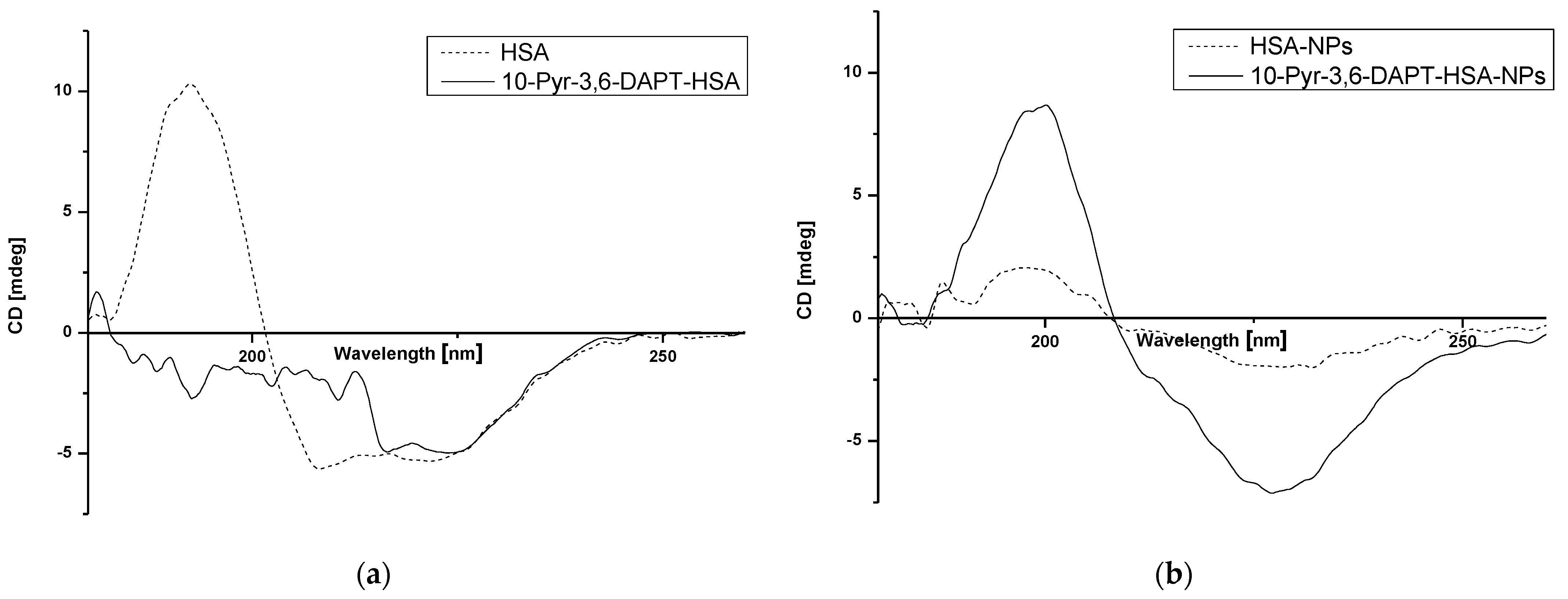
2.3. Study of HSA and HSA-NPs Secondary Structure
3. Discussion
3.1. Study of HSA as a Carrier of 10-Pyr-3,6-DAPT
3.2. Study of 10-Pyr-3,6-DAPT Encapsulation into HSA-NPs
3.3. Study of HSA and HSA-NPs Secondary Structure
4. Materials and Methods
4.1. Study of HSA as a Carrier of 10-Pyr-3,6-DAPT
4.1.1. Sample Preparation
4.1.2. Steady State Fluorescence Measurements
4.2. Study of 10-Pyr-3,6-DAPT Encapsulation into HSA-NPs
4.3. Study of HSA and HSA-NPs Secondary Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bojko, B.; Vuckovic, D.; Pawliszyn, J. Comparison of Solid Phase Microextraction versus Spectroscopic Techniques for Binding Studies of Carbamazepine. J. Pharm. Biomed. Anal. 2012, 66, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U.; Chuang, V.T.G.; Otagiri, M. Practical Aspects of the Ligand-Binding and Enzymatic Properties of Human Serum Albumin. Biol. Pharm. Bull. 2002, 25, 695–704. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic Structure and Chemistry of Human Serum Albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human Serum Albumin: From Bench to Bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar]
- Varga, B.; Csonka, Á.; Csonka, A.; Molnár, J.; Amaral, L.; Spengler, G. Possible Biological and Clinical Applications of Phenothiazines. Anticancer Res. 2017, 37, 5983–5993. [Google Scholar]
- Ohlow, M.J.; Moosmann, B. Phenothiazine: The Seven Lives of Pharmacology’s First Lead Structure. Drug Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef]
- Sudeshna, G.; Parimal, K. Multiple Non-Psychiatric Effects of Phenothiazines: A Review. Eur. J. Pharmacol. 2010, 648, 6–14. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, D.; Liu, Z.; Liu, F. Repurposing Psychiatric Drugs as Anti-Cancer Agents. Cancer Lett. 2018, 419, 257–265. [Google Scholar] [CrossRef]
- González-González, A.; Vazquez-Jimenez, L.K.; Paz-González, A.D.; Bolognesi, M.L.; Rivera, G. Recent Advances in the Medicinal Chemistry of Phenothiazines: New Anticancer and Antiprotozoal Agents. Curr. Med. Chem. 2021, 28, 7910–7936. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Jeleń, M.; Pluta, K. Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents. Life 2021, 11, 206. [Google Scholar] [CrossRef]
- Okafor, C. Studies in the Heterocyclic Series. XIV. The Chemistry and Biological Activity of New Aza- and Thia-Phenothiazines, and Related Dibenzothiazepines. Phosphorus Sulfur Silicon Relat. Elem. 1978, 4, 79. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Suwińska, K.; Jeleń, M.; Kuśmierz, D. 3,6-Diazaphenothiazines as Potential Lead Molecules—Synthesis, Characterization, and Anticancer Activity. J. Enzyme Inhib. Med. Chem. 2016, 31, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Wenzhi, Z.; Okechukwu, P.N.; Morak-Młodawska, B.; Pluta, K.; Jeleń, M.; Md Akim, A.; Ang, K.-P.; Ooi, K.K. 10H-3,6-Diazaphenothiazines Induce G2/M Phase Cell Cycle Arrest, Caspase-Dependent Apoptosis, and Inhibit Cell Invasion of A2780 Ovarian Carcinoma Cells through Regulation on NF-κB and [BIRC6-XIAP] Complexes. Drug Des. Dev. Ther. 2017, 11, 3045–3060. [Google Scholar] [CrossRef] [PubMed]
- Owczarzy, A.; Zięba, A.; Pożycka, J.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Spectroscopic Studies of Quinobenzothiazine Derivative in Terms of the In Vitro Interaction with Selected Human Plasma Proteins. Part 1. Molecules 2021, 26, 4776. [Google Scholar] [CrossRef] [PubMed]
- Owczarzy, A.; Rogóż, W.; Kulig, K.; Pożycka, J.; Zięba, A.; Maciążek-Jurczyk, M. Spectroscopic Studies of Quinobenzothiazine Derivative in Terms of the In Vitro Interaction with Selected Human Plasma Proteins. Part 2. Molecules 2023, 28, 698. [Google Scholar] [CrossRef]
- Owczarzy, A.; Trzepacz, M.; Kulig, K.; Rogóż, W.; Zięba, A.; Maciążek-Jurczyk, M. In Vitro Spectroscopic Studies of 9-Amino-5-Alkyl-12(H)-Quino [3,4-b][1,4]benzothiazine Chloride with Main Carrier Plasma Proteins. Chem.-Biol. Interact. 2025, 405, 111289. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Tasciotti, E.; Liu, X.; Bhavane, R.; Plant, K.; Leonard, A.D.; Price, B.K.; Cheng, M.M.-C.; Decuzzi, P.; Tour, J.M.; Robertson, F.; et al. Mesoporous Silicon Particles as a Multistage Delivery System for Imaging and Therapeutic Applications. Nat. Nanotechnol. 2008, 3, 151–157. [Google Scholar] [CrossRef]
- Kiarashi, M.; Yasamineh, S. Albumin nanoparticles are a promising drug delivery system in dentistry. Biomed. Eng. Online 2024, 23, 122. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, S.; Peng, X.; Zhao, M.; Li, S.; Pu, Y.; He, B. Human Serum Albumin-Based Doxorubicin Prodrug Nanoparticles with Tumor pH-Responsive Aggregation-Enhanced Retention and Reduced Cardiotoxicity. J. Mater. Chem. B 2020, 8, 3939–3948. [Google Scholar] [CrossRef]
- Kulig, K.; Ziąbka, M.; Pilarczyk, K.; Owczarzy, A.; Rogóż, W.; Maciążek-Jurczyk, M. Physicochemical Study of Albumin Nanoparticles with Chlorambucil. Processes 2022, 10, 1170. [Google Scholar] [CrossRef]
- Van de Weert, M.; Stella, L. Fluorescence Quenching and Ligand Binding: A Critical Discussion of a Popular Methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2009; pp. 34–123. [Google Scholar]
- Maciążek-Jurczyk, M.; Morak-Młodawska, B.; Jeleń, M.; Kopeć, W.; Szkudlarek, A.; Owczarzy, A.; Kulig, K.; Rogóż, W.; Pożycka, J. The Influence of Oxidative Stress on Serum Albumin Structure as a Carrier of Selected Diazaphenothiazine with Potential Anticancer Activity. Pharmaceuticals 2021, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Bteich, M. An Overview of Albumin and Alpha-1-Acid Glycoprotein Main Characteristics: Highlighting the Roles of Amino Acids in Binding Kinetics and Molecular Interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. Further Characterization of Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar]
- Maciążek-Jurczyk, M. Phenylbutazone and Ketoprofen Binding to Serum Albumin: Fluorescence Study. Pharmacol. Rep. 2014, 66, 727–731. [Google Scholar] [CrossRef]
- Kirby, E.P. Experimental Techniques, Chapter 2. In Excited States of Protein and Nucleic Acids; Steiner, R.F., Weinryb, I., Eds.; Plenum Press: New York, NY, USA, 1971; pp. 27–61. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 1–26, 277–330, 529–535. [Google Scholar]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.; Matic, M.; Djukic, M.; Avdovic, E. Redox Status, DNA, and HSA Binding Study of Naturally Occurring Naphthoquinone Derivatives. EXCLI J. 2020, 19, 48–70. [Google Scholar]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The Characterization of Two Specific Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar]
- Maciążek-Jurczyk, M.; Janas, K.; Pożycka, J.; Szkudlarek, A.; Rogóż, W.; Owczarzy, A.; Kulig, K. Human Serum Albumin Aggregation/Fibrillation and Its Abilities to Bind Drugs. Molecules 2020, 25, 618. [Google Scholar] [CrossRef]
- Ryan, A.J.; Ghuman, J.; Zunszain, P.A.; Chung, C.; Curry, S. Structural Basis of Binding of Fluorescent, Site-Specific Dansylated Amino Acids to Human Serum Albumin. J. Struct. Biol. 2011, 174, 84–91. [Google Scholar] [CrossRef]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation Process and Surface Characterization of Protein Nanoparticles. J. Pharm. 2000, 194, 91–102. [Google Scholar]
- Teran-Saavedra, N.G.; Sarabia-Sainz, J.A.; Velázquez-Contreras, E.F.; Ramos-Clamont Montfort, G.; Pedroza-Montero, M.; Vazquez-Moreno, L. Albumin-Albumin/Lactosylated Core-Shell Nanoparticles: Therapy to Treat Hepatocellular Carcinoma for Controlled Delivery of Doxorubicin. Molecules 2020, 25, 5432. [Google Scholar] [CrossRef]
- Agudelo, D.; Bourassa, P.; Bruneau, J.; Bérubé, G.; Asselin, E.; Tajmir-Riahi, H.A. Probing the Binding Sites of Antibiotic Drugs Doxorubicin and N-(Trifluoroacetyl) Doxorubicin with Human and Bovine Serum Albumins. PLoS ONE 2012, 7, e43814. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Z.; Li, C.; Xie, H.; Zhao, Q.; Zhao, M. Molecular Imprinting of Doxorubicin by Refolding Thermally Denatured Bovine Serum Albumin and Cross-Linking with Hydrogel Network. React. Funct. Polym. 2021, 168, 105036. [Google Scholar] [CrossRef]
- Śliwińska-Hill, U.; Krzyżak, E.; Czyżnikowska, Ż. The Effect of Simultaneous Binding of Doxorubicin and Cyclophosphamide on the Human Serum Albumin Structure. J. Mol. Liq. 2024, 404, 125003. [Google Scholar] [CrossRef]
- Saufi, A.N.M.; Ridzwan, N.F.W.; Mohamad, S.B.; Tayyab, S.; Halim, A.A.A. Fluorometric and Docking Analysis of the Complex Formation between an Anti-Cancer Drug, Chlorambucil, and Bovine Serum Albumin. Indian J. Pharm. Educ. Res. 2019, 53, 682–687. [Google Scholar] [CrossRef]
- Kulig, K.; Morak-Młodawska, B.; Jeleń, M.; Ziąbka, M.; Owczarzy, A.; Rogóż, W.; Maciążek-Jurczyk, M. Bovine Serum Albumin Nanoparticles as a Proposed Drug Formulation for the Delivery of 10H-2,7-Diazaphenothiazine. J. Clust. Sci. 2024, 35, 2353–2362. [Google Scholar] [CrossRef]
- Kelly, M.S.; Jess, T.J.; Price, N.C. How to Study Proteins by Circular Dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Kulig, K.; Denisiuk, Z.; Kłósek, M.; Owczarzy, A.; Rogóż, W.; Sędek, Ł.; Maciążek-Jurczyk, M. Circular Dichroism as a Rapid Method for Analyzing the Binding of a Targeting Ligand to the Surface of Albumin Nanoparticles. Pharmaceuticals 2023, 16, 1423. [Google Scholar] [CrossRef]
- Ciepluch, K.; Biehl, R.; Bryszewska, M.; Arabski, M. Poly(propylene imine) Dendrimers Can Bind to PEGylated Albumin at PEG and Albumin Surface: Biophysical Examination of a PEGylated Platform to Transport Cationic Dendritic Nanoparticles. Biopolymers 2020, 111, e23386. [Google Scholar] [CrossRef]
- Klotz, I.M.; Hunston, D.L. Properties of Graphical Representations of Multiple Classes of Binding Sites. Biochemistry 1971, 10, 3065–3069. [Google Scholar] [CrossRef]


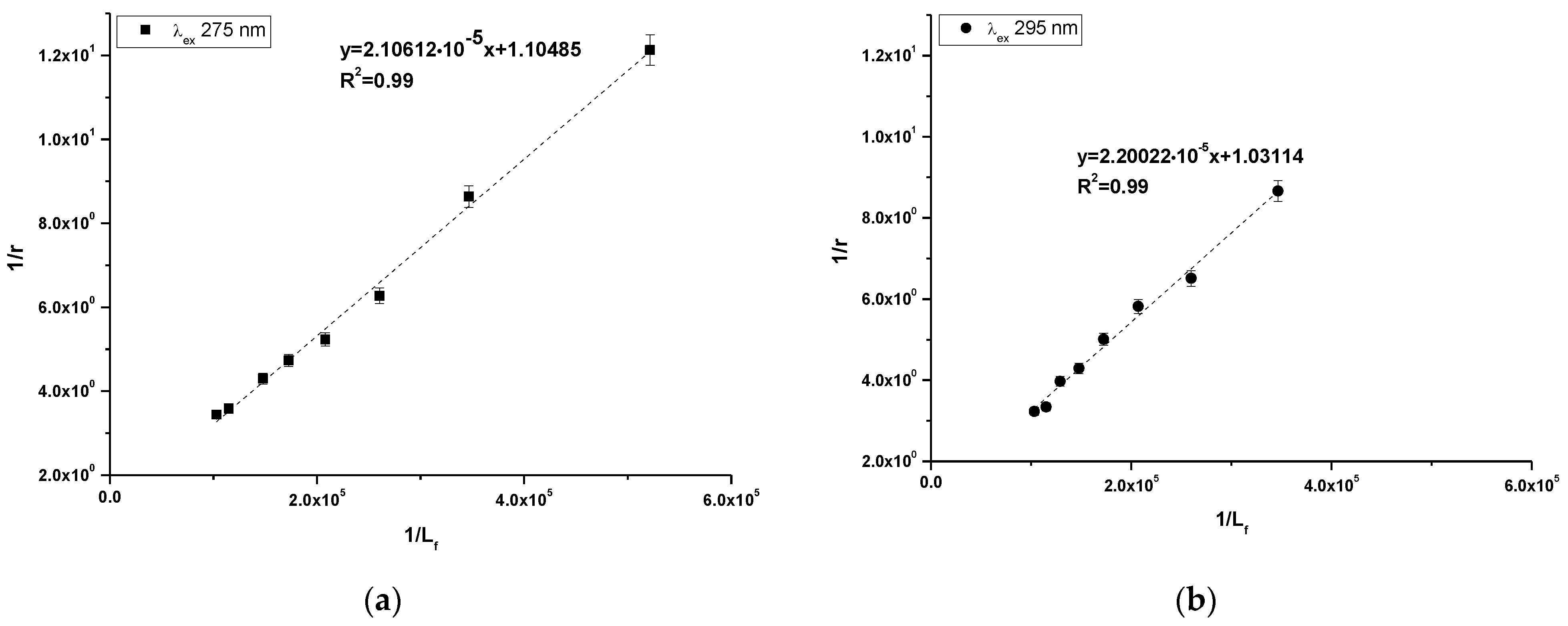
| [10-Pyr-3,6-DAPT]:[HSA] Molar Ratio | λex 275 nm | λex 295 nm | ||||
|---|---|---|---|---|---|---|
| λmax [nm] | Parameter A ±SD 1 | FWHM [nm] ±SD 1 | λmax [nm] | Parameter A ±SD 1 | FWHM [nm] ±SD 1 | |
| 0:1 | 329 | 0.71 ± 0.01 | 64.87 ± 0.41 | 336 | 0.99 ± 0.04 | 52.43 ± 0.12 |
| 10:1 | 324 | 0.70 ± 0.03 | 62.14 ± 0.23 | 335 | 0.86 ± 0.02 | 52.33 ± 0.09 |
| [10-Pyr-3,6-DAPT]:[HSA] Molar Ratio | Percentage [%] of HSA Fluorescence Quenching ±SD 1 | |
|---|---|---|
| λex 275 nm | λex 295 nm | |
| 10:1 | 15.33 ± 1.13 | 10.65 ± 2.34 |
| [10-Pyr-3,6-DAPT]:[HSA] Molar Ratio | λex 275 nm | λex 295 nm | ||
|---|---|---|---|---|
| KS-V ± SD 1 [mol−1·L] | kq ± SD 1 [mol−1·L·s−1] | KS-V ± SD 1 [mol−1·L] | kq ± SD 1 [mol−1·L·s−1] | |
| 10:1 | 1.89 × 104 ± 0.09 | 3.16 × 1012 ± 0.09 | 1.22 × 104 ± 0.04 | 2.04 × 1012 ± 0.07 |
| λex 275 nm | λex 275 nm | |||
|---|---|---|---|---|
| [10-Pyr-3,6-DAPT]:[HSA] Molar Ratio | Ka [mol−1·L] ± SD 1 | n ± SD 1 | Ka [mol−1·L] ± SD1 | n ± SD 1 |
| 10:1 | (5.24 ± 0.57) × 104 | 0.91 ± 0.13 | (4.67 ± 0.59) × 104 | 1.03 ± 0.17 |
| C10-Pyr-3,6-DAPT [mol·L−1] | [HSA]:[dGlu] Molar Ratio | [HSA]:[dPro] Molar Ratio |
|---|---|---|
| 1:1 | ||
| Percentage [%] of Displacement ± SD 1 | ||
| 0 | - | - |
| 1 × 10−5 | 39.86 ± 1.09 | 56.21 ± 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarzy, A.; Kulig, K.; Morak-Młodawska, B.; Jeleń, M.; Muhammetoglu, T.; Rogóż, W.; Maciążek-Jurczyk, M. Human Serum Albumin and Human Serum Albumin Nanoparticles as Carriers of 10-(2′-Pyrimidyl)-3,6-diazaphenothiazine: In Vitro Spectroscopic Studies. Molecules 2025, 30, 315. https://doi.org/10.3390/molecules30020315
Owczarzy A, Kulig K, Morak-Młodawska B, Jeleń M, Muhammetoglu T, Rogóż W, Maciążek-Jurczyk M. Human Serum Albumin and Human Serum Albumin Nanoparticles as Carriers of 10-(2′-Pyrimidyl)-3,6-diazaphenothiazine: In Vitro Spectroscopic Studies. Molecules. 2025; 30(2):315. https://doi.org/10.3390/molecules30020315
Chicago/Turabian StyleOwczarzy, Aleksandra, Karolina Kulig, Beata Morak-Młodawska, Małgorzata Jeleń, Tammam Muhammetoglu, Wojciech Rogóż, and Małgorzata Maciążek-Jurczyk. 2025. "Human Serum Albumin and Human Serum Albumin Nanoparticles as Carriers of 10-(2′-Pyrimidyl)-3,6-diazaphenothiazine: In Vitro Spectroscopic Studies" Molecules 30, no. 2: 315. https://doi.org/10.3390/molecules30020315
APA StyleOwczarzy, A., Kulig, K., Morak-Młodawska, B., Jeleń, M., Muhammetoglu, T., Rogóż, W., & Maciążek-Jurczyk, M. (2025). Human Serum Albumin and Human Serum Albumin Nanoparticles as Carriers of 10-(2′-Pyrimidyl)-3,6-diazaphenothiazine: In Vitro Spectroscopic Studies. Molecules, 30(2), 315. https://doi.org/10.3390/molecules30020315







