Encapsulation of Vecuronium and Rocuronium by Sugammadex Investigated by Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
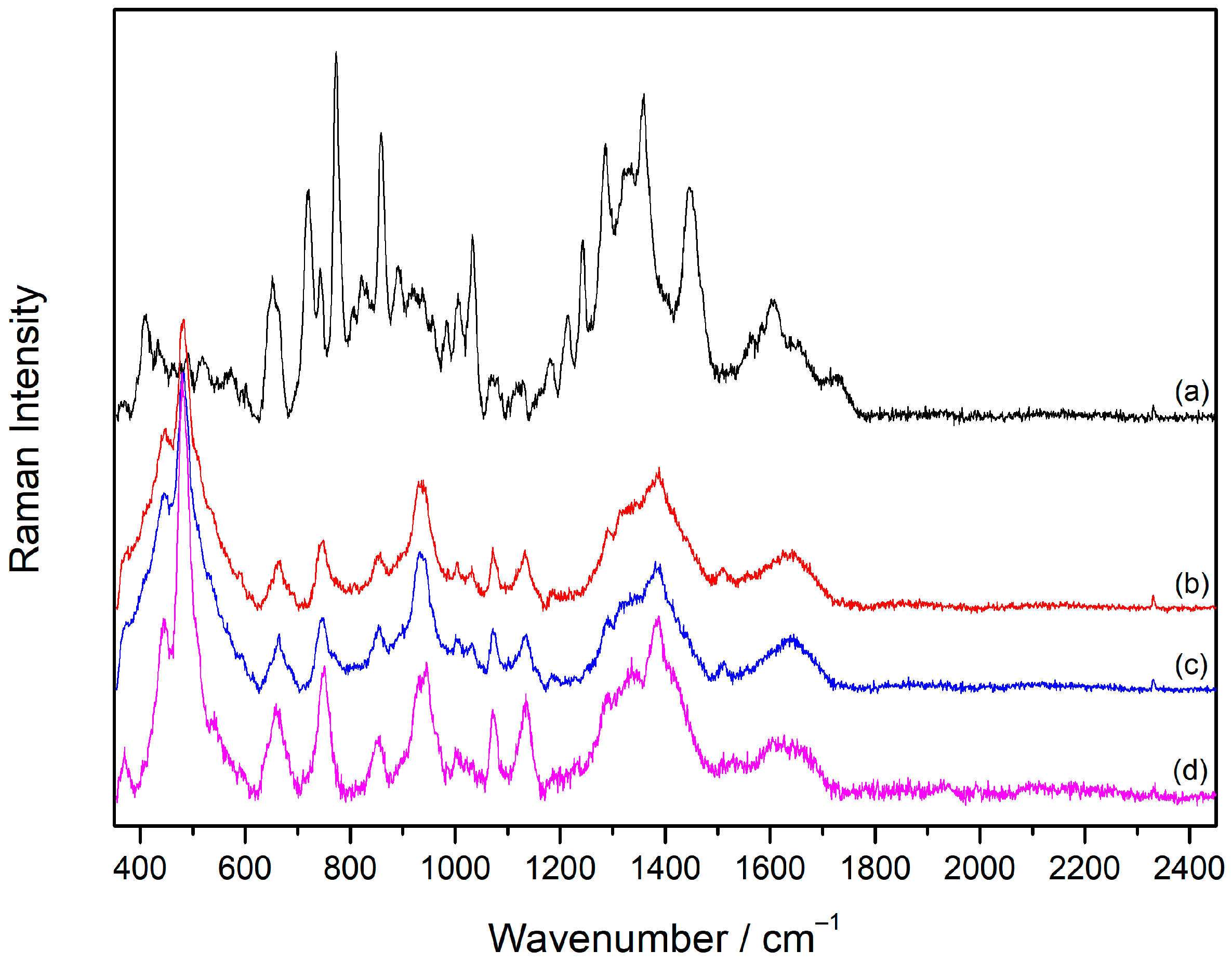
2.1. Raman Spectrum of Sugammadex
2.2. SERS Spectra of Sugammadex
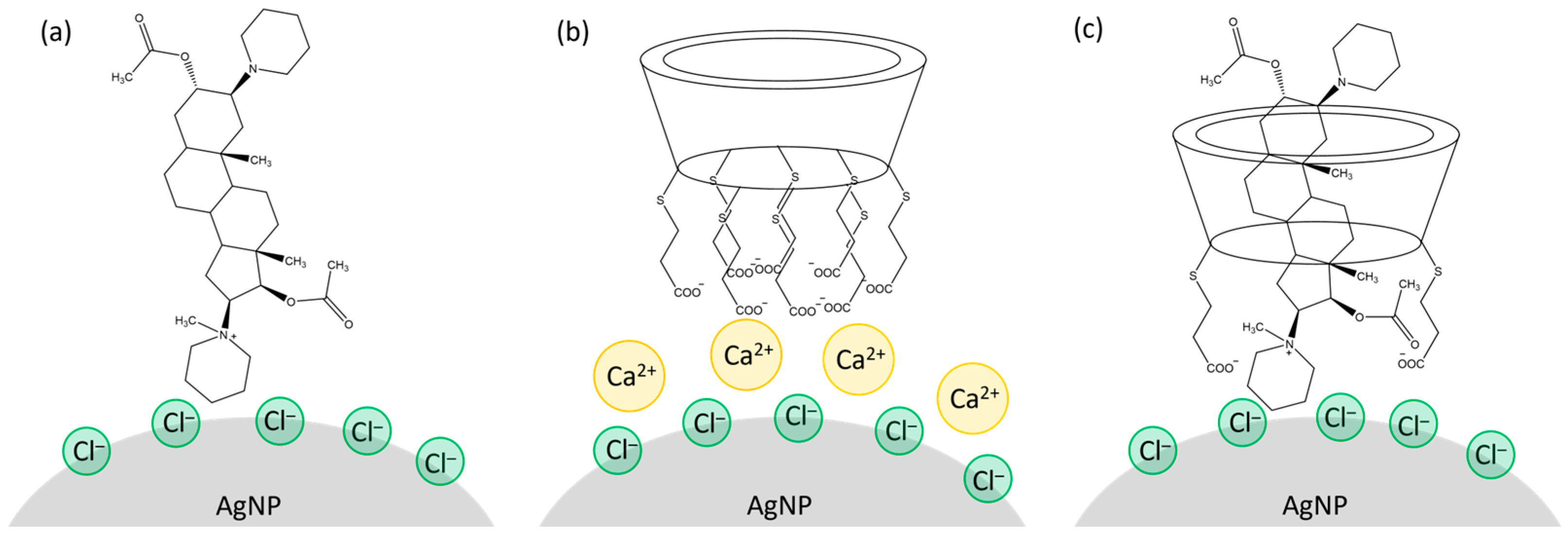
2.2.1. Aggregation of the Silver Colloid with Inorganic Salts
| Wavenumber/cm−1 | Assignment | |
|---|---|---|
| Raman | SERS | |
| 1512 | νasym COO– | |
| 1401 | δ CH | |
| 1383 | 1388 | δ CH |
| 1334 | 1330 | δ CH, δ CH2 |
| 1311 | 1316 | δ CH, δ CH2 |
| 1290 | νsym COO– | |
| 1132 | 1135 | ν C–C |
| 1115 | ν C–C | |
| 1074 | 1073 | ν C–O |
| 1014 | νsym C–O–C | |
| 937 | 940 | νsym C–O–C |
| 854 | δ ring “breathing” glucose | |
| 765 | 749 | δip ring glucose |
| 663 | 660 | ν C–S |
| 481 | 484 | δoop ring glucose |
| 440 | 444 | δoop OH |
2.2.2. Concentration-Dependent SERS Spectra of Sugammadex
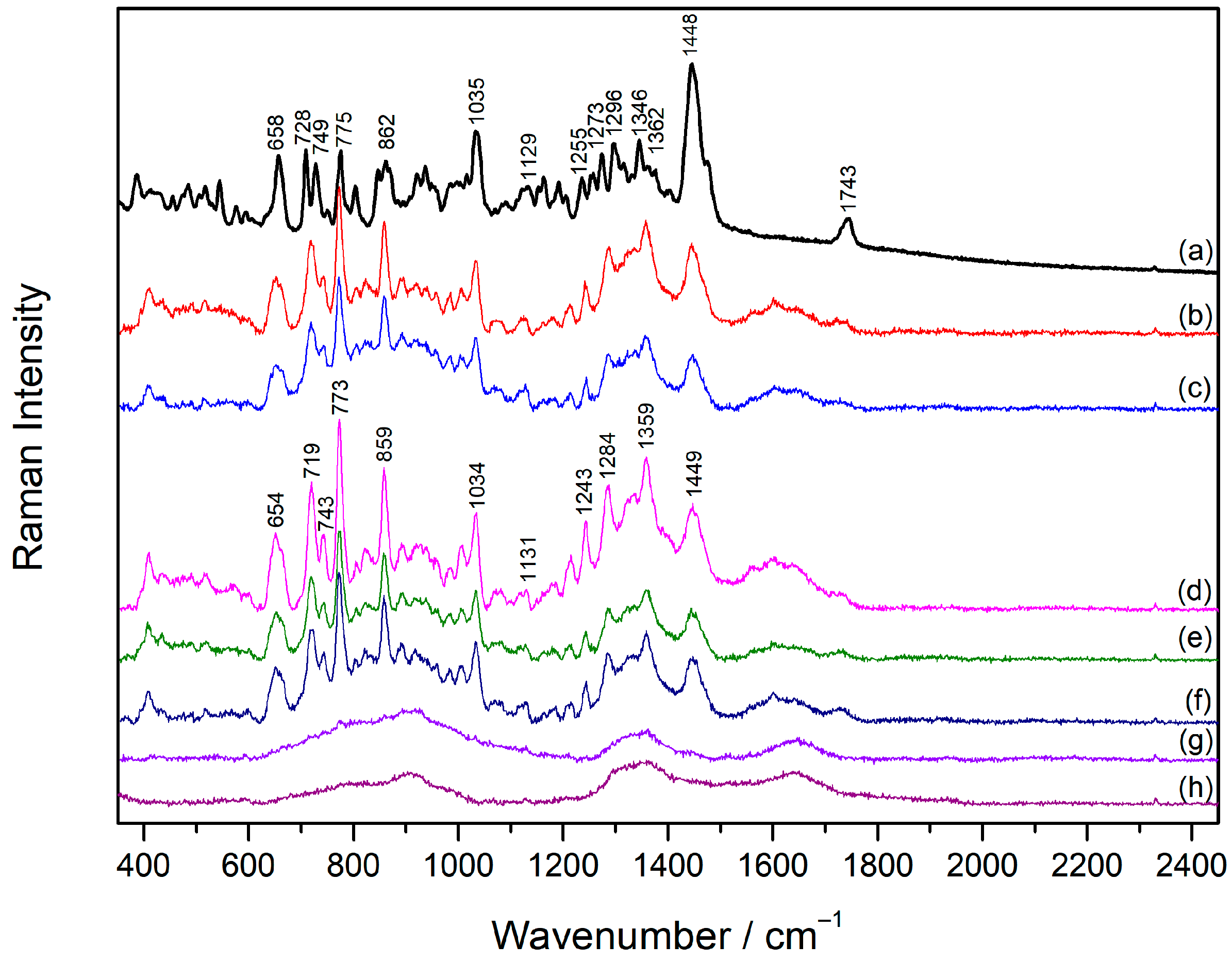
2.3. Raman Spectra of Vecuronium and Rocuronium
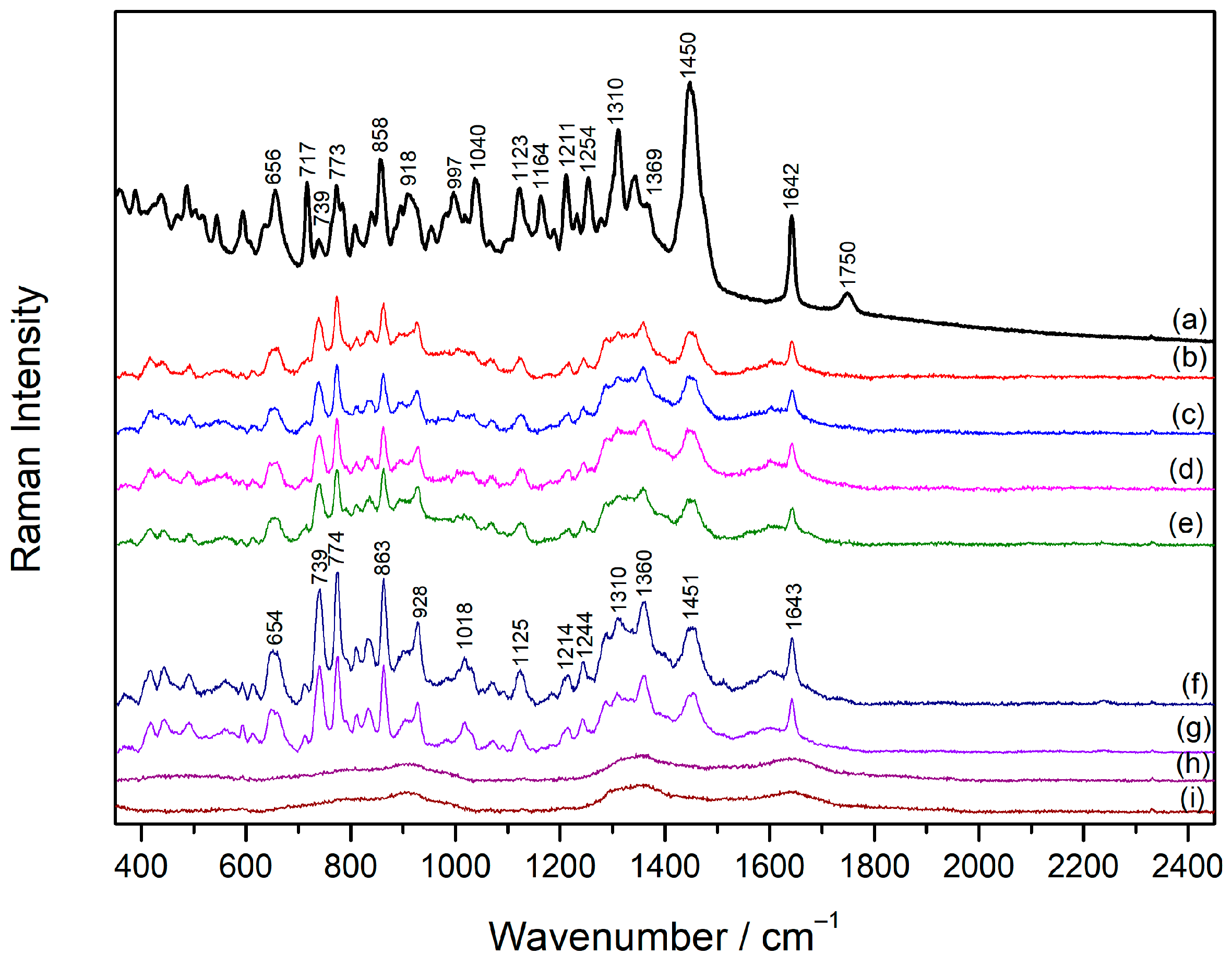
2.4. Concentration-Dependent SERS Spectra of Vecuronium and Rocuronium
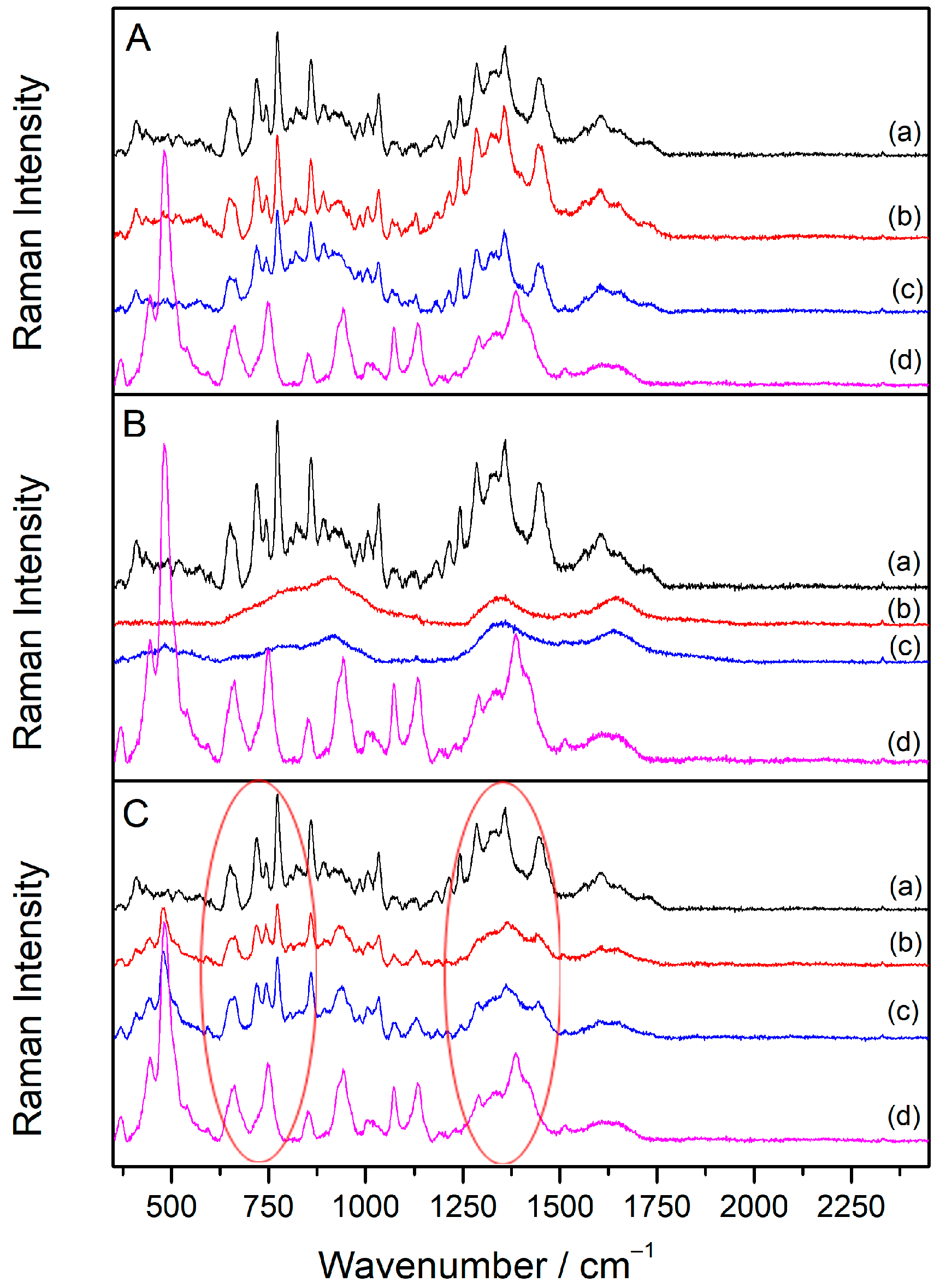
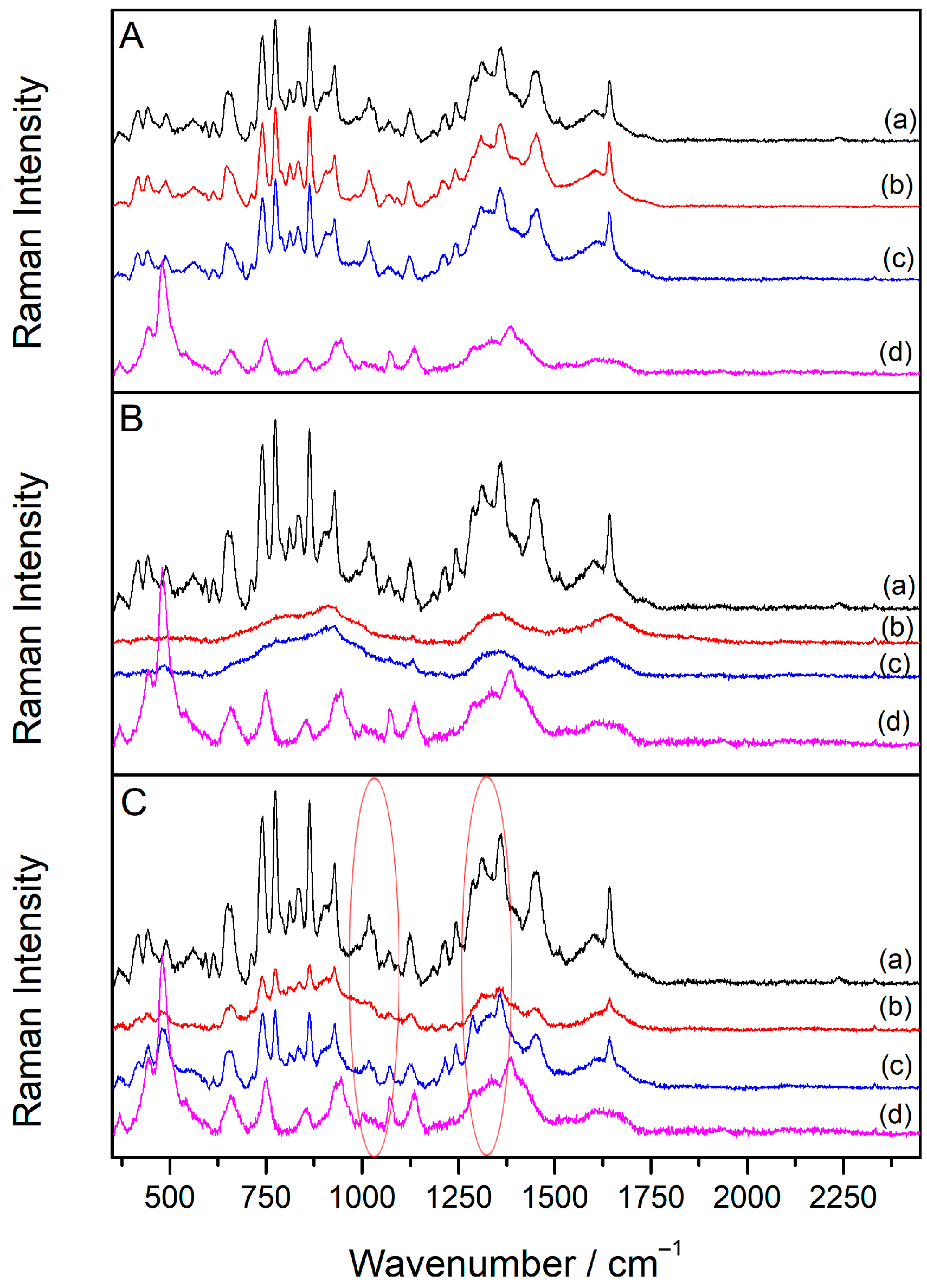
2.5. SERS Spectra of Sugammadex/Drug Complexes
3. Materials and Methods
3.1. Chemicals and Solutions
3.2. Colloids Preparations
3.3. Samples’ Preparation
3.4. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, J.M. New neuromuscular blocking drugs. N. Engl. J. Med. 1995, 332, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Bom, A.; Bradley, M.; Cameron, K.; Clark, J.K.; van Egmond, J.; Feilden, H.; MacLean, E.J.; Muir, A.W.; Palin, R.; Rees, D.C.; et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of Rocuronium Bromide by a cyclodextrin-based synthetic host. Angew. Chem. Int. Ed. 2002, 41, 265–270. [Google Scholar] [CrossRef]
- Hunter, J.M. Neuromuscular blocking agents and reversal agents. Anaesth. Intensiv. Care Med. 2021, 22, 380–384. [Google Scholar] [CrossRef]
- Hunter, J.M. Rocuronium: The newest aminosteroid neuromuscular blocking drug. Br. J. Anaesth. 1996, 76, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Irani, A.H.; Voss, L.; Whittle, N.; Sleigh, J.W. Encapsulation dynamics of neuromuscular blocking drugs by Sugammadex. Anesthesiology 2023, 138, 152–163. [Google Scholar] [CrossRef]
- Stäuble, C.G.; Blobner, M. The future of neuromuscular blocking agents. Curr. Opin. Anaesthesiol. 2020, 33, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Zhao, X.; Su, F.; Lu, H.; Zhang, D.; Sun, L.; Wang, F.; Xu, L. New advances in clinical application of neostigmine: No longer focusing solely on increasing skeletal muscle strength. Front. Pharmacol. 2023, 14, 1227496. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, Y.; Wang, Z.; Wu, C.; Li, Z.; Sun, C.; Sun, T. Theoretical studies on themechanismof sugammadex for the reversal of aminosteroid-induced neuromuscular blockade. J. Mol. Liq. 2018, 265, 450–456. [Google Scholar] [CrossRef]
- Bom, A.; Hope, F.; Rutherford, S.; Thomson, K. Preclinical pharmacology of sugammadex. J. Crit. Care 2009, 24, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zwiers, A.; van den Heuvel, M.; Smeets, J.; Rutherford, S. Assessment of the potential for displacement interactions with Sugammadex. A pharmacokinetic-pharmacodynamic modelling approach. Clin. Drug Investig. 2011, 31, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Herring, W.J.; Woo, T.; Assaid, C.A.; Lupinacci, R.J.; Lemmens, H.J.; Blobner, M.; Khuenl-Brady, K.S. Sugammadex efficacy for reversal of rocuronium- and vecuronium-induced neuromuscular blockade: A pooled analysis of 26 studies. J. Clin. Anesth. 2017, 41, 84–91. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, R.; Wang, Y.; Ma, J.; Li, K.; Li, Z. Biophysical study on the interaction of an anesthetic, vecuronium bromide with human serum albumin using spectroscopic and calorimetric methods. J. Photochem. Photobiol. B 2014, 140, 381–389. [Google Scholar] [CrossRef] [PubMed]
- García, P.L.; Gomes, F.P.; Santoro, M.I.R.M.; Kedor-Hackmann, E.R.M.; Quero, J.L.V.; Montón, A.N.; Montoya, G.C.; Cabrera, M.A. Determination of Vecuronium Bromide in Pharmaceuticals: Development, Validation and Comparative Study of HPLC and CZE Analytical Methods. Chromatographia 2011, 73, 799–805. [Google Scholar] [CrossRef]
- Kooijman, H.; van Geerestein, V.J.; van der Sluis, P.; Kanters, J.A.; Kroon, J.; Funke, C.W.; Kelder, J. Molecular structure of vecuronium bromide, a neuromuscular blocking agent. Crystal structure, molecular mechanics and NMR investigations. J. Chem. Soc. Perkin Trans. 2 1991, 1581–1586. [Google Scholar] [CrossRef]
- Ciceri, S.; Colombo, D.; Ferraboschi, P.; Grisenti, P.; Iannone, M.; Mori, M.; Meneghetti, F. Vecuronium bromide and its ad-vanced intermediates: A crystallographic and spectroscopic study. Steroids 2021, 176, 108928. [Google Scholar] [CrossRef]
- Nakov, N.; Petkovska, R.; Ugrinova, L.; Trajkovic-Jolevska, S.; Dimitrovska, A. Determination of Rocuronium bromide by hydrophilic interaction liquid chromatography (HILIC). Maced. Pharm. Bull. 2011, 57, 17–24. [Google Scholar] [CrossRef]
- Błażewicz, A.; Fijałek, Z.; Warowna-Grześkiewicz, M.; Boruta, M. Simultaneous determination of rocuronium and its eight impurities in pharmaceutical preparation using high-performance liquid chromatography with amperometric detection. J. Chromatogr. A 2007, 1149, 66–72. [Google Scholar] [CrossRef]
- Cho, H.E.; Park, M.J.; Kim, S.C.; Hong, R.S.; Moon, D.C.; Ahn, S.Y. Analysis of rocuronium in human whole blood and tissues using liquid chromatography–tandem mass spectrometry. J. Chromatogr. Sci. 2013, 51, 297–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valadares de Moraes, N.; Lauretti, G.R.; Campos de Oliveira Filgueira, G.; Lopes, B.C.P.; Lanchote, V.L. Analysis of rocuronium in human plasma by liquid chromatography–tandem mass spectrometry with application in clinical pharmacokinetics. J. Pharm. Biomed. Anal. 2014, 90, 180–185. [Google Scholar] [CrossRef]
- Cameron, K.S.; Fletcher, D.; Fielding, L. An NMR study of cyclodextrin complexes of the steroidal neuromuscular blocker drug rocuronium bromide. Magn. Reson. Chem. 2002, 40, 251–260. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, B.; Wen, X.; Su, J.; Jia, B.; Fu, F.; Zhang, Y.; Yu, Q.; Liu, X. One-Pot Synthesis of a Three-Dimensional Au-Decorated Cellulose Nanocomposite as a Surface-Enhanced Raman Scattering Sensor for Selective Detection and in Situ Monitoring. ACS Sustain. Chem. Eng. 2021, 9, 3324–3336. [Google Scholar] [CrossRef]
- Somer, A.; Roik, J.R.; Ribeiro, M.A.; Urban, A.M.; Schoeffel, A.; Urban, V.M.; Farago, P.V.; Vizioli de Castro, L.; Sato, F.; Jacinto, C.; et al. Nystatin complexation with β-cyclodextrin: Spectroscopic evaluation of inclusion by FT-Raman, photoacoustic spectroscopy, and 1H NMR. Mater. Chem. Phys. 2020, 239, 122117. [Google Scholar] [CrossRef]
- Li, W.; Lu, B.; Sheng, A.; Yang, F.; Wang, Z. Spectroscopic and theoretical study on inclusion complexation of beta-cyclodextrin with permethrin. J. Mol. Struct. 2010, 981, 194–203. [Google Scholar] [CrossRef]
- Egyed, O. Spectroscopic studies on β-cyclodextrin. Vib. Spectrosc. 1990, 1, 225–227. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef]
- Han, S.; Hong, S.; Li, X. Effects of cations and anions as aggregating agents on SERS detection of cotinine (COT) and trans-3′-hydroxycotinine (3HC). J. Colloid Interface Sci. 2013, 410, 74–80. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Sirimuthu, N.M.S. Surface-enhanced Raman spectroscopy as a probe of competitive binding by anions to citrate-reduced silver colloids. J. Phys. Chem. A 2005, 109, 7405–7410. [Google Scholar] [CrossRef] [PubMed]
- Stefancu, A.; Iancu, S.D.; Moisoiu, V.; Leopold, N. Specific and selective SERS active sites generation on silver nanoparticles by cationic and anionic adatoms. Rom. Rep. Phys. 2018, 70, 509. [Google Scholar]
- Dollish, F.R.; Fateley, W.G.; Bentley, F.F. Characteristic Raman Frequencies of Organic Compounds, 1st ed.; John Wiley & Sons: New York, NY, USA, 1974; pp. 105–110. [Google Scholar]
- Butler, I.S.; Doyon, M. Raman spectra of steroids. In Analysis of Sterols and Other Biologically Significant Steroids, 1st ed.; Nes, W.D., Parish, E.J., Eds.; Academia Press, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Pouskouleli, G.; Butler, I.S.; Kourounakis, P. Laser Raman spectra of some representative hormonal steroids. J. Mol. Struct. 1983, 102, 93–101. [Google Scholar] [CrossRef]
- Chandra, S.; Saleem, H.; Erdogdu, Y.; Subashchandrabose, S.; Krishnan, A.R.; Gulluoglu, M. FT-IR, FT-Raman spectra and scaled quantum mechanical study of 4-amino-1-benzylpiperidine. J. Mol. Struct. 2011, 998, 69–78. [Google Scholar] [CrossRef]
- Hao, Y.; Fang, Y. Piperidine adsorption on two different silver electrodes: A combined surface enhanced Raman spectroscopy and density functional theory study. J. Nanoparticle Res. 2007, 9, 817–824. [Google Scholar] [CrossRef]
- SenGupta, S.; Maiti, N.; Chadha, R.; Kapoor, S. Conformational analysis of morpholine studied using Raman spectroscopy and density functional theoretical calculations. Chem. Phys. Lett. 2015, 639, 1–6. [Google Scholar] [CrossRef]
- Xie, M.; Zhu, G.; Hu, Y.; Gu, H. Conformations of morpholine in liquid and adsorbed on gold nanoparticles explored by raman spectroscopy and theoretical calculations. J. Phys. Chem. C 2011, 115, 20596–20602. [Google Scholar] [CrossRef]
- Singh, D.K.; Srivastava, S.K.; Ojha, A.K.; Asthana, B.P. pH-dependent Raman study of pyrrole and its vibrational analysis using DFT calculations. Spectrochim. Acta A 2008, 71, 823–829. [Google Scholar] [CrossRef]
- Abdullah, H.S. Electrochemical polymerization and Raman study of polypyrrole and polyaniline thin films. Int. J. Phys. Sci. 2012, 7, 5468–5476. [Google Scholar]
- Raffaini, G.; Elli, S.; Catauro, M.; D’angelo, A. Different drug mobilities in hydrophobic cavities of host–guest complexes between β-Cyclodextrin and 5-fluorouracil at different stoichiometries: A molecular dynamics study in water. Int. J. Mol. Sci. 2024, 25, 5888. [Google Scholar] [CrossRef]
- Del Valle, M.E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]



| Wavenumber/cm−1 | Assignment | |||||
|---|---|---|---|---|---|---|
| Raman | SERS | Raman | SERS | |||
| Vec | Vec 10−5 M | SDX/Vec | Roc | Roc 10−6 M | SDX/Roc | |
| 1743 | 1750 | ν C=O ester | ||||
| 1642 | 1643 | 1643 | ν C=C chain | |||
| 1450 | 1451 | 1455 | δsc CH2, δas CH3 (steroid, morpholine) | |||
| 1448 | 1449 | 1445 | δsc CH2, δas CH3 (steroid, piperidine) | |||
| 1362 | 1359 | 1365 | 1369 | 1360 | 1359 | δwg CH2, δs CH3 |
| 1346 | δwg CH2, δs CH3 | |||||
| 1310 | 1310 | δwg CH2 (steroid, morpholine) | ||||
| 1296 | 1284 | 1285 | δwg CH2 (steroid, piperidine) | |||
| 1273 | 1278 | 1285 | 1285 | δwg CH2 (steroid) | ||
| 1255 | 1243 | 1243 | 1254 | 1244 | 1243 | δtw CH2 |
| 1234 | 1214 | 1212 | 1232 | δtw CH2 | ||
| 1211 | 1214 | 1215 | δ ring pyrrole | |||
| 1206 | δtw CH2 | |||||
| 1192 | 1189 | δrc CH2 | ||||
| 1164 | δrc CH2 (morpholine) | |||||
| 1163 | 1179 | 1185 | δrc CH2 (piperidine) | |||
| 1129 | 1131 | 1131 | ν C–O (Vec), ν C–C (SDX) | |||
| 1123 | 1125 | 1125 | δ CH pyrrole | |||
| 1066 w | 1070 | 1072 | ν C–C, δ CH (pyrrole) | |||
| 1040 | ν C–C (morpholine) | |||||
| 1035 | 1034 | 1034 | ν C–C (piperidine) | |||
| 1018 | δ ring pyrrole | |||||
| 997 | ν C–C (morpholine) | |||||
| 937 | νsym C–O–C (SDX) | |||||
| 918 w | 928 | 928 | δ ring pyrrole | |||
| 862 | 859 | 859 | 858 | 863 | 863 | cyclopenthane ring “breathing” |
| 839 | 835 | 832 | ν C–C, ν C–O (morpholine), δ ring pyrrole | |||
| 775 | 773 | 774 | 773 | 774 | 774 | cyclohexane ring “breathing” |
| 749 | 743 | 743 | 739 | 739 | 740 | cyclohexane ring “breathing” |
| 728 | 719 | 719 | 717 | 713 w | 714 w | cyclohexane ring “breathing” |
| 658 | 654 | 656 | 656 | 654 | 661 | δ skeleton steroid |
| 481 | 484 | δoop ring glucose (SDX) | ||||
| 441 | 444 | δoop OH (SDX) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenđel, A.; Piantanida, I.; Miljanić, S. Encapsulation of Vecuronium and Rocuronium by Sugammadex Investigated by Surface-Enhanced Raman Spectroscopy. Molecules 2025, 30, 231. https://doi.org/10.3390/molecules30020231
Kenđel A, Piantanida I, Miljanić S. Encapsulation of Vecuronium and Rocuronium by Sugammadex Investigated by Surface-Enhanced Raman Spectroscopy. Molecules. 2025; 30(2):231. https://doi.org/10.3390/molecules30020231
Chicago/Turabian StyleKenđel, Adriana, Ivo Piantanida, and Snežana Miljanić. 2025. "Encapsulation of Vecuronium and Rocuronium by Sugammadex Investigated by Surface-Enhanced Raman Spectroscopy" Molecules 30, no. 2: 231. https://doi.org/10.3390/molecules30020231
APA StyleKenđel, A., Piantanida, I., & Miljanić, S. (2025). Encapsulation of Vecuronium and Rocuronium by Sugammadex Investigated by Surface-Enhanced Raman Spectroscopy. Molecules, 30(2), 231. https://doi.org/10.3390/molecules30020231








