Stereoelectronic Effect of Protecting Groups on the Stability of Galactosyl Donor Intermediates
Abstract
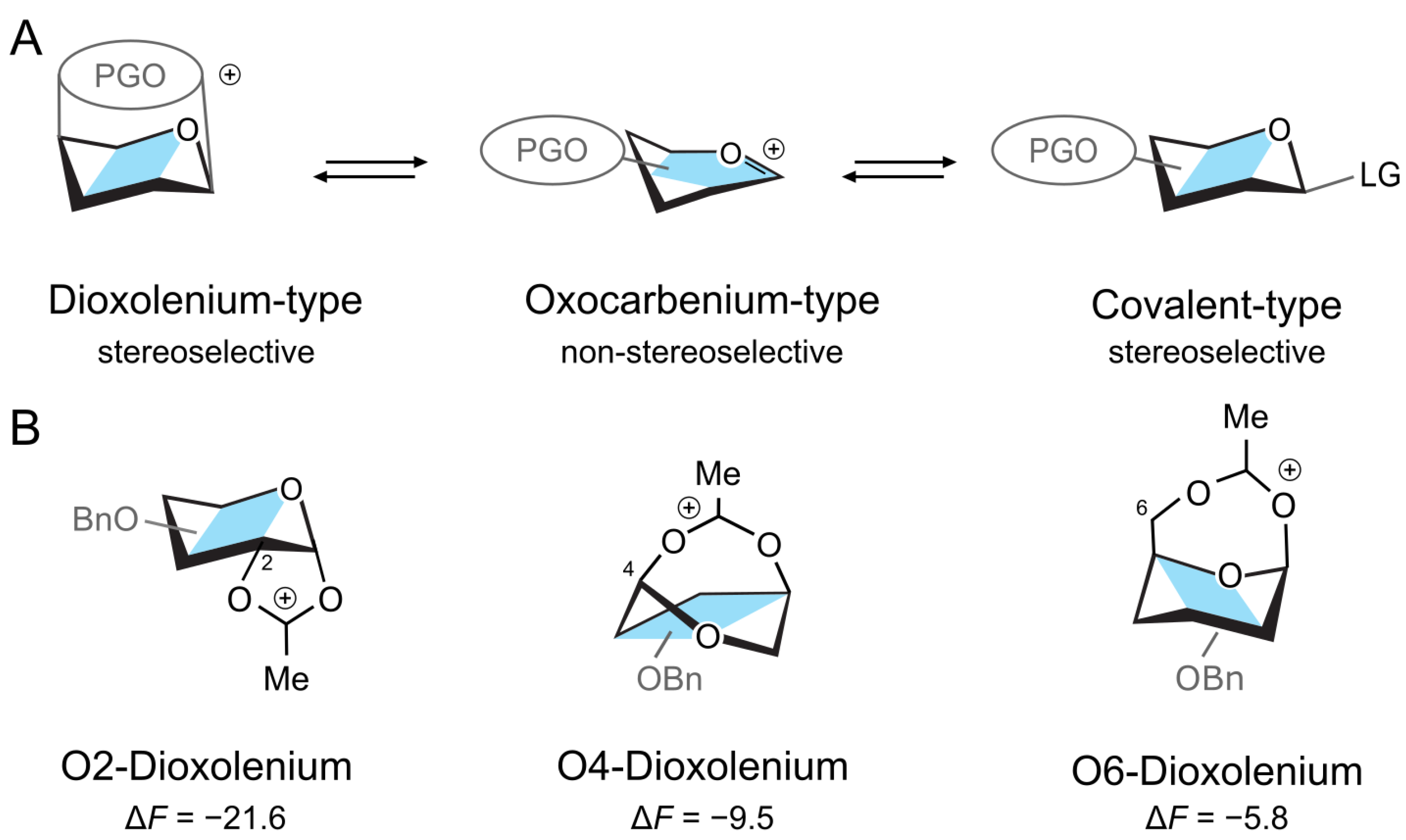
1. Introduction
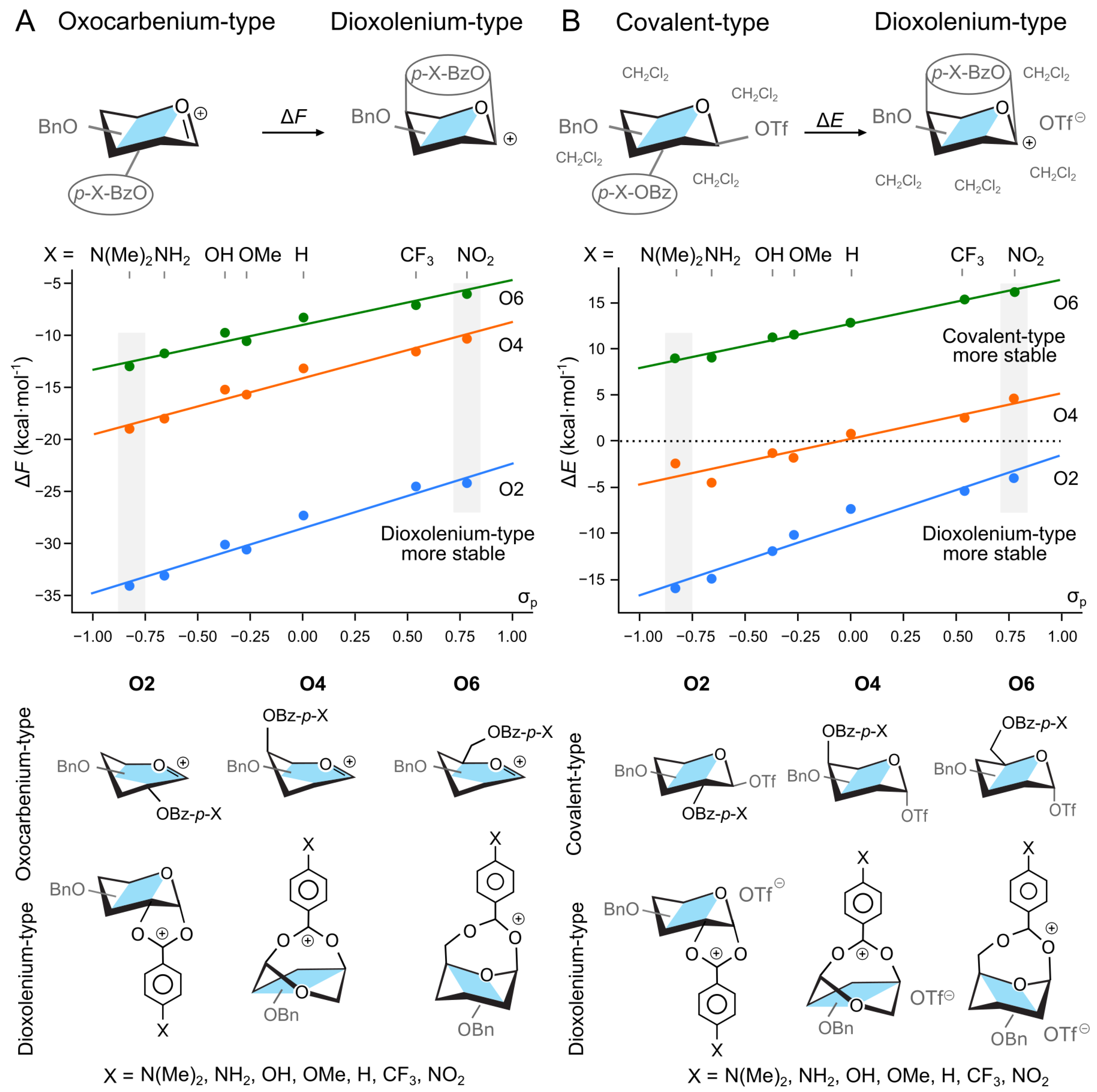
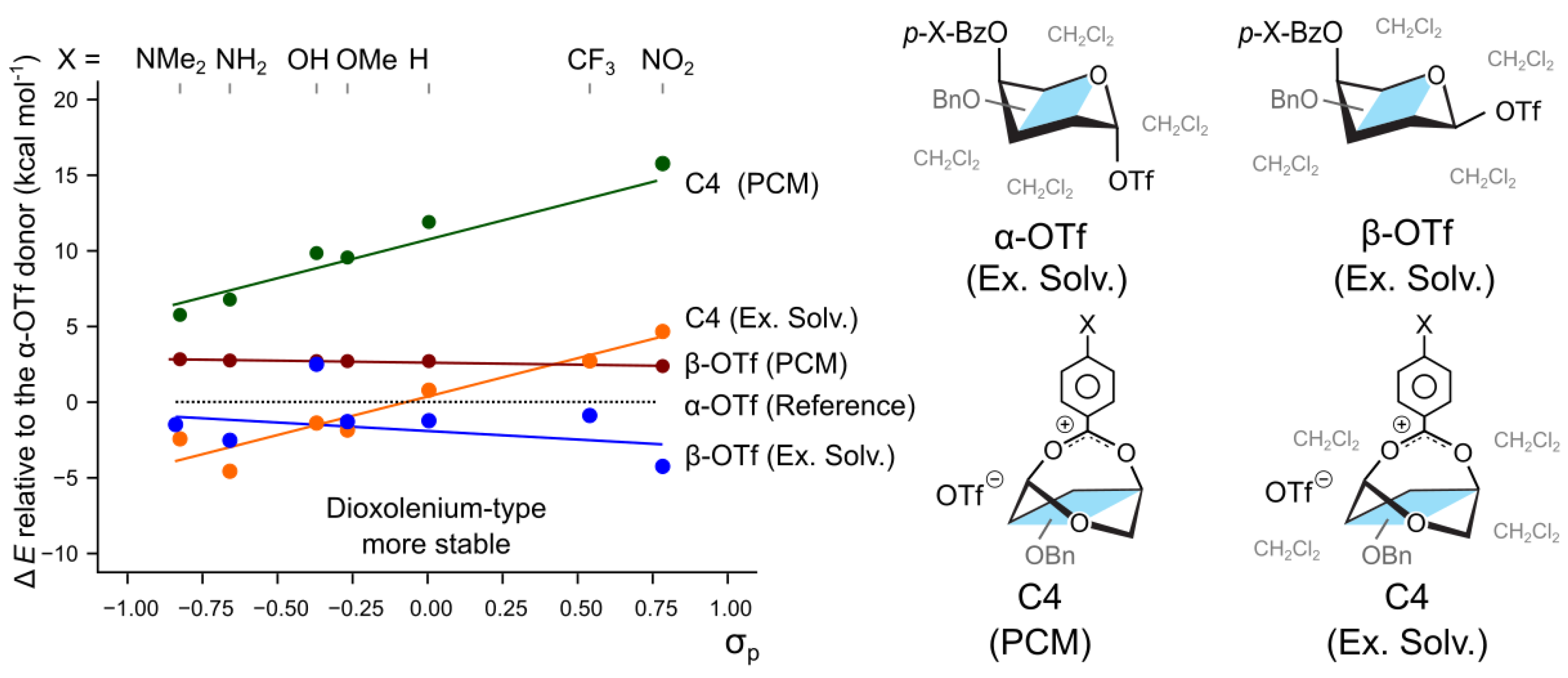
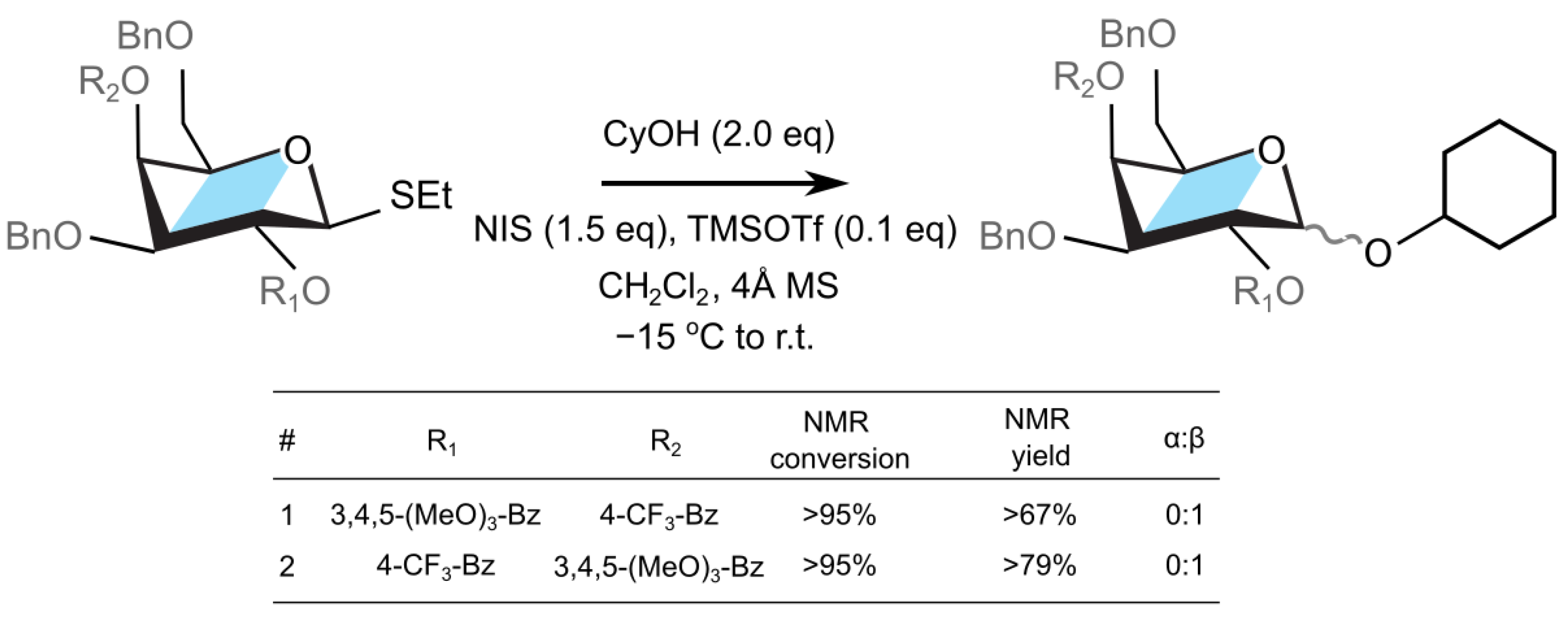
2. Results
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Pan, L.; Cai, C.; Liu, C.; Liu, D.; Li, G.; Linhardt, R.J.; Yu, G. Recent progress and advanced technology in carbohydrate-based drug development. Curr. Opin. Biotechnol. 2021, 69, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Gim, S.; Zhu, Y.; Seeberger, P.H.; Delbianco, M. Carbohydrate-based nanomaterials for biomedical applications. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1558. [Google Scholar] [CrossRef] [PubMed]
- Adero, P.O.; Amarasekara, H.; Wen, P.; Bohé, L.; Crich, D. The Experimental Evidence in Support of Glycosylation Mechanisms at the SN1–SN2 Interface. Chem. Rev. 2018, 118, 8242–8284. [Google Scholar] [CrossRef]
- Hansen, T.; Lebedel, L.; Remmerswaal, W.A.; van der Vorm, S.; Wander, D.P.A.; Somers, M.; Overkleeft, H.S.; Filippov, D.V.; Désiré, J.; Mingot, A.; et al. Defining the SN1 Side of Glycosylation Reactions: Stereoselectivity of Glycopyranosyl Cations. ACS Cent. Sci. 2019, 5, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Zulueta, M.M. Glycochemical Synthesis: Strategies and Applications; Wiley: Hoboken, NJ, USA, 2016; pp. 35–130. [Google Scholar]
- Andreana, P.R.; Crich, D. Guidelines for O-glycoside Formation from First Principles. ACS Cent. Sci. 2021, 7, 1454–1462. [Google Scholar] [CrossRef]
- Martin, A.; Arda, A.; Désiré, J.; Martin-Mingot, A.; Probst, N.; Sinaÿ, P.; Jiménez-Barbero, J.; Thibaudeau, S.; Blériot, Y. Catching elusive glycosyl cations in a condensed phase with HF/SbF5 superacid. Nat. Chem. 2016, 8, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Tanikawa, S.; Saito, R.; Sasaki, K. β-Stereoselective Mannosylation Using 2,6-Lactones. J. Am. Chem. Soc. 2016, 138, 14840–14843. [Google Scholar] [CrossRef]
- Mucha, E.; Marianski, M.; Xu, F.F.; Thomas, D.A.; Meijer, G.; von Helden, G.; Seeberger, P.H.; Pagel, K. Unravelling the structure of glycosyl cations via cold-ion infrared spectroscopy. Nat. Commun. 2018, 9, 4174. [Google Scholar] [CrossRef] [PubMed]
- Elferink, H.; Severijnen, M.E.; Martens, J.; Mensink, R.A.; Berden, G.; Oomens, J.; Rutjes, F.P.J.T.; Rijs, A.M.; Boltje, T.J. Direct Experimental Characterization of Glycosyl Cations by Infrared Ion Spectroscopy. J. Am. Chem. Soc. 2018, 140, 6034–6038. [Google Scholar] [CrossRef]
- ter Braak, F.; Elferink, H.; Houthuijs, K.J.; Oomens, J.; Martens, J.; Boltje, T.J. Characterization of Elusive Reaction Intermediates Using Infrared Ion Spectroscopy: Application to the Experimental Characterization of Glycosyl Cations. Acc. Chem. Res. 2022, 55, 1669–1679. [Google Scholar] [CrossRef]
- Crich, D.; Dai, Z.; Gastaldi, S. On the Role of Neighboring Group Participation and Ortho Esters in β-Xylosylation: 13C NMR Observation of a Bridging 2-Phenyl-1,3-dioxalenium Ion. J. Org. Chem. 1999, 64, 5224–5229. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yang, H.; Boons, G.J. Stereoselective Glycosylation Reactions with Chiral Auxiliaries. Angew. Chem. Int. Ed. 2005, 44, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Müller, M.; Schmidt, R.R. Intramolecular O-Glycoside Bond Formation. Chem. Rev. 2000, 100, 4423–4442. [Google Scholar] [CrossRef] [PubMed]
- Nigudkar, S.S.; Demchenko, A.V. Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem. Sci. 2015, 6, 2687–2704. [Google Scholar] [CrossRef]
- Shadrick, M.; Singh, Y.; Demchenko, A.V. Stereocontrolled α-galactosylation under cooperative catalysis. J. Org. Chem. 2020, 85, 15936–15944. [Google Scholar] [CrossRef]
- Shadrick, M.; Stine, K.J.; Demchenko, A.V. Expanding the scope of stereoselective α-galactosylation using glycosyl chlorides. Bioorg. Med. Chem. 2022, 73, 117031. [Google Scholar] [CrossRef]
- Marianski, M.; Mucha, E.; Greis, K.; Moon, S.; Pardo, A.; Kirschbaum, C.; Thomas, D.A.; Meijer, G.; von Helden, G.; Gilmore, K.; et al. Remote Participation during Glycosylation Reactions of Galactose Building Blocks: Direct Evidence from Cryogenic Vibrational Spectroscopy. Angew. Chem. Int. Ed. 2020, 59, 6166–6171. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Elferink, H.; van Hengst, J.; Houthuijs, K.J.; Remmerswaal, W.A.; Kromm, A.; Berden, G.; van der Vorm, S.; Rijs, A.M.; Overkleeft, H.S.; et al. Characterization of glycosyl dioxolenium ions and their role in glycosylation reactions. Nat. Commun. 2020, 11, 2664. [Google Scholar] [CrossRef] [PubMed]
- Greis, K.; Leichnitz, S.; Kirschbaum, C.; Chang, C.W.; Lin, M.H.; Meijer, G.; von Helden, G.; Seeberger, P.H.; Pagel, K. The Influence of the Electron Density in Acyl Protecting Groups on the Selectivity of Galactose Formation. J. Am. Chem. Soc. 2022, 144, 20258–20266. [Google Scholar] [CrossRef]
- Hettikankanamalage, A.A.; Lassfolk, R.; Ekholm, F.S.; Leino, R.; Crich, D. Mechanisms of Stereodirecting Participation and Ester Migration from Near and Far in Glycosylation and Related Reactions. Chem. Rev. 2020, 120, 7104–7151. [Google Scholar] [CrossRef]
- de Kleijne, F.F.J.; ter Braak, F.; Piperoudis, D.; Moons, P.H.; Moons, S.J.; Elferink, H.; White, P.B.; Boltje, T.J. Detection and Characterization of Rapidly Equilibrating Glycosylation Reaction Intermediates Using Exchange NMR. J. Am. Chem. Soc. 2023, 145, 26190–26201. [Google Scholar] [CrossRef] [PubMed]
- Lettow, M.; Greis, K.; Mucha, E.; Lambeth, T.R.; Yaman, M.; Kontodimas, V.; Manz, C.; Hoffmann, W.; Meijer, G.; Julian, R.R.; et al. Decoding the Fucose Migration Product during Mass-Spectrometric analysis of Blood Group Epitopes. Angew. Chem. Int. Ed. 2023, 62, e202302883. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Remmerswaal, W.A.; Codée, J.D.C.; Woerpel, K.A. Neighboring-Group Participation by C-2 Acyloxy Groups: Influence of the Nucleophile and Acyl Group on the Stereochemical Outcome of Acetal Substitution Reactions. Chem. A Eur. J. 2023, 29. [Google Scholar] [CrossRef]
- Kwan, E.E.; Zeng, Y.; Besser, H.A.; Jacobsen, E.N. Concerted nucleophilic aromatic substitutions. Nat. Chem. 2018, 10, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, S.; Murphy, J.A.; Tuttle, T. Computational Study on the Boundary Between the Concerted and Stepwise Mechanism of Bimolecular SNAr Reactions. J. Am. Chem. Soc. 2020, 142, 14871–14876. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Lee, Y.; Kwak, Y.; Mishra, A.; Yu, E.; Ryou, B.; Park, C.M. Alkyne–Alkene [2 + 2] cycloaddition based on visible light photocatalysis. Nat. Commun. 2020, 11, 2509. [Google Scholar] [CrossRef]
- Loco, D.; Chataigner, I.; Piquemal, J.P.; Spezia, R. Efficient and Accurate Description of Diels-Alder Reactions Using Density Functional Theory**. ChemPhysChem 2022, 23, e202200349. [Google Scholar] [CrossRef] [PubMed]
- Zholdassov, Y.S.; Yuan, L.; Garcia, S.R.; Kwok, R.W.; Boscoboinik, A.; Valles, D.J.; Marianski, M.; Martini, A.; Carpick, R.W.; Braunschweig, A.B. Acceleration of Diels-Alder reactions by mechanical distortion. Science 2023, 380, 1053–1058. [Google Scholar] [CrossRef]
- Marianski, M.; Supady, A.; Ingram, T.; Schneider, M.; Baldauf, C. Assessing the Accuracy of Across-the-Scale Methods for Predicting Carbohydrate Conformational Energies for the Examples of Glucose and α-Maltose. J. Chem. Theory Comput. 2016, 12, 6157–6168. [Google Scholar] [CrossRef]
- Kontodimas, V.; Yaman, M.; Greis, K.; Lettow, M.; Pagel, K.; Marianski, M. Reinvestigation of the internal glycan rearrangement of Lewis a and blood group type H1 epitopes. Phys. Chem. Chem. Phys. 2024, 26, 14160–14170. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Bernasconi, L.; Liu, P. Ab Initio Molecular Dynamics Simulations of the SN1/SN2 Mechanistic Continuum in Glycosylation Reactions. J. Am. Chem. Soc. 2021, 143, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Remmerswaal, W.A.; Elferink, H.; Houthuijs, K.J.; Hansen, T.; ter Braak, F.; Berden, G.; van der Vorm, S.; Martens, J.; Oomens, J.; van der Marel, G.A.; et al. Anomeric Triflates versus Dioxanium Ions: Different Product-Forming Intermediates from 3-Acyl Benzylidene Mannosyl and Glucosyl Donors. J. Org. Chem. 2024, 89, 1618–1625. [Google Scholar] [CrossRef]
- Ardévol, A.; Rovira, C. Reaction Mechanisms in Carbohydrate-Active Enzymes: Glycoside Hydrolases and Glycosyltransferases. Insights from ab Initio Quantum Mechanics/Molecular Mechanics Dynamic Simulations. J. Am. Chem. Soc. 2015, 137, 7528–7547. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vorm, S.; Hansen, T.; Van Hengst, J.M.; Overkleeft, H.S.; Van Der Marel, G.A.; Codée, J.D. Acceptor reactivity in glycosylation reactions. Chem. Soc. Rev. 2019, 48, 4688–4706. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Luo, Y.; Mo, J.; Noguchi, M.; Jing, J.; Luo, Z.; Shoda, S.I.; Ye, X.S. Hydrogen bond-assisted 1,2-cis O-glycosylation under mild hydrogenolytic conditions. Chin. Chem. Lett. 2023, 34, 107754. [Google Scholar] [CrossRef]
- Gou, X.Y.; Li, Y.; Shi, W.Y.; Luan, Y.Y.; Ding, Y.N.; An, Y.; Huang, Y.C.; Zhang, B.S.; Liu, X.Y.; Liang, Y.M. Ruthenium-Catalyzed Stereo-and Site-Selective ortho-and meta-C- H Glycosylation and Mechanistic Studies. Angew. Chem. Int. Ed. 2022, 134, e202205656. [Google Scholar] [CrossRef]
- Pal, K.B.; Guo, A.; Das, M.; Báti, G.; Liu, X.W. Superbase-Catalyzed Stereo- and Regioselective Glycosylation with 2-Nitroglycals: Facile Access to 2-Amino-2-deoxy-O-glycosides. ACS Catal. 2020, 10, 6707–6715. [Google Scholar] [CrossRef]
- Colombo, M.I.; Rúveda, E.A.; Stortz, C.A. Regioselectivity of the glycosylation of N-dimethylmaleoyl-protected hexosamine acceptors. An experimental and DFT approach. Org. Biomol. Chem. 2011, 9, 3020–3025. [Google Scholar] [CrossRef]
- Elferink, H.; Mensink, R.A.; Castelijns, W.W.A.; Jansen, O.; Bruekers, J.P.J.; Martens, J.; Oomens, J.; Rijs, A.M.; Boltje, T.J. The Glycosylation Mechanisms of 6,3-Uronic Acid Lactones. Angew. Chem. Int. Ed. 2019, 58, 8746–8751. [Google Scholar] [CrossRef]
- Huang, M.; Garrett, G.E.; Birlirakis, N.; Bohé, L.; Pratt, D.A.; Crich, D. Dissecting the mechanisms of a class of chemical glycosylation using primary 13C kinetic isotope effects. Nat. Chem. 2012, 4, 663–667. [Google Scholar] [CrossRef]
- Van der Vorm, S.; Hansen, T.; Overkleeft, H.; Van der Marel, G.; Codée, J. The influence of acceptor nucleophilicity on the glycosylation reaction mechanism. Chem. Sci. 2017, 8, 1867–1875. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakagawa, A.; Nishi, N.; Iijima, K.; Sawa, R.; Takahashi, D.; Toshima, K. Boronic-Acid-Catalyzed Regioselective and 1,2-cis-Stereoselective Glycosylation of Unprotected Sugar Acceptors via SNi-Type Mechanism. J. Am. Chem. Soc. 2018, 140, 3644–3651. [Google Scholar] [CrossRef]
- Whitfield, D.M.; Nukada, T. DFT studies of the role of C-2–O-2 bond rotation in neighboring-group glycosylation reactions. Carbohydr. Res. 2007, 342, 1291–1304. [Google Scholar] [CrossRef]
- Alibay, I.; Bryce, R.A. Ring Puckering Landscapes of Glycosaminoglycan-Related Monosaccharides from Molecular Dynamics Simulations. J. Chem. Inf. Model. 2019, 59, 4729–4741. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Chatterjee, S.; Seeberger, P.H.; Gilmore, K. Predicting glycosylation stereoselectivity using machine learning. Chem. Sci. 2021, 12, 2931–2939. [Google Scholar] [CrossRef]
- Pardo-Vargas, A.; Delbianco, M.; Seeberger, P.H. Automated glycan assembly as an enabling technology. Curr. Opin. Chem. Biol. 2018, 46, 48–55. [Google Scholar] [CrossRef]
- Panza, M.; Pistorio, S.G.; Stine, K.J.; Demchenko, A.V. Automated Chemical Oligosaccharide Synthesis: Novel Approach to Traditional Challenges. Chem. Rev. 2018, 118, 8105–8150. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Bennett, C.S. New chemical processes to streamline carbohydrate synthesis. Curr. Opin. Chem. Biol. 2022, 70, 102184. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Grimme, S. Density functional theory with London dispersion corrections. WIREs Comp. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, V.S.; Diallo, M.S.; Goddard III, W.A. Calculation of Solvation Free Energies of Charged Solutes Using Mixed Cluster/Continuum Models. J. Phys. Chem. B 2008, 112, 9709–9719. [Google Scholar] [CrossRef] [PubMed]
- Garreffi, B.P.; Kwok, R.W.; Marianski, M.; Bennett, C.S. Origins of Selectivity in Glycosylation Reactions with Saccharosamine Donors. Org. Lett. 2023, 25, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, Z.; Sathe, D.; Zhou, J.; Dym, S.; Zhao, Z.; Wang, J.; Niu, J. Precision native polysaccharides from living polymerization of anhydrosugars. Nat. Chem. 2023, 15, 1276–1284. [Google Scholar] [CrossRef]
- Komarova, B.S.; Tsvetkov, Y.E.; Nifantiev, N.E. Design of α-Selective Glycopyranosyl Donors Relying on Remote Anchimeric Assistance. Chem. Rec. 2016, 16, 488–506. [Google Scholar] [CrossRef] [PubMed]
- Greis, K.; Kirschbaum, C.; Fittolani, G.; Mucha, E.; Chang, R.; von Helden, G.; Meijer, G.; Delbianco, M.; Seeberger, P.H.; Pagel, K. Neighboring Group Participation of Benzoyl Protecting Groups in C3- and C6-Fluorinated Glucose. Eur. J. Org. Chem. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Lee, B.Y.; Jo, M.G.; Kim, K.S. β-Directing Effect of Electron-Withdrawing Groups at O-3, O-4, and O-6 Positions and α-Directing Effect by Remote Participation of 3-O-Acyl and 6-O-Acetyl Groups of Donors in Mannopyranosylations. J. Am. Chem. Soc. 2010, 132, 7229. [Google Scholar] [CrossRef]
- Chen, J.; Hansen, T.; Zhang, Q.J.; Liu, D.Y.; Sun, Y.; Yan, H.; Codée, J.D.; Schmidt, R.R.; Sun, J.S. 1-Picolinyl-5-azido Thiosialosides: Versatile Donors for the Stereoselective Construction of Sialyl Linkages. Angew. Chem. Int. Ed. 2019, 131, 17156–17164. [Google Scholar] [CrossRef]
- Upadhyaya, K.; Subedi, Y.P.; Crich, D. Direct Experimental Characterization of a Bridged Bicyclic Glycosyl Dioxacarbenium Ion by 1 H and 13 C·NMR Spectroscopy: Importance of Conformation on Participation by Distal Esters. Angew. Chem. Int. Ed. 2021, 60, 25397–25403. [Google Scholar] [CrossRef]
- Ande, C.; Crich, D. Stereodirecting Effect of Esters at the 4-Position of Galacto- and Glucopyranosyl Donors: Effect of 4- C -Methylation on Side-Chain Conformation and Donor Reactivity, and Influence of Concentration and Stoichiometry on Distal Group Participation. J. Org. Chem. 2023, 88, 13883–13893. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.S.; Liao, G.; Guo, Z. Chemical synthesis of the tumor-associated globo H antigen. RSC Adv. 2015, 5, 23311–23319. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwok, R.W.; Rutkoski, R.; Nagorny, P.; Marianski, M. Stereoelectronic Effect of Protecting Groups on the Stability of Galactosyl Donor Intermediates. Molecules 2025, 30, 218. https://doi.org/10.3390/molecules30020218
Kwok RW, Rutkoski R, Nagorny P, Marianski M. Stereoelectronic Effect of Protecting Groups on the Stability of Galactosyl Donor Intermediates. Molecules. 2025; 30(2):218. https://doi.org/10.3390/molecules30020218
Chicago/Turabian StyleKwok, Ryan W., Ryan Rutkoski, Pavel Nagorny, and Mateusz Marianski. 2025. "Stereoelectronic Effect of Protecting Groups on the Stability of Galactosyl Donor Intermediates" Molecules 30, no. 2: 218. https://doi.org/10.3390/molecules30020218
APA StyleKwok, R. W., Rutkoski, R., Nagorny, P., & Marianski, M. (2025). Stereoelectronic Effect of Protecting Groups on the Stability of Galactosyl Donor Intermediates. Molecules, 30(2), 218. https://doi.org/10.3390/molecules30020218






