Cuphea hookeriana: Phytochemical Profile and the Cosmeceutical and Dermatological Properties of Its Active Fraction from the Whole Plant
Abstract
1. Introduction
2. Results and Discussion
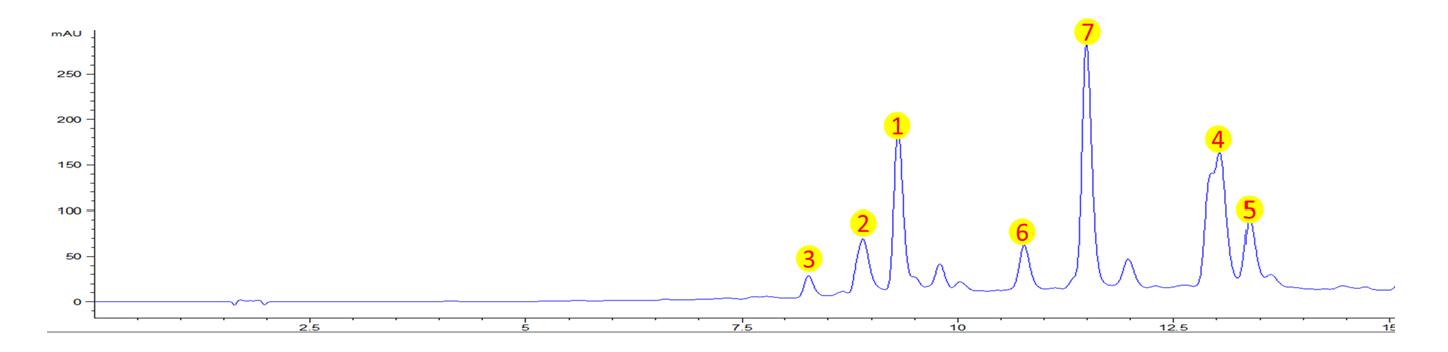
2.1. Phytochemical Profiling by HPLC
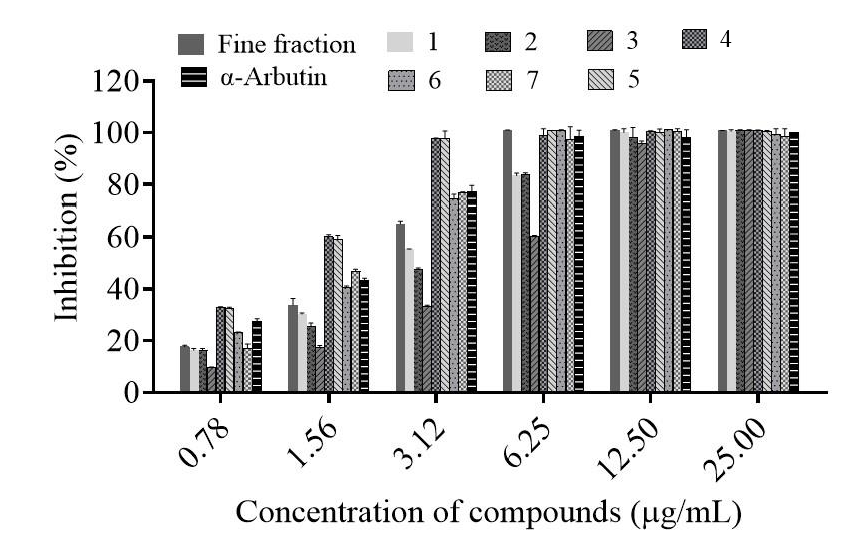
2.2. In Vitro Antioxidant Assay
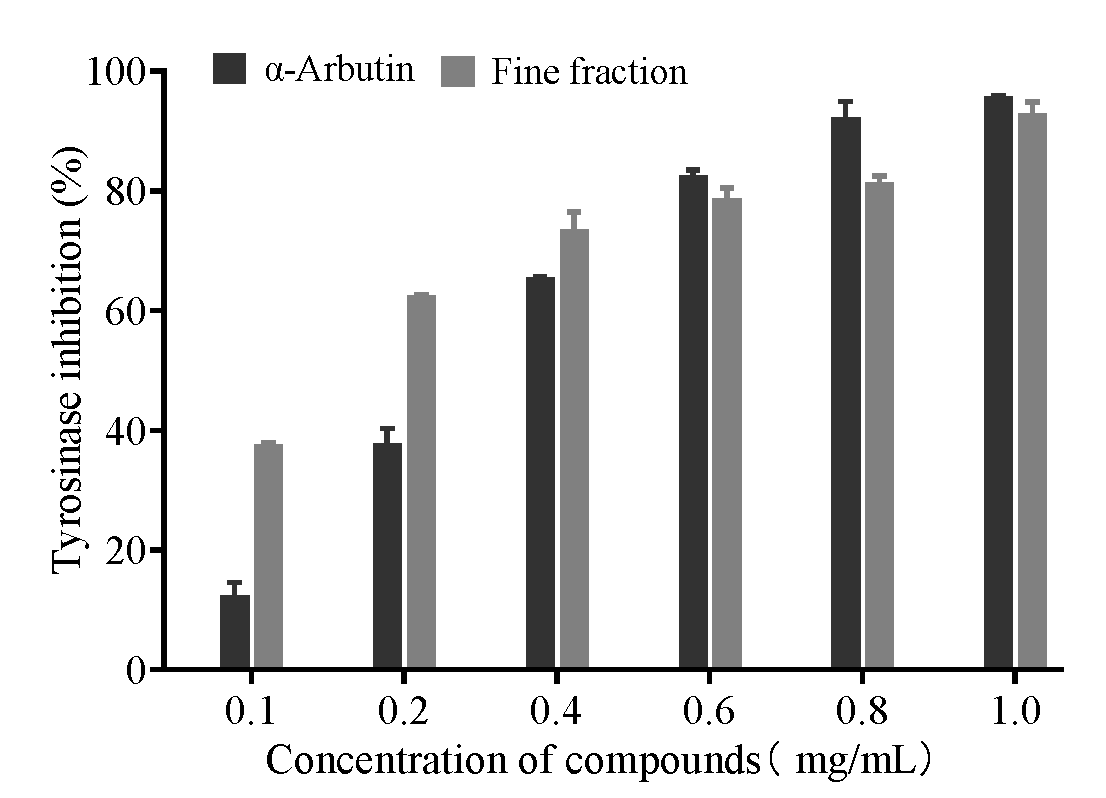
2.3. Effect of Tyrosinase Inhibition
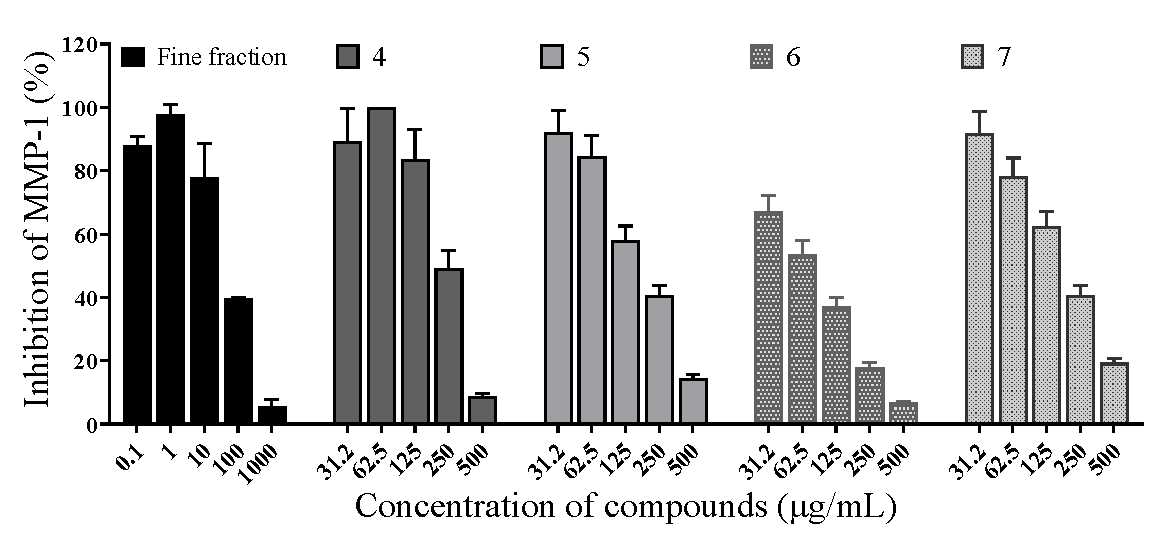
2.4. Effects of Matrix Metalloproteinase-1
2.5. Acute Toxicity Test in Mice
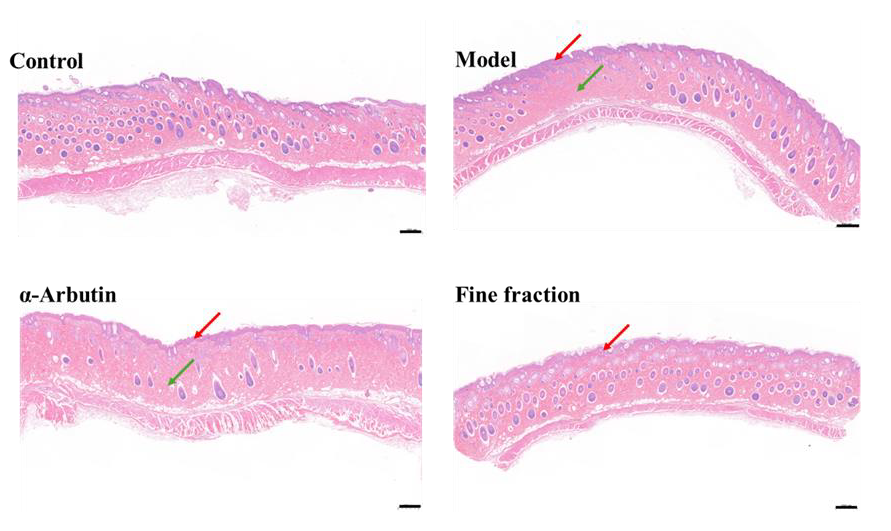
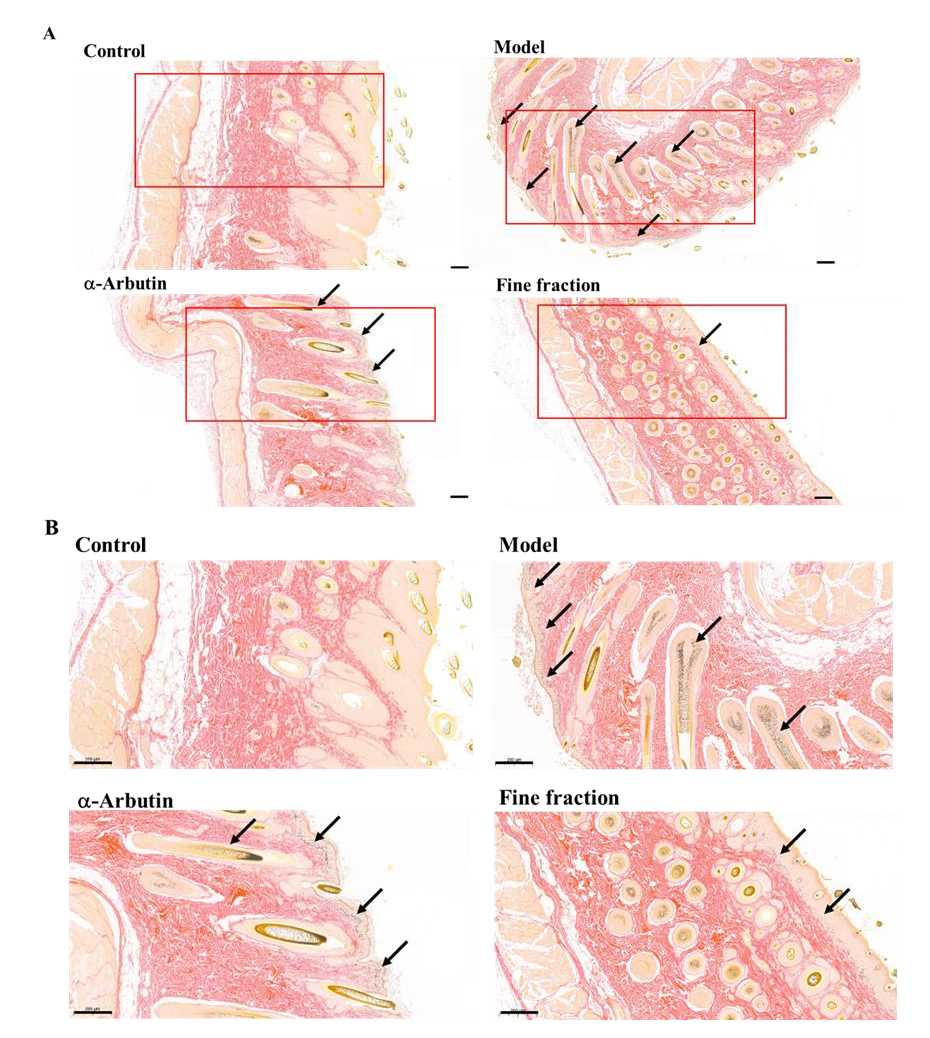
2.6. UVB-Induced Skin Pigmentation in Guinea Pigs
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
- Myricitrin (1): yellow powders, 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 6.95 (s, H-2′/H-6′), 6.36 (d, J = 2.1, H-8), 6.20 (d, J = 2.1, H-6), 5.32 (d, J = 1.6, H-1”), 4.22 (dd, J = 3.4, 1.6, H-2″), 3.79 (dd, J = 9.6, 3.4, H-3″), 3.34 (t, J = 9.6, H-4″), 3.52 (dd, J = 9.6, 6.2, H-5″), 0.96 (d, J = 6.2, H-6″). 13C NMR (150 MHz, δ, ppm): 178.3 (C-4), 164.5 (C-7), 161.7 (C-5), 158.0 (C-2), 157.1(C-9), 145.5 (C-3′/5′), 136.5 (C-4′), 134.9 (C-3), 120.5 (C-1′), 108.2 (C-2′/6′), 104.5 (C-10), 102.2 (C-1″), 98.4 (C-6), 93.3 (C-8), 71.9 (C-4″), 70.7 (C-3″), 70.6 (C-5″), 70.5 (C-2″), 16.3 (C-6″) [18].
- Tellimoside (2): yellow powders, 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 7.78 (d, J = 2.2, H-2′), 7.56 (dd, J = 8.4, 2.2, H-6′), 6.89 (s, H-2″′/6″′), 6.81 (d, J = 8.4, H-5′), 6.37 (d, J = 2.0, H-8), 6.17 (d, J = 2.0, H-6), 5.10 (d, J = 7.7, H-1″), 4.32 (dd, J = 11.0, 6.9, H-6″), 4.20 (dd, J = 11.0, 6.0, H-6″), 3.88 (d, J = 3.7, H-4″), 3.85 (m, dd, J = 9.7, 7.7 H-2″), 3.80 (dd, J = 6.9, 6.0 H-5″), 3.60 (dd, J = 9.7, 3.7, H-3″). 13C NMR (150 MHz, δ, ppm): 178.1 (C-4), 166.6 (C-7′″), 164.6 (C-7), 161.4 (C-5), 157.6(C-2), 156.9 (C-9), 148.5 (C-4′), 144.9 (C-3′″/5′″), 144.3 (C-3′), 138.4 (C-4′″), 134.3 (C-3), 121.6 (C-1′), 121.4 (C-6′), 119.6 (C-1′″), 116.4 (C-2′), 114.7 (C-5′), 108.7 (C-2′″/6′″), 104.1 (C-10), 104.1 (C-1″), 98.6 (C-6), 93.4 (C-8), 73.6 (C-5″), 73.1 (C-3″), 71.6 (C-2″), 68.6 (C-4″), 62.3 (C-6″) [19].
- Myricetin 3-O-(6″-O-galloyl)-β-D-glucopyranoside (3): yellow powders, 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 7.27 (s, H-2′/H-6′), 7.14 (s, H-2″′/6″′), 6.32 (d, J = 2.2, H-6), 6.15 (d, J = 2.2, H-8), 5.78 (d, J = 7.9, H-1”), 5.45 (dd, J = 9.9, 7.9, H-2”), 3.96 (dd, J = 3.5, 1.2, H-4”),3.85 (dd, J = 9.9, 3.5, H-3”), 3.70 (d, J = 6.1, H-6”), 3.61 (td, J = 6.1, 1.2, H-5′). 13C NMR (150 MHz, δ, ppm): 177.5 (C-4), 166.9 (C-7′″), 164.3 (C-7), 161.7 (C-9), 156.8 (C-5), 156.6 (C-2), 144.9 (C-3′/5′), 144.8 (C-3′″/5′″), 138.4 (C-4′″), 136.5 (C-4′), 133.9 (C-3), 120.6 (C-1′), 120.2 (C-1′″), 109.2 (C-2′″/6′″), 108.4 (C-2′/6′), 104.5 (C-10), 99.9 (C-1″), 98.2 (C-8), 93.1 (C-6), 76.1 (C-5″), 73.2 (C-2″), 72.1 (C-3″), 69.1 (C-4″), 60.6 (C-6″) [20].
- Myricetin (4): yellow powders, 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 7.34 (s, H-2′/H-6′), 6.38 (d, J = 2.2, H-6), 6.18 (d, J = 2.2, H-8). 13C NMR (150 MHz, δ, ppm): 175.9 (C-4), 164.2 (C-7), 161.1 (C-5), 156.8 (C-9), 146.6 (C-2), 145.3 (C-3′/5′), 136.0 (C-3), 135.5 (C-4′), 121.7 (C-1′), 107.1 (C-2′/6′), 103.1 (C-10), 97.8 (C-8), 93.0 (C-6) [21].
- Desmanthin 1 (5): yellow powders, 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 7.08 (s, H-2″′/6″′), 6.98 (s, H-2′/H-6′), 6.36 (d, J = 2.2, H-6), 6.19 (d, J = 2.2, H-8), 5.63 (dd, J = 3.4, 1.8 H-2”), 5.51 (d, J = 1.8, H-1”), 4.05 (dd, J = 9.1, 3.4, H-3”),3.52 (dq, J = 9.1, 5.8, H-5”), 3.48 (t, J = 9.1, H-4”), 1.04 (d, J = 5.8, H-6”). 13C NMR (150 MHz, δ, ppm): 177.9 (C-4), 166.1 (C-7′″), 164.5 (C-7), 161.8 (C-9), 158.1 (C-2), 157.1 (C-5), 145.5 (C-3′/5′), 145.1 (C-3′″/5′″), 138.5 (C-4′″), 136.6 (C-4′), 134.2 (C-3), 120.4 (C-1′), 119.8 (C-1′″), 108.9 (C-2′″/6′″, 108.2 (C-2′/6′), 104.5 (C-10), 99.1 (C-1″), 98.2 (C-8), 93.3 (C-6), 72.5 (C-4″), 72.1 (C-2″), 70.8 (C-5″), 39.6 (C-3″), 16.4 (C-6″) [22].
- Penta-O-galloyl-β-D-glucose (6): yellow powders, 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 7.02, 6.96, 6.88, 6.86, 6.80 (5×2′/6′), 6.14 (d, J = 8.3 Hz, H-1), 5.81 (t, J = 9.9 Hz, H-3), 5.51 (t, J = 9.9 Hz, H-4), 5.48 (dd, J = 9.9, 8.3 Hz, H-2), 4.41 (dd, J = 12.1, 1.9 Hz, H-6), 4.32 (dd, J = 4.3, 1.9 Hz, H-5), 4.28 (dd, J = 12.1, 4.3 Hz, H-6). 13C NMR (150 MHz, δ, ppm): 166.6, 165.9, 165.6, 165.5, 164.8 (5×C-7′), 145.2, 145.1, 145.1, 145.0, 144.9 (5×C-3′/5′), 139.4, 139.0, 138.9, 138.7, 138.6 (5×C-4′), 119.7, 119.0, 118.9, 118.8, 118.3 (5×C-1′), 109.3, 109.1, 109.1, 109.0, 109.0 (5×C-2′/6′), 92.4 (C-1), 73.0 (C-5), 72.7 (C-3), 70.8 (C-2), 68.4 (C-4), 61.8 (C-6) [23].
- Quercetin 3-O-β-(2”-O-galloylxylopyranoside) (7): yellow powders, 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 7.61 (d, J = 2.3 Hz, H-2′), 7.53 (dd, J = 8.4, 2.3 Hz, H-6′), 7.13 (s, H-2′″, H-6′″), 6.84 (d, J = 8.4 Hz, H-5′), 6.18 (d, J = 2.2 Hz, H-6), 6.36 (d, J = 2.2 Hz, H-8), 5.55 (d, J = 5.9 Hz, H-1″), 5.46 (dd, J = 7.6, 5.9 Hz, H-2″), 3.50 (m, H-4″), 3.89 (m, H-5″). 13C NMR (150 MHz, δ, ppm): 177.8 (C-4), 166.3 (C-7′″), 164.4 (C-7), 161.7 (C-5), 157.1 (C-2), 156.9 (C-9), 148.4 (C-4′, 145.0 (C-3′″/5′″), 144.7 (C-3′), 138.6 (C-4′″), 133.7 (C-3), 121.9 (C-1′), 121.5 (C-6′), 120.0 (C-1′″), 115.7 (C-2′), 114.9 (C-5′), 109.1 (C-2/6′″), 104.4 (C-10), 99.5 (C-6), 98.4 (C-1″), 93.2 (C-8), 72.5 (C-2″), 70.4 (C-3″), 67.5 (C-4″), 64.8 (C-5″) [24].
3.4. In Vitro Colorimetric Assay
3.5. Tyrosinase Inhibitory Assay
3.6. Matrix Metalloproteinase-1 Inhibitory Assay
3.7. Acute Toxicity Test in Mice
3.8. UVB-Induced Skin Pigmentation in Guinea Pigs
3.9. Histopathological Examination
3.10. Fontana–Masson Staining
3.11. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637–1297652. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Halai, P.; Griffiths, C.E.; Sherratt, M.J.; Watson, R.E. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.S.I.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar]
- Wölfle, U.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Ski. Pharmacol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Liu, J.K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in dermatology: An evidence-based review. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef]
- Tawfeek, N.; Fikry, E.; Mahdi, I.; Ochieng, M.A.; Bakrim, W.B.; Taarji, N.; Mahmoud, M.F.; Sobeh, M. Cupressus arizonica Greene: Phytochemical Profile, Cosmeceutical and Dermatological Properties of its Leaf Extracts. Molecules 2023, 28, 1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Hwang, S.-J.; Lee, S.-K.; Choi, Y.; Byun, C.K.; Son, C.-G. Anti-Melanogenic Effects of Fractioned Cynanchum atratum by Regulation of cAMP/MITF Pathway in a UVB-Stimulated Mice Model. Cells 2023, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, D.; Michalska, K.; Wróbel-Biedrawa, D.; Grabowska, K.; Owczarek-Januszkiewicz, A.; Olszewska, M.A.; Podolak, I. The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond—A Review. Int. J. Mol. Sci. 2023, 24, 6614. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.M.; Crane, J.; Caspersen, A.M.; Kovach, D.; Gardner, C.; Walters, C. Hydration of Cuphea seeds containing crystallized triacylglycerols. Funct. Plant Biol. 2007, 34, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.A. Revision of Cuphea Section Diploptychia (Lythraceae). Syst. Bot. Monogr. 1998, 53, 1–96. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Chung, S.K.; Kim, Y.C.; Takaya, Y.; Terashima, K.; Niwa, M. Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J. Agric. Food Chem. 2004, 52, 4664–4668. [Google Scholar] [CrossRef]
- Braca, A.; Politi, M.; Sanogo, R.; Sanou, H.; Morelli, I.; Pizza, C.; De Tommasi, N. Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves. J. Agric. Food Chem. 2003, 51, 6689–6695. [Google Scholar] [CrossRef]
- Korul’kina, L.M.; Shul’ts, E.E.; Zhusupova, G.E.; Abilov, Z.A.; Erzhanov, K.B.; Chaudri, M.I. Biologically active compounds from Limonium Gmeliniii and L-Popoviii. I. Chem. Nat. Compd. 2004, 40, 465–471. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, J.; Zhang, S. Flavonoids from Leaves of Heritiera littoralis D. J. Chin. Phar. Sci. 2004, 133, 214–216. [Google Scholar]
- Furusawa, M.; Ito, T.; Nakaya, K.; Tanaka, T.; Ibrahim, I.; Iinuma, M.; Murata, H.; Inatomi, Y.; Nakanishi, T. Flavonol glycosides in two Diospyros plants and their radical scavenging activity. Heterocycles 2003, 60, 2557–2563. [Google Scholar] [CrossRef]
- Cui, C.B.; Zhao, Q.C.; Cai, B.; Yao, X.S.; Osadsa, H. Two new and four known polyphenolics obtained as new cell-cycle inhibitors from Rubus aleaefolius Poir. J. Asian Nat. Prod. Res. 2002, 4, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.H.; Wang, H.; Zhi, D.; Shen, Y.M. CE analysis of endogenous flavonoid gallate esters from Nepenthes gracilis (Nepenthaceae). Chromatographia 2010, 72, 1013–1016. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, K.; Wang, Y.H.; Ma, R. Effects of myricitrin and relevant molecular mechanisms. Curr. Stem Cell Res. Ther. 2020, 15, 11–17. [Google Scholar]
- Masuda, T.; Iritani, K.; Yonemori, S.; Oyama, Y.; Takeda, Y. Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant, Pemphis acidula. Biosci. Biotechnol. Biochem. 2001, 65, 1302–1309. [Google Scholar] [CrossRef]
- Sohretoglu, D.; Sakar, M.K.; Sterner, O. New galloylated flavonoid glycosides from Geranium stepporum Davis. Helv. Chim. Acta 2009, 92, 520–524. [Google Scholar] [CrossRef]
- Song, X.M.; Tan, L.; Wang, M.; Ren, C.X.; Guo, C.J.; Yang, B.; Ren, Y.L.; Cao, Z.X.; Li, Y.Z.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Guilhon, G.M.S.P.; Andrade, E.H.d.A.; Zoghbi, M.d.G.B.; Santos, L.d.S. Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Himmeldirk, K.B.; Qian, Y.R.; Marki, A.; Chen, X.Z. Biological and biomedical functions of penta-O-galloyl-D-glucose and its derivatives. J. Nat. Med. 2014, 68, 465–472. [Google Scholar] [CrossRef]
- Zhou, Q.; Feng, C.; Ruan, Z. Inhibitory effect of a genistein derivative on pigmentation of guinea pig skin. RSC Adv. 2017, 7, 7914. [Google Scholar] [CrossRef]
- Kandi, S.; Albert, L.C. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar]
- Song, W.; Zhao, Y.Y.; Ren, Y.J.; Liu, L.L.; Wei, S.D.; Yang, H.B. Proanthocyanidins isolated from the leaves of Photinia fraseri block the cell cycle and induce apoptosis by inhibiting tyrosinase activity in melanoma cells. Food Funct. 2021, 12, 3978. [Google Scholar] [CrossRef] [PubMed]
- Amer, R.I.; Ezzat, S.M.; Aborehab, N.M.; Ragab, M.F.; Mohamed, D.; Hashad, A.; Attia, D.; Salama, M.M.; Bishbishy, M.H.E. Downregulation of MMP1 expression mediates the anti-aging activity of Citrus sinensis peel extract nanoformulation in UV induced photoaging in mice. Biomed. Pharmacother. 2021, 138, 111537. [Google Scholar] [CrossRef]
- Lao, Y.; Wang, Y.; Chen, J.; Huang, P.; Su, R.; Shi, J.; Jiang, C.; Zhang, J. Synthesis and biological evaluation of 1,2,4-triazole derivatives as potential Nrf2 activators for the treatment of cerebral ischemic injury. Eur. J. Med. Chem. 2022, 236, 114315. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.H.; Nan, X.J.; Zhang, J.; Dong, L.C.; Ji, W.; Sheng, G.H.; Zhou, Q.C. Inhibition of persimmon tannin extract on guinea pig skin pigmentation. J. Cosmet. Dermatol. 2021, 20, 2648–2656. [Google Scholar] [CrossRef]
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory Effects of Resveratrol on Melanin Synthesis in Ultraviolet B-Induced Pigmentation in Guinea Pig Skin. Biomol. Ther. 2014, 22, 35–40. [Google Scholar] [CrossRef]

| Sample | IC50 | Sample | IC50 |
|---|---|---|---|
| Fine fraction | 1.66 | 4 | 1.15 |
| 1 | 2.52 | 5 | 1.16 |
| 2 | 2.98 | 6 | 1.73 |
| 3 | 4.42 | 7 | 1.68 |
| α-arbutin | 1.52 |
| Samples. | Inhibition Rate | Samples | Inhibition Rate |
|---|---|---|---|
| Fine fraction | 70.5 ± 1.9 | 4 | 66.0 ± 4.9 |
| 1 | 52.5 ± 0.5 | 5 | 49.8 ± 7.6 |
| 2 | 50.9 ± 0.7 | 6 | 63.1 ± 2.1 |
| 3 | 61.4 ± 5.2 | 7 | 47.1 ± 1.4 |
| α-arbutin | 54.6 ± 2. | Kojic acid | 97.9 ± 1.3 |
| Samples | A1 | A2 | B1 | B2 | |
|---|---|---|---|---|---|
| Reagents | |||||
| Phosphate-buffered solution | 20 | 140 | 40 | 160 | |
| Tyrosinase solution | 40 | 40 | 40 | 40 | |
| Sample solution | 20 | 20 | 0 | 0 | |
| L-Dopa | 120 | 0 | 120 | 0 | |
| Enzyme Control | Negative Control | Samples | |
|---|---|---|---|
| MMP-1 stock solution | 65 | 0 | 65 |
| Sample solution | — | — | 10 |
| Test buffer | 10 | 75 | — |
| FRET substrate | 25 | 25 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Wanyan, M.-F.; Shi, B.-B.; Huang, R.; Yang, H.-X.; Wang, X.; Liu, J.-K. Cuphea hookeriana: Phytochemical Profile and the Cosmeceutical and Dermatological Properties of Its Active Fraction from the Whole Plant. Molecules 2025, 30, 311. https://doi.org/10.3390/molecules30020311
Wu X, Wanyan M-F, Shi B-B, Huang R, Yang H-X, Wang X, Liu J-K. Cuphea hookeriana: Phytochemical Profile and the Cosmeceutical and Dermatological Properties of Its Active Fraction from the Whole Plant. Molecules. 2025; 30(2):311. https://doi.org/10.3390/molecules30020311
Chicago/Turabian StyleWu, Xing, Meng-Fei Wanyan, Bao-Bao Shi, Rong Huang, Hui-Xiang Yang, Xian Wang, and Ji-Kai Liu. 2025. "Cuphea hookeriana: Phytochemical Profile and the Cosmeceutical and Dermatological Properties of Its Active Fraction from the Whole Plant" Molecules 30, no. 2: 311. https://doi.org/10.3390/molecules30020311
APA StyleWu, X., Wanyan, M.-F., Shi, B.-B., Huang, R., Yang, H.-X., Wang, X., & Liu, J.-K. (2025). Cuphea hookeriana: Phytochemical Profile and the Cosmeceutical and Dermatological Properties of Its Active Fraction from the Whole Plant. Molecules, 30(2), 311. https://doi.org/10.3390/molecules30020311






