LC-HRMS/MS-Guided Profiling and Biological Evaluation of Stachys duriaei Extracts: Anticancer and Vasorelaxant Mechanisms via Apoptosis and Endothelium-Dependent Pathways
Abstract
1. Introduction
2. Results
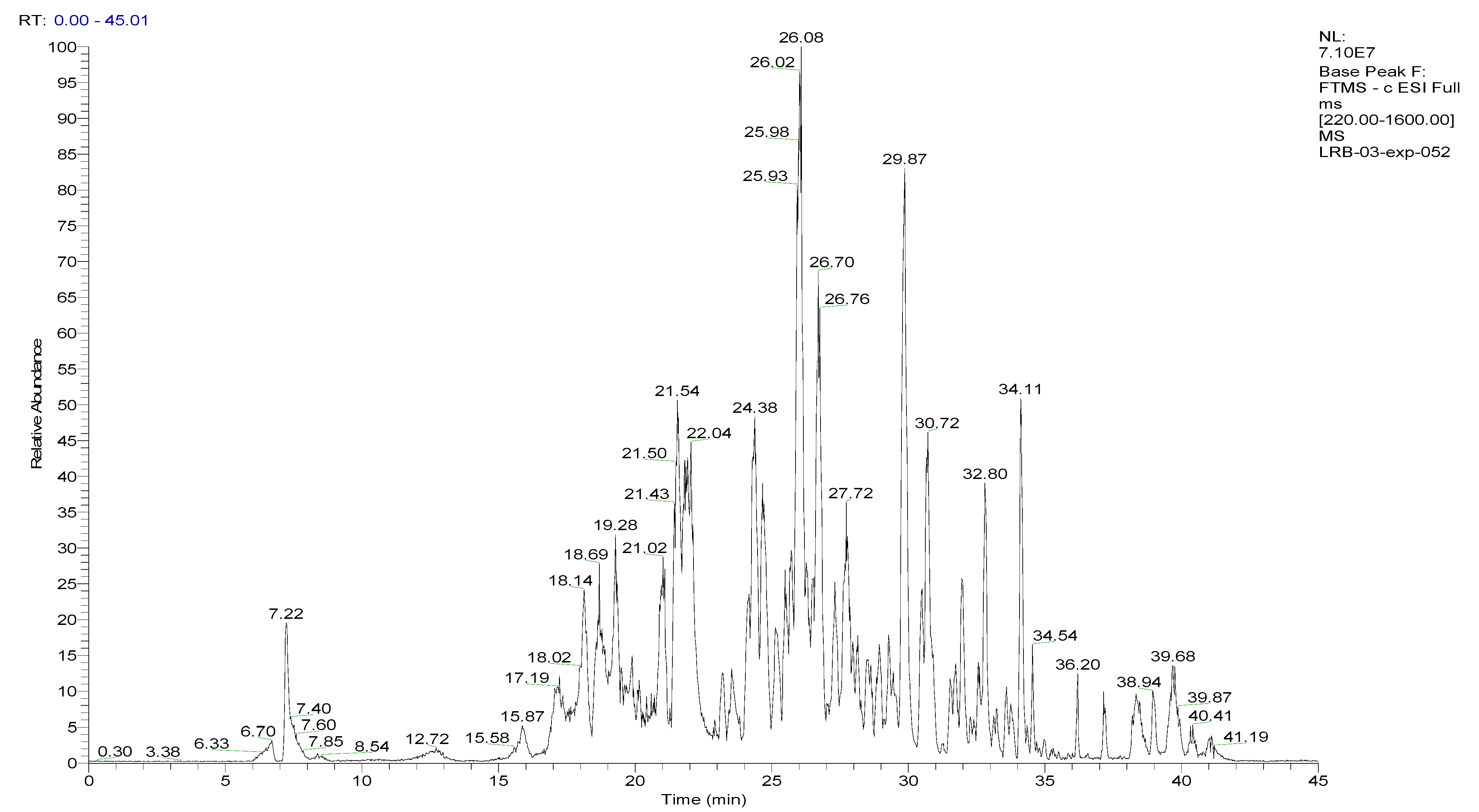
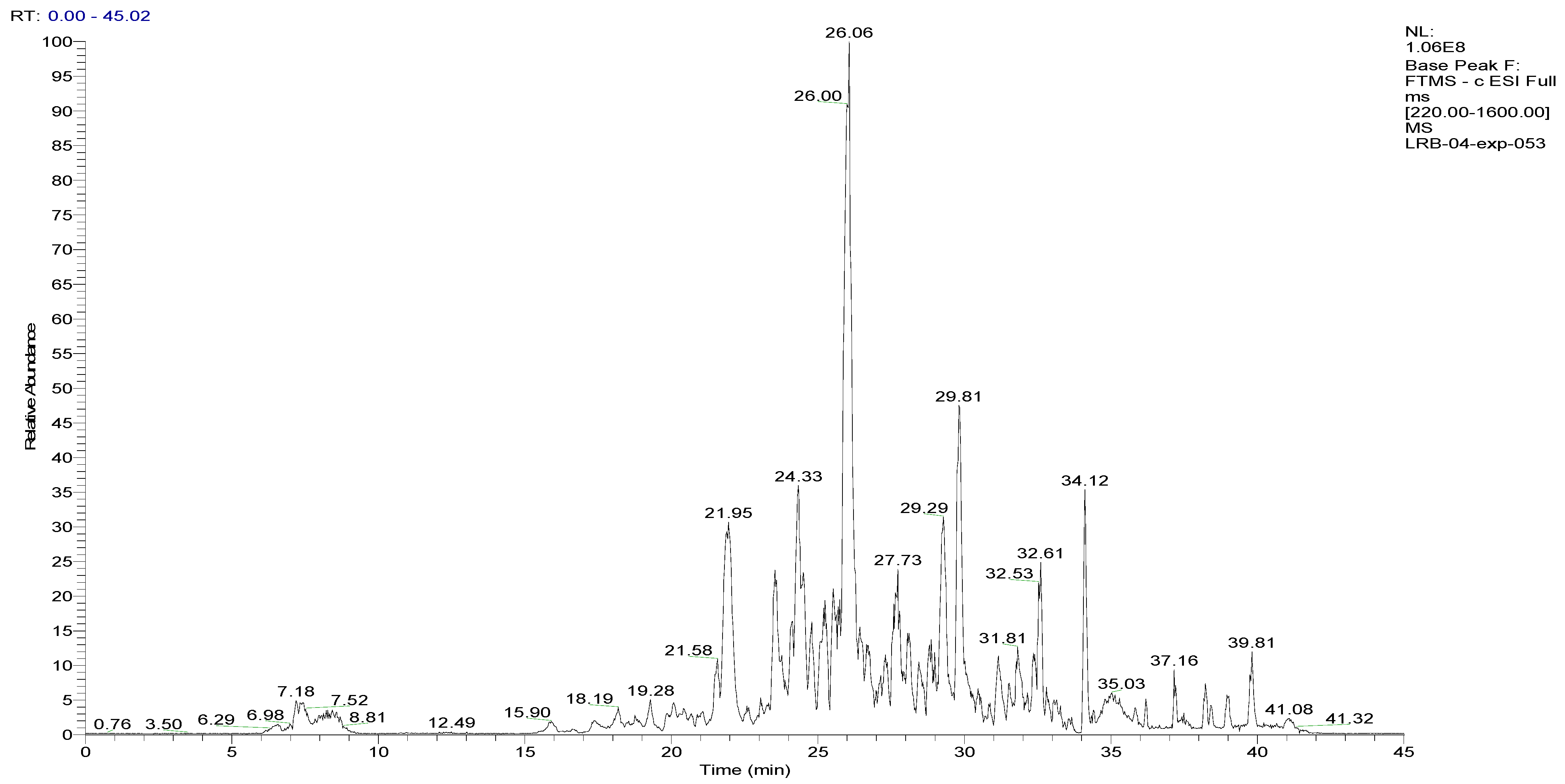
2.1. LC-HRMS/MS Analyses of the BESD and BF1SD
2.2. Compounds Detected in the BESD
2.3. Compounds Detected in the BF1SD
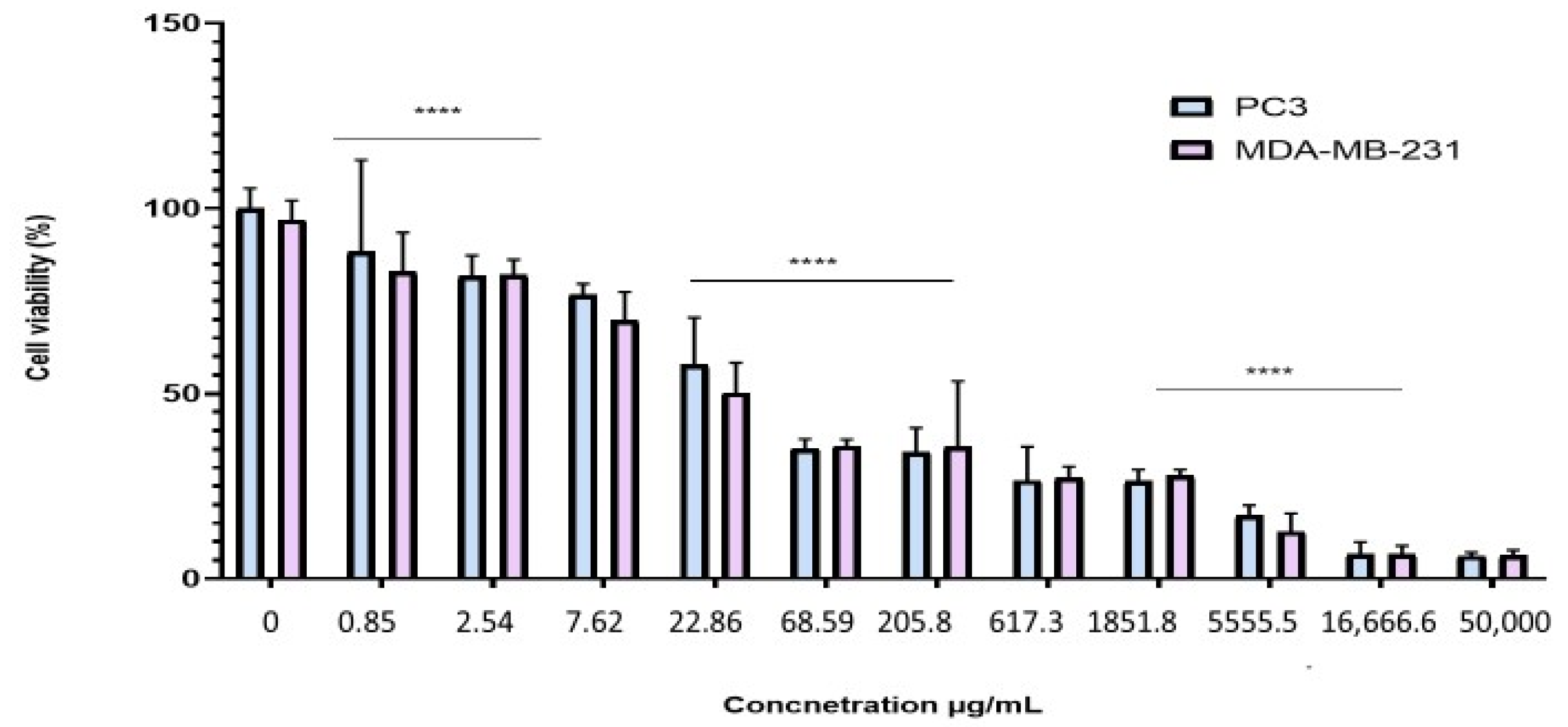
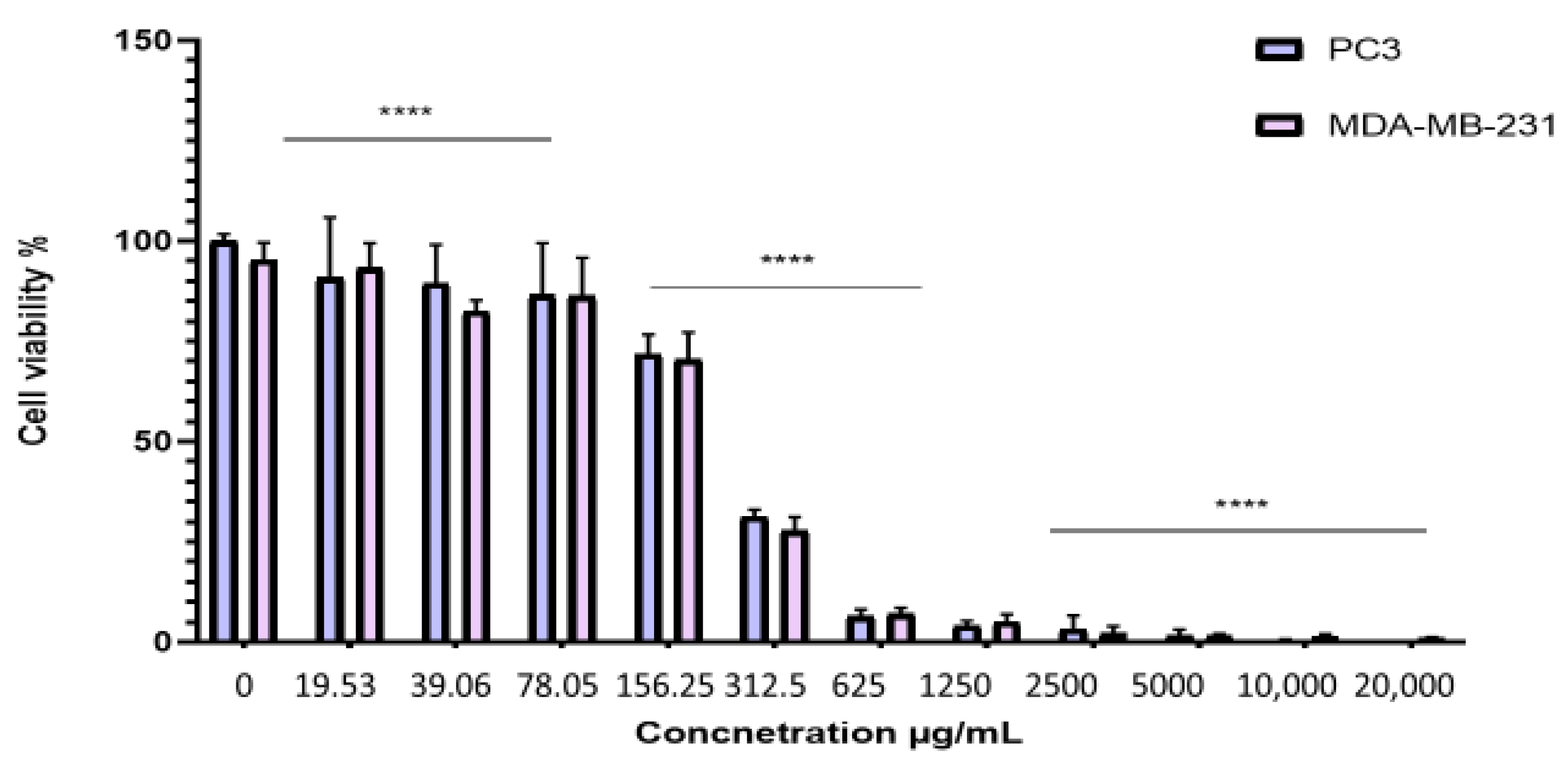
2.4. Antiproliferative Effect of the BESD and BF1SD on PC3 and MDA-MB-231 Cell Lines
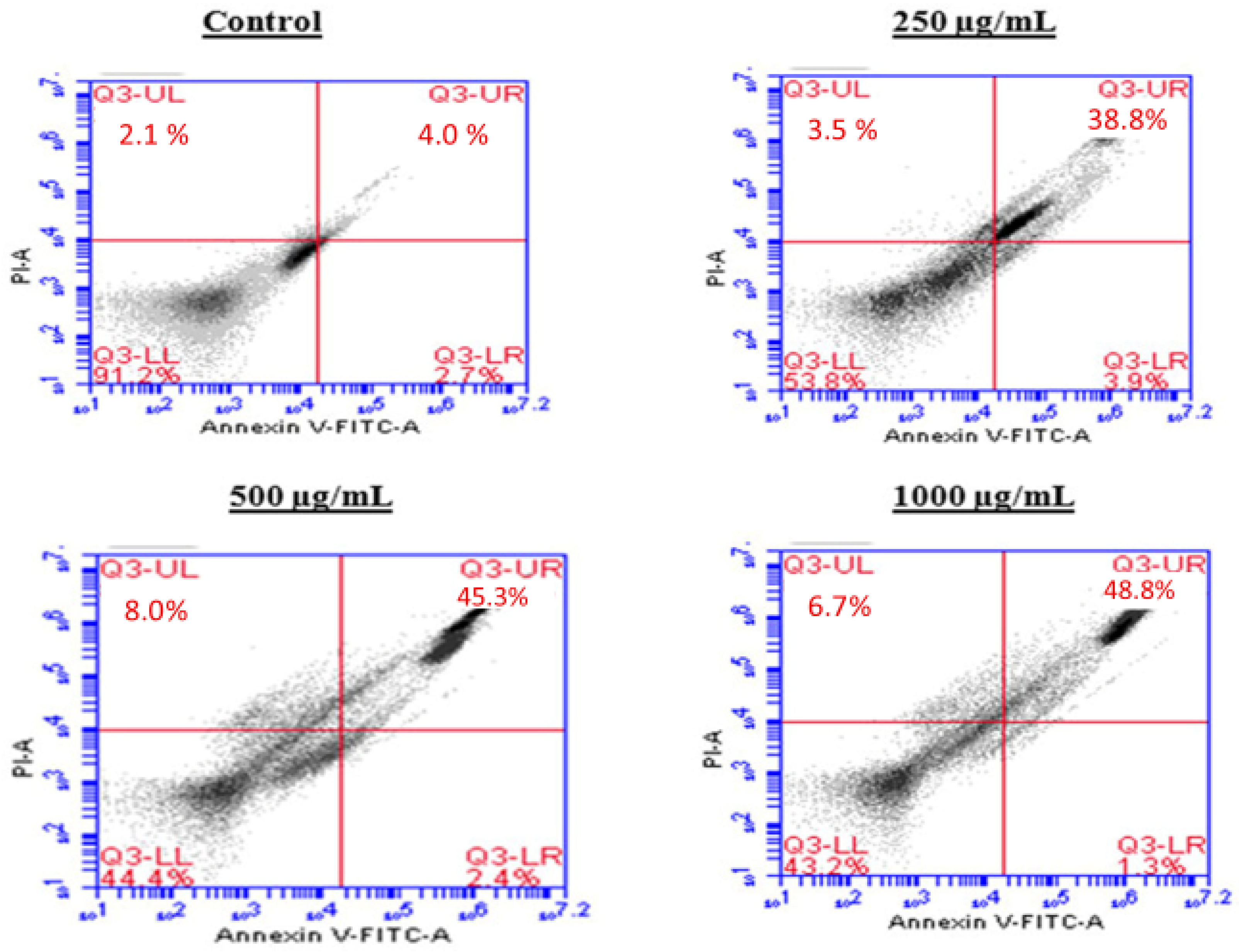
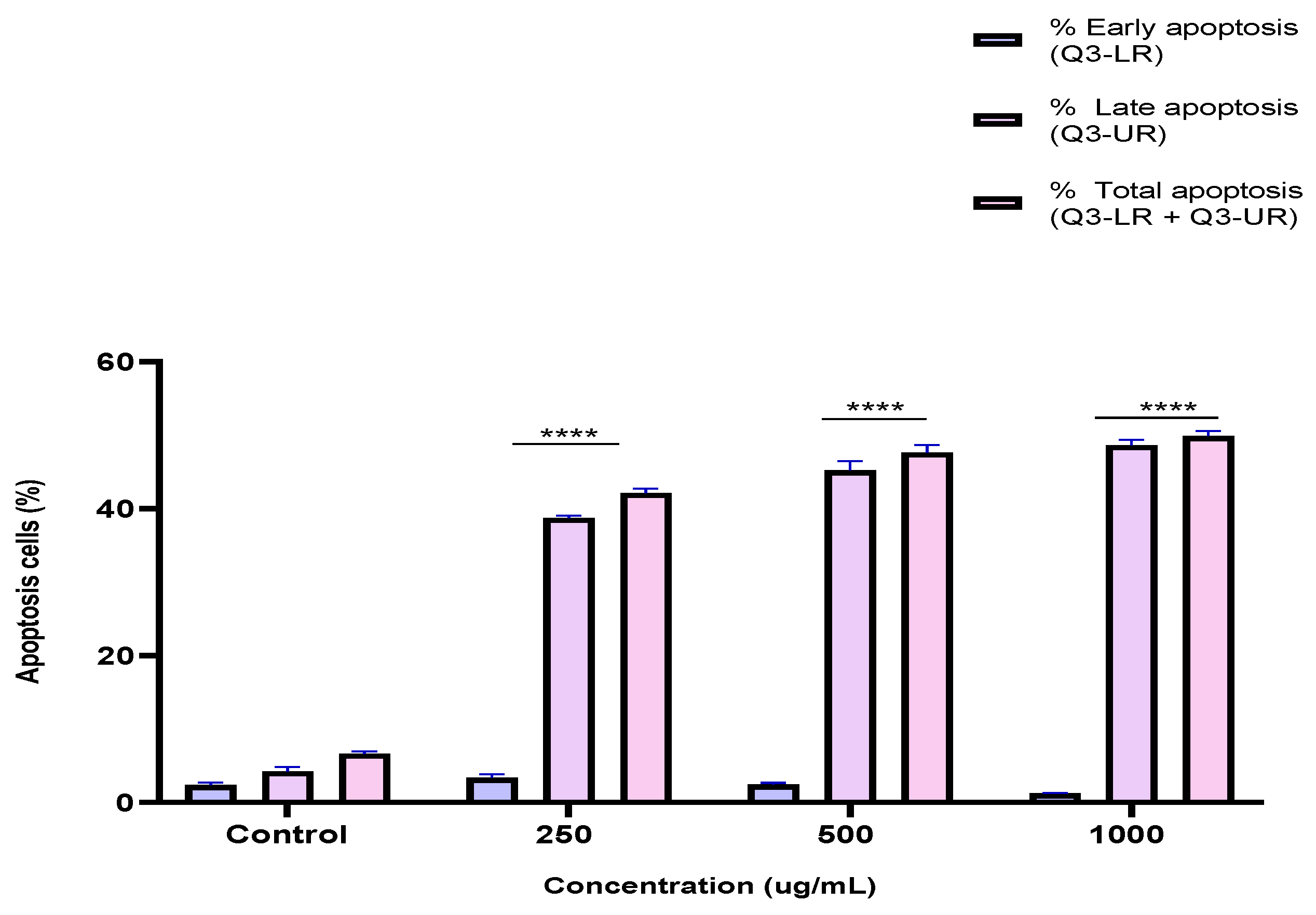
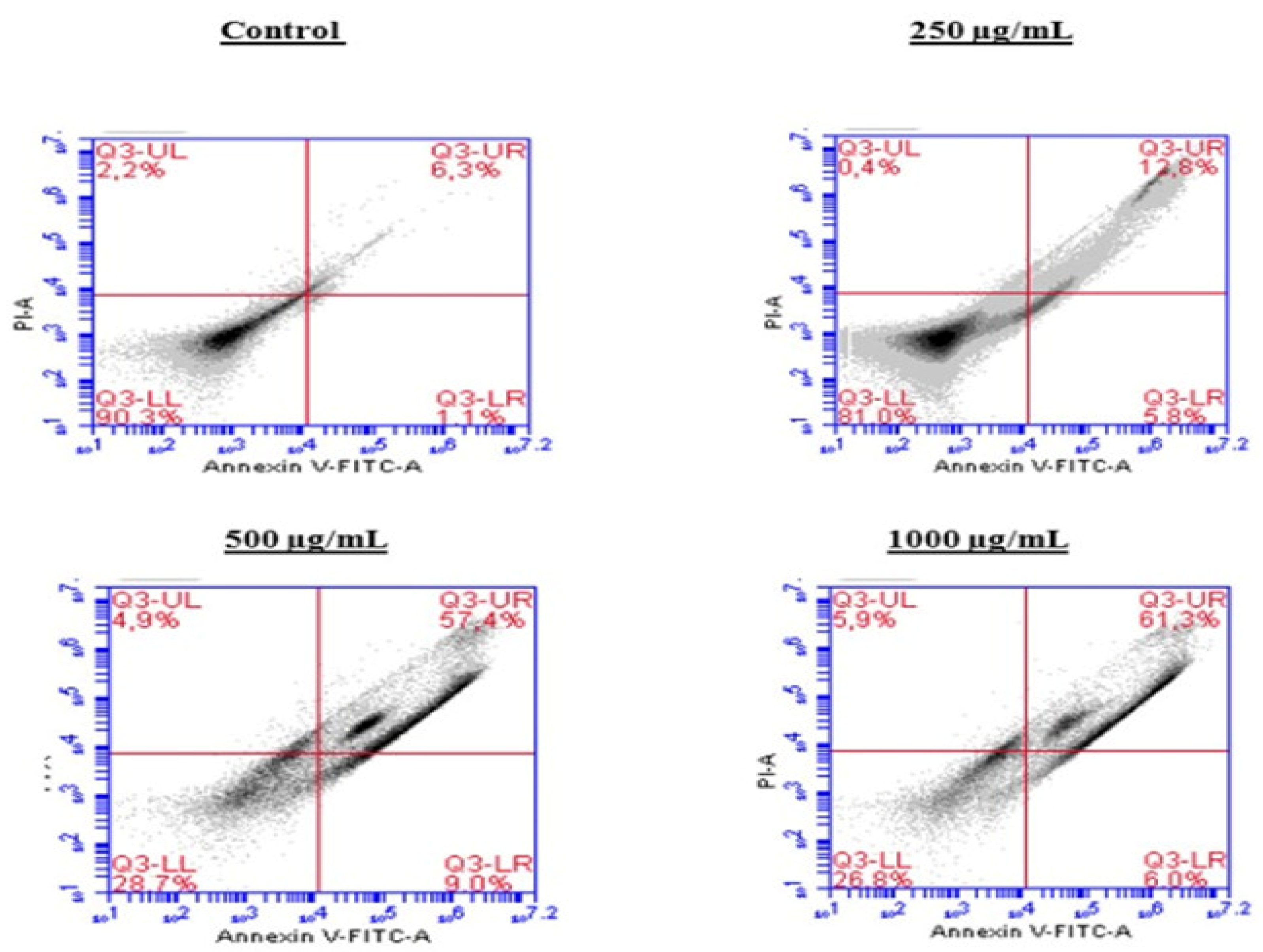
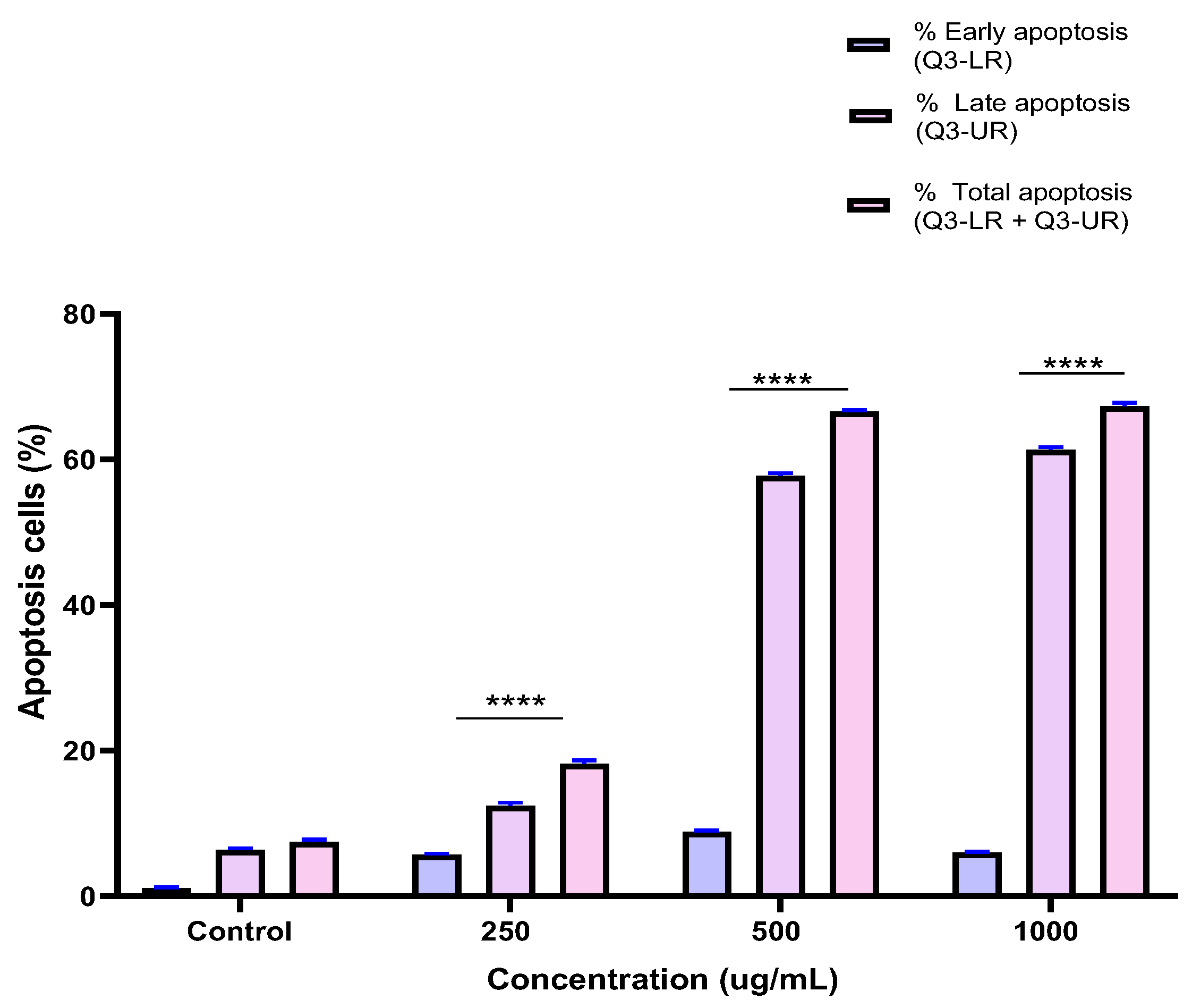
2.5. Induction of Apoptosis by the BESD and BF1SD in PC3 Cells
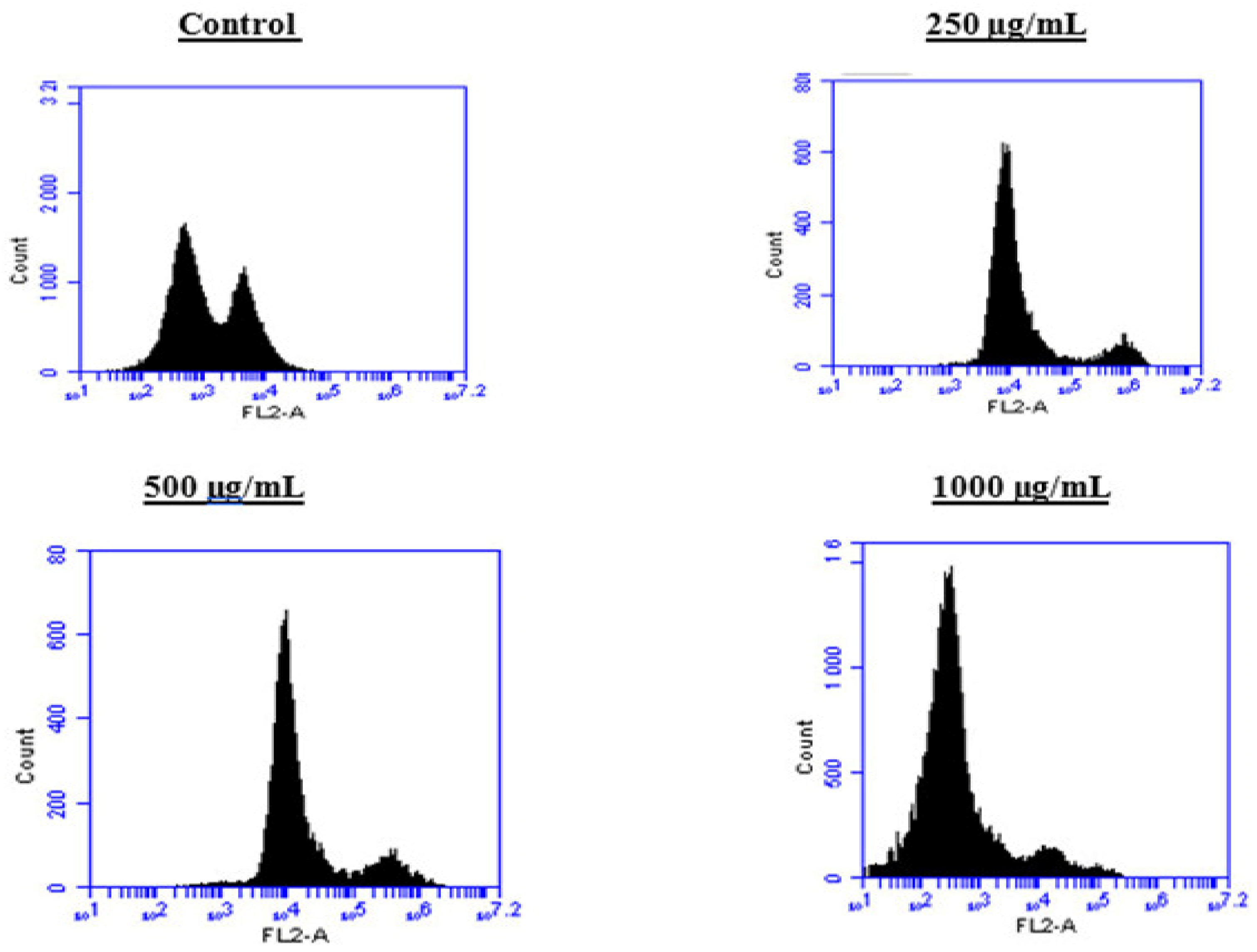
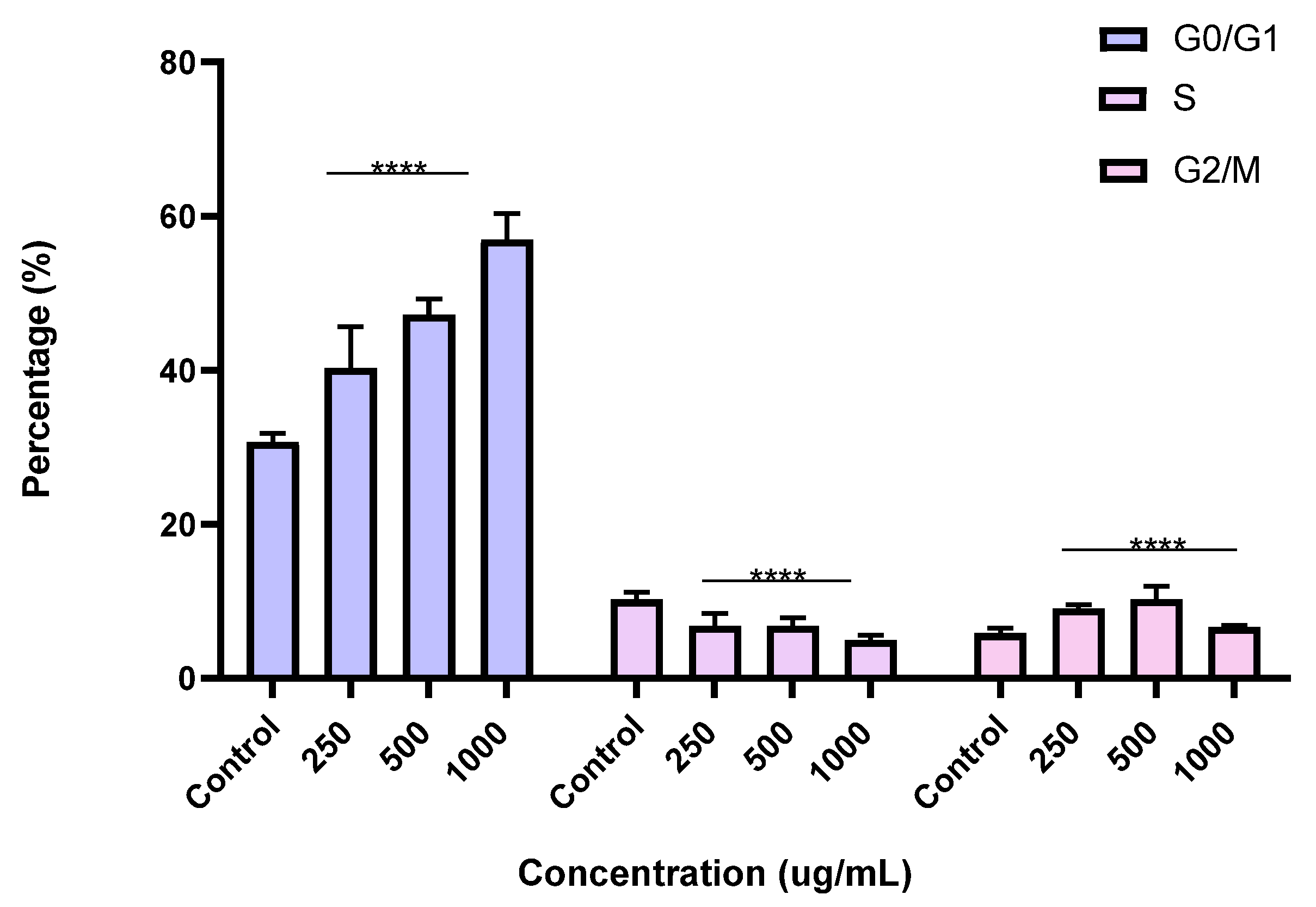
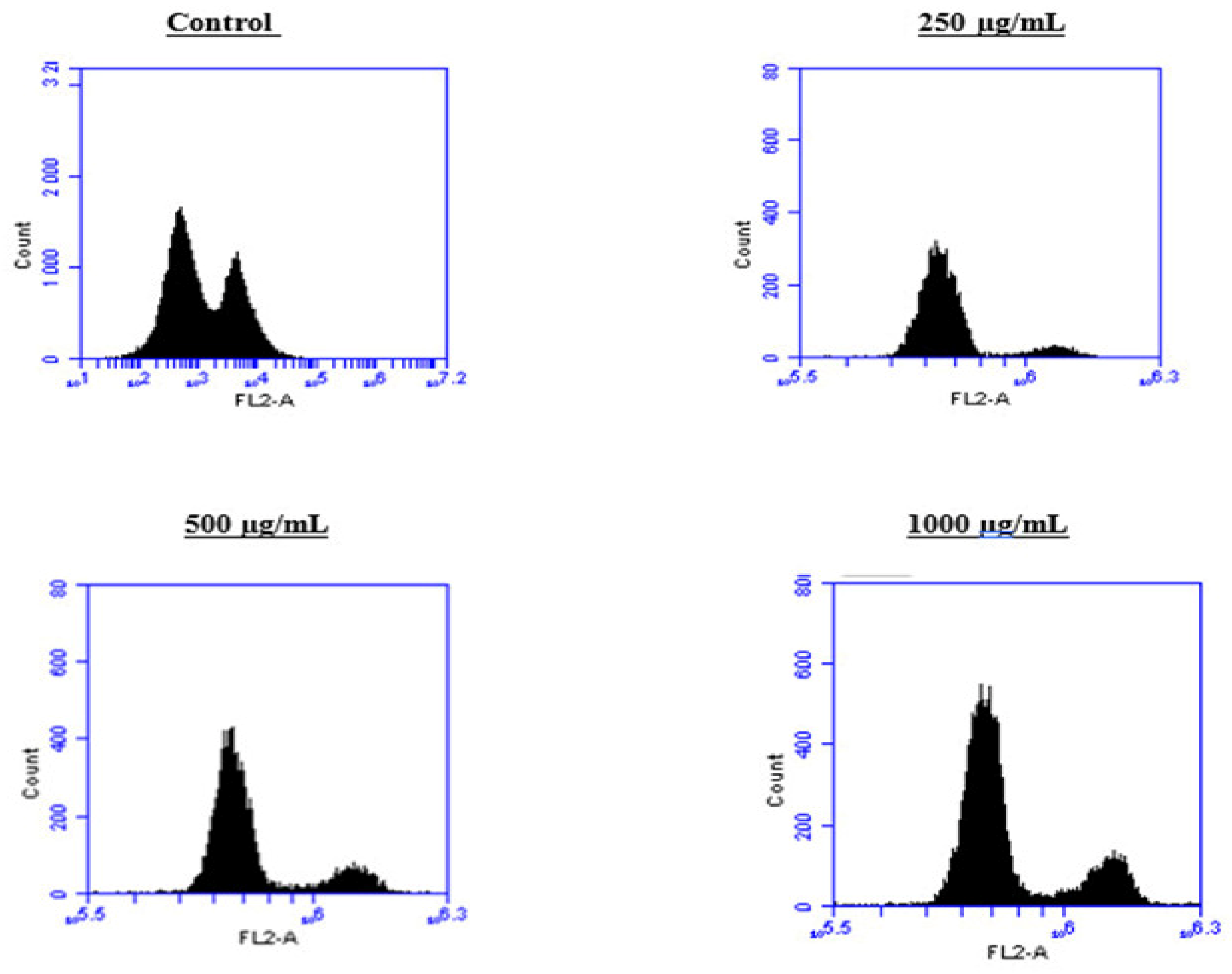
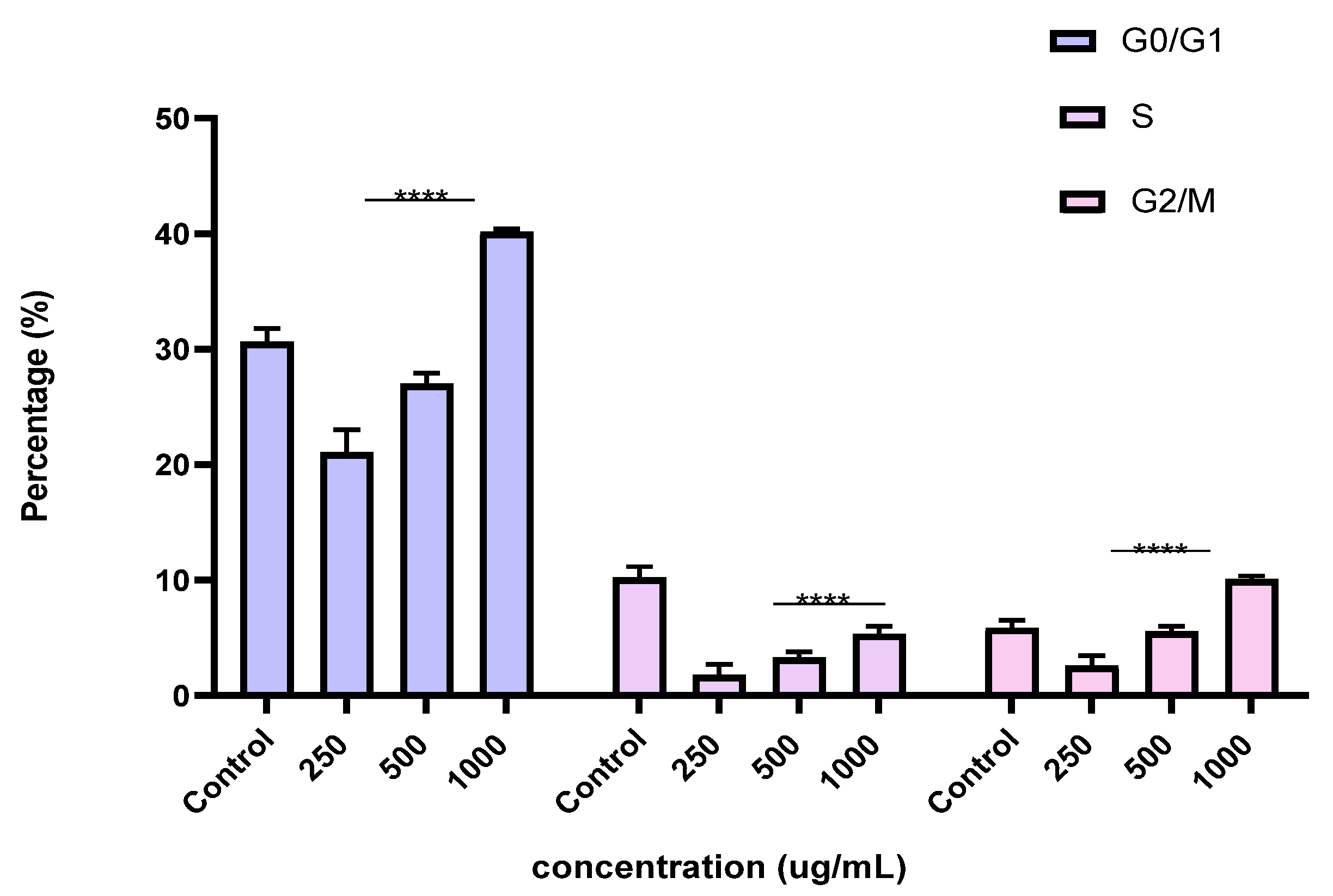
2.6. Induction of Cell Cycle Arrest in PC3 Cells by the BESD and BF1SD
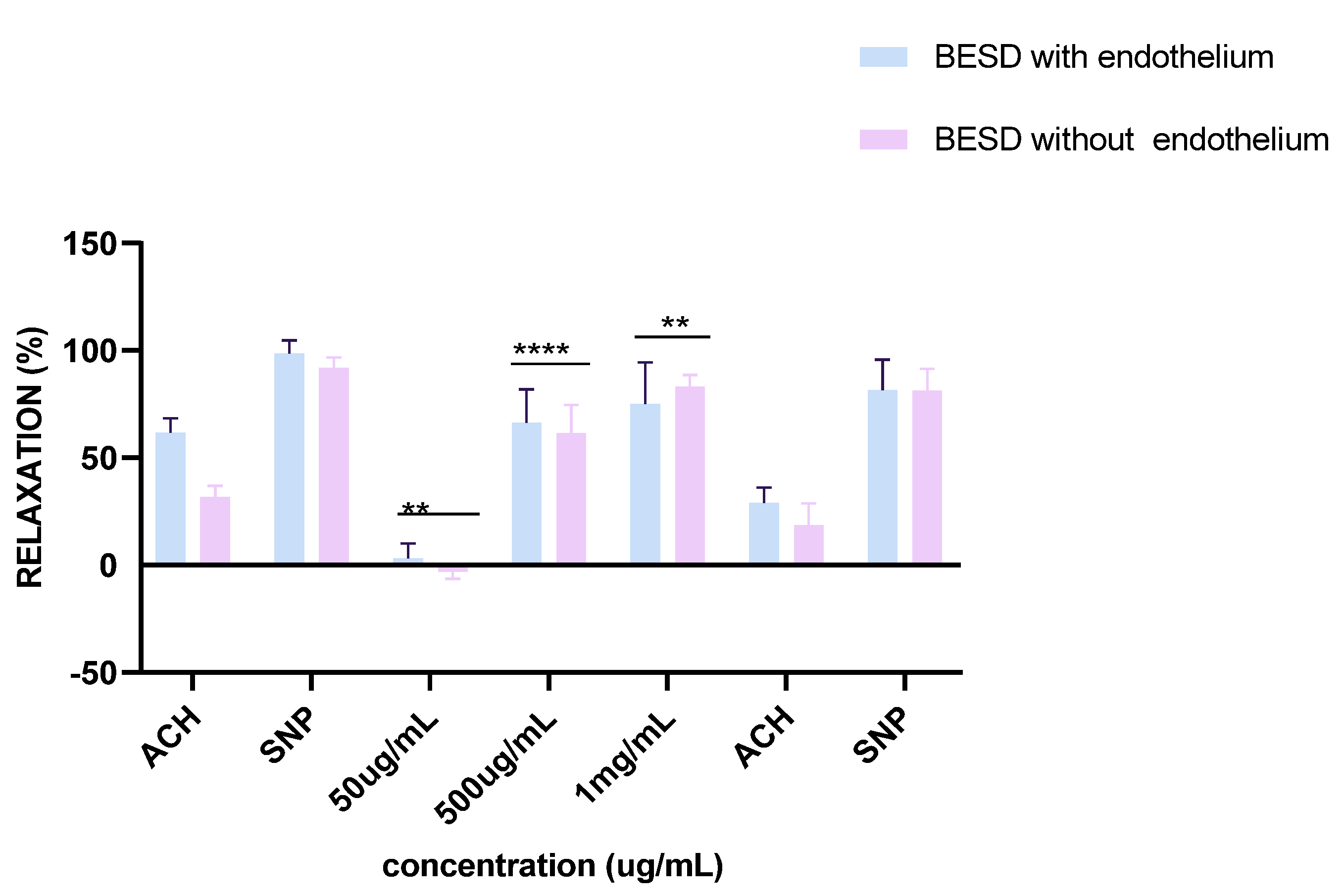
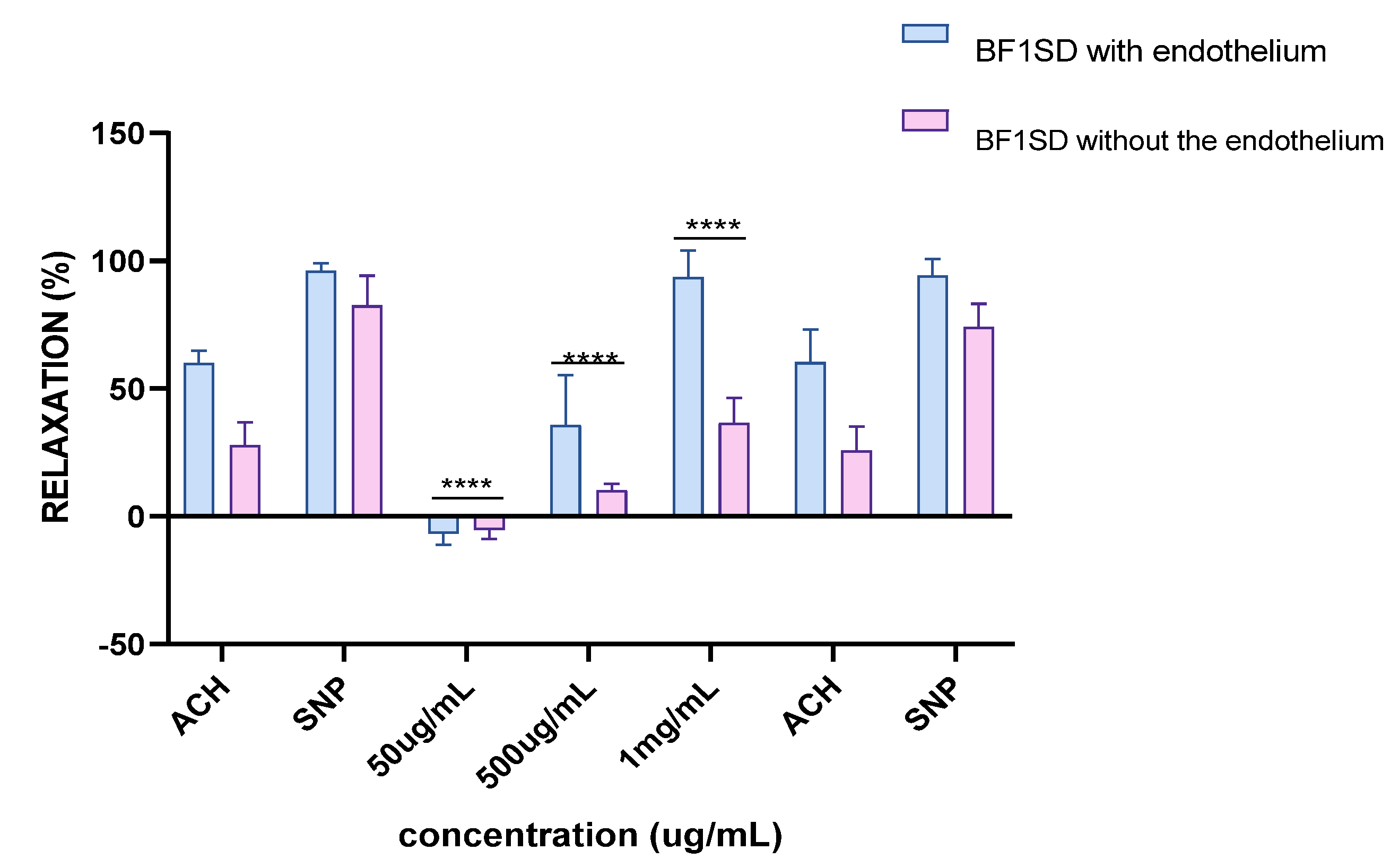
2.7. Ex Vivo Studies: Vascular Reactivity
2.7.1. Vascular Reactivity to Acetylcholine (ACh)
2.7.2. Vascular Reactivity to Sodium Nitroprusside (SNP)
3. Discussion
3.1. Antitumoral Activity (Antiproliferative—Induction of Apoptosis—Induction of Cell Cycle Arrest)
3.2. Vascular Reactivity
4. Materials and Methods
4.1. Plant Material
4.2. Fractionation of the n-Butanolic Extract of Stachys duriaei by Vacuum Liquid Chromatography (VLC)
4.3. Sample Preparation
4.4. LC-HRMS/MS Analyses (Liquid Chromatography—High Resolution Tandem Mass Spectrometry)
4.5. Cell Culture
4.6. MTT Assay
4.7. Apoptosis Assay
4.8. Cell Cycle Analysis
4.9. Ex Vivo Studies: Vascular Reactivity
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Cesare, M.; Bixby, H.; Gaziano, T.; Hadeed, L.; Kabudula, C.; Vaca McGhie, D.; Mwangi, J.; Pervan, B.; Perel, P.; Piñeiro, D.; et al. World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023; Available online: https://world-heart-federation.org/resource/world-heart-report-2023/ (accessed on 8 June 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Tomou, E.; Barda, C.; Skaltsa, H. Genus Stachys: A Review of Traditional Uses, Phytochemistry and Bioactivity. Medicines 2020, 7, 63. [Google Scholar] [CrossRef]
- Laggoune, S.; Kabouche, A.; Kabouche, Z.; Lakhal, H. Biological Activities and Polyphenolic Profile of Stachys arvensis (L.) L. Nat. Prod. Res. 2023, 38, 4044–4049. [Google Scholar] [CrossRef]
- Benmebarek, A.; Zerizer, S.; Laggoune, S.; Kabouche, Z. Immunostimulatory Activity of Stachys mialhesi de Noé. Allergy Asthma Clin. Immunol. 2013, 9, 2. [Google Scholar] [CrossRef]
- Laggoune, S.; Kabouche, A.; Kabouche, Z.; El-Azzouny, A.M. Analysis of the Essential Oil of Stachys circinnata L’Her. from Algeria. J. Essent. Oil Res. 2009, 21, 67–68. [Google Scholar] [CrossRef]
- Laggoune, S.; Zeghib, A.; Kabouche, A.; Kabouche, Z.; Leon, F.; Brouard, I.; Bermejo, J.; Calliste, C.-A.; Duroux, J.-L. Components and Antioxidant Activity of the Roots of the Endemic Species Stachys mialhesi de Noé. Rec. Nat. Prod. 2011, 5, 238–241. [Google Scholar]
- Lakhal, H.; Boudiar, T.; Kabouche, A.; Laggoune, S.; Kabouche, Z.; Topçu, G. Antioxidant activity and flavonoids of Stachys ocymastrum. Chem. Nat. Compd. 2011, 46, 964–965. [Google Scholar] [CrossRef]
- Laggoune, S.; Zeghib, A.; Kabouche, A.; Kabouche, Z.; Maklad, Y.; Leon, F.; Brouard, I.; Bermejo, J.; Calliste, C.-A.; Duroux, J.-L. Components and Antioxidant, Anti-Inflammatory, Anti-Ulcer, and Antinociceptive Activities of the Endemic Species Stachys mialhesi de Noé. Arab. J. Chem. 2016, 9, S191–S197. [Google Scholar] [CrossRef]
- Ferhat, M.; Erol, E.; Beldjila, K.; Çetintaş, Y.; Duru, M.; Öztürk, M.; Kabouche, A.; Kabouche, Z. Antioxidant, Anticholinesterase, and Antibacterial Activities of Stachys guyoniana and Mentha aquatica. Pharm. Biol. 2016, 55, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Slimani, W.; Maioli, M.; Cruciani, S.; Zerizer, S.; Santaniello, S.; Kabouche, Z.; Coradduzza, D.; Chessa, M.; Fancello, S.; Migheli, R.; et al. Antioxidant, Anti-Inflammatory, and Anti-Proliferative Properties of Stachys circinata on HepG2 and MCF7 Cells. Plants 2023, 12, 2272. [Google Scholar] [CrossRef]
- Efferth, T. Cancer Therapy with Natural Products and Medicinal Plants. Planta Med. 2010, 76, 1035–1036. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Ferri, C. Protective Effects of Dark Chocolate on Endothelial Function and Diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 662–668. [Google Scholar] [CrossRef]
- Douglas, H.; Weinberg, R.-A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fulda, S. Targeting Apoptosis for Anticancer Therapy. Semin. Cancer Biol. 2015, 31, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.V.; Kim, S.; Wang, M.-H. Chemical Composition, Antioxidant, and Anti-Diabetic Activities of Ethyl Acetate Fraction of Stachys riederi var. japonica (Miq.) in Streptozotocin-Induced Type 2 Diabetic Mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, C.; Tringali, C. Natural-Derived Polyphenols as Potential Anticancer Agents. Anti-Cancer Agents Med. Chem. 2012, 12, 902–918. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Bhattacharjee, M.; Yeo, B.T.; Chen, S.-H. Maximizing dissimilarity in resting state detects heterogeneous subtypes in healthy population associated with high substance use and problems in antisocial personality. Hum. Brain Mapp. 2020, 41, 1261–1273. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Pommier, Y.; Kohn, K.W. Cycle Cellulaire et Points de Contrôle en Oncologie: Nouvelles Cibles Thérapeutiques. Med. Sci. 2003, 19, 173–186. [Google Scholar] [CrossRef][Green Version]
- Kong, Y.; Sun, W.; Wu, P. Hyperoside Exerts Potent Anticancer Activity in Skin Cancer. Front. Biosci. (Landmark Ed.) 2020, 25, 463–479. [Google Scholar] [CrossRef]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Yang, Y.; Tantai, J.; Sun, Y.; Zhong, C.; Li, Z. Effect of Hyperoside on the Apoptosis of A549 Human Non-Small Cell Lung Cancer Cells and the Underlying Mechanism. Mol. Med. Rep. 2017, 16, 6483–6488. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qi, Z.-X.; Sun, M.X.; Xie, L.-P. Hyperoside Induces Cell Cycle Arrest and Suppresses Tumorigenesis in Bladder Cancer through the Interaction of EGFR-Ras and Fas Signaling Pathways. Int. J. Med. Sci. 2024, 21, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-M.; Song, Y.-S. Luteolin and Luteolin-7-O-Glucoside Inhibit Lipopolysaccharide-Induced Inflammatory Responses through Modulation of NF-κB/AP-1/PI3K-Akt Signaling Cascades in RAW 264.7 Cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Lee, E.-J.; Kim, H.-R.; Hwang, K.-A. Molecular Mechanisms of Luteolin-7-O-Glucoside-Induced Growth Inhibition on Human Liver Cancer Cells: G2/M Cell Cycle Arrest and Caspase-Independent Apoptotic Signaling Pathways. BMB Rep. 2013, 46, 611–616. [Google Scholar] [CrossRef]
- Chen, S.; Yang, B.; Xu, Y.; Rong, Y.; Qiu, Y. Protection of Luteolin-7-O-Glucoside against Apoptosis Induced by Hypoxia/Reoxygenation through the MAPK Pathways in H9c2 Cells. Mol. Med. Rep. 2018, 17, 7156–7162. [Google Scholar] [CrossRef]
- Ho, H.-Y.; Chen, P.; Lo, Y.-S.; Lin, C.; Chuang, Y.; Hsieh, M.; Chen, M. Luteolin-7-O-Glucoside Inhibits Cell Proliferation and Modulates Apoptosis through the AKT Signaling Pathway in Human Nasopharyngeal Carcinoma. Environ. Toxicol. 2021, 36, 2013–2024. [Google Scholar] [CrossRef]
- Li, Y.; Guan, Y.; Wang, Z.; Zhang, L. Hydroxysafflor Yellow A Induces Apoptosis in MCF-7 Cells by Blocking NFκB/p65 Pathway and Disrupting Mitochondrial Transmembrane Potential. RSC Adv. 2014, 4, 47576–47586. [Google Scholar] [CrossRef]
- Wu, N.; Li, J.; Luo, H.; Wang, D.; Bai, X. Hydroxysafflor Yellow A Promotes Apoptosis via Blocking Autophagic Flux in Liver Cancer. Biomed. Pharmacother. 2021, 136, 111227. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Qari, H.; Oves, M. Rutin (Bioflavonoid) as a Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Nasiri, F.; Kismali, G.; Alpay, M.; Kosova, F.; Cakir, D.; Sel, T. Rutin Enhances the Antiproliferative Effect of 5-FU and Oxaliplatin in Colon Cancer Cells. Cancer Res. 2016, 76, 2177. [Google Scholar] [CrossRef]
- Yan, X.; Hao, Y.; Chen, S.; Jia, G.; Guo, Y.; Zhang, G.; Wang, C.; Cheng, R.; Hu, T.; Zhang, X.; et al. Rutin Induces Apoptosis via P53 Up-Regulation in Human Glioma CHME Cells. Transl. Cancer Res. 2019, 8, 1763–1773. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-Tumor Effect of Rutin on Human Neuroblastoma Cell Lines through Inducing G2/M Cell Cycle Arrest and Promoting Apoptosis. Sci. World J. 2013, 2013, 269165. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.; et al. Myricetin: A Comprehensive Review on Its Biological Potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, P.; Jiang, M.; Yu, S.; Wang, L. Myricetin Induces Apoptosis and Autophagy by Inhibiting PI3K/Akt/mTOR Signalling in Human Colon Cancer Cells. BMC Complement. Med. Ther. 2020, 20, 209. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Wang, Y.; Du, Y.; Sun, Q.; Zang, W.; Zhao, G. Myricetin Inhibits Proliferation and Induces Apoptosis and Cell Cycle Arrest in Gastric Cancer Cells. Mol. Cell. Biochem. 2015, 408, 163–170. [Google Scholar] [CrossRef]
- Yang, W.; Su, J.; Li, M.; Li, T.; Wang, X.; Zhao, M.; Hu, X. Myricetin Induces Autophagy and Cell Cycle Arrest of HCC by Inhibiting MARCH1-Regulated p38 MAPK/Stat3 Signaling Pathways. Front. Pharmacol. 2021, 12, 709526. [Google Scholar] [CrossRef]
- Prata, M.N.L.; Charlie-Silva, I.; Gomes, J.M.M.; Barra, A.; Berg, B.B.; Paiva, I.R.; Perez, A.C. Anti-Inflammatory and Immune Properties of the Peltatoside, Isolated from the Leaves of Annona crassiflora Mart, in a New Experimental Model Zebrafish. Fish Shellfish Immunol. 2020, 101, 234–243. [Google Scholar] [CrossRef]
- Piozzi, F.; Bruno, M. Diterpenoids from Roots and Aerial Parts of the Genus Stachys. Rec. Nat. Prod. 2011, 5, 1–14. [Google Scholar]
- Li, Y.; Yu, X.; Wang, Y.; Zheng, X.; Chu, Q. Kaempferol-3-O-rutinoside, a Flavone Derived from Tetrastigma hemsleyanum, Suppresses Lung Adenocarcinoma via the Calcium Signaling Pathway. Food Funct. 2021, 12, 8351–8365. [Google Scholar] [CrossRef]
- James, A.R.; Jayaprakash, S.; Sundeep, L.M. In-Vitro Cytotoxicity, Apoptotic Property, and Gene Expression Changes Induced by Naringenin-7-O-Glucoside in Triple-Negative Breast Cancer. Cureus 2024, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, C. The Inhibitory Role of Kaempferol-3-O-Rutinoside Induced AMPK Activation on the Growth of Human Breast Cancer Cell Lines. Cancer Res. 2017, 77, 2323. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Q.; Duncan, J.W.; Bakrania, B.A.; Bradshaw, J.L.; Granger, J.P.; Rana, S.; Spradley, F.T. Luteolin-Induced Vasorelaxation in Uterine Arteries from Normal Pregnant Rats. Pregnancy Hypertens. 2021, 23, 11–17. [Google Scholar] [CrossRef]
- Si, H.; Wyeth, R.P.; Liu, D. The Flavonoid Luteolin Induces Nitric Oxide Production and Arterial Relaxation. Eur. J. Nutr. 2014, 53, 269–275. [Google Scholar] [CrossRef]
- Li, W.; Dong, M.; Guo, P.; Liu, Y.; Jing, Y.; Chen, R.; Zhang, M. Luteolin-Induced Coronary Arterial Relaxation Involves Activation of the Myocyte Voltage-Gated K+ Channels and Inward Rectifier K+ Channels. Life Sci. 2019, 221, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.; Vázquez, L.; Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M. Vasodilator Compounds Derived from Plants and Their Mechanisms of Action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef]
- Adaramoye, O.; Medeiros, I. Endothelium-Independent Vasodilation Induced by Kolaviron, a Biflavonoid Complex from Garcinia kola Seeds, in Rat Superior Mesenteric Arteries. J. Smooth Muscle Res. 2009, 45, 39–53. [Google Scholar] [CrossRef]
- Shahlehi, S.; Azizi, A.; Tengah, A.; Amdani, S.; Petalcorin, M.-I. Anti-Hypertensive Vasodilatory Action of Gynura procumbens Mediated by Kaempferol 3-O-Rutinoside. F1000Research 2020, 9, 1226. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
| No | Compounds | Rt (Min) | m/z (Molecular Ion) | Intensity BESD | Intensity BF1SD |
|---|---|---|---|---|---|
| 1 | 2′,3′-Epoxyindicolactone | 4.14 | 383.1147 | 50,238,147 (1.01%) | 233,411,187 (5.06%) |
| 2 | Melibiose | 4.35 | 365.1042 | 45,638,908 (0.91%) | 135,487,001 (2.94%) |
| 3 | Trans 4-O-Caffeoylquinic acid | 7.41 | 353.1073 | 5,454,199 (0.11%) | 97,332,808 (2.11%) |
| 4 | Adenosine | 10.24 | 268.1030 | 1,613,449 (0.03%) | 33,593,786 (0.73%) |
| 5 | 4-p-Coumaroylquinic acid | 17.31 | 339.1067 | 920,113 (0.02%) | 2,140,260 (0.05%) |
| 6 | Chlorogenic acid | 17.60 | 355.1015 | 16,240,683 (0.33%) | 6,546,218 (0.14%) |
| 7 | Aloesin | 17.85 | 395.1325 | 1,033,557 (0.02%) | 3,631,532 (0.08%) |
| 8 | N-(6-methoxyquinolin-8-yl)alanine | 18.23 | 247.1071 | 108,964 (0.00%) | 682,061 (0.01%) |
| 9 | 8-[5-(5,7-Dihydroxy-4-oxo-chromen-2-yl)-2-methoxy-phenyl]-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-chromen-4-one | 18.60 | 397.1470 | 18,970,320 (0.38%) | 31,610,243 (0.69%) |
| 10 | 1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid | 18.99 | 231.1136 | 11,824,363 (0.24%) | 16,993,092 (0.37%) |
| 11 | Vicenin 2 | 19.13 | 595.1651 | 1,233,768 (0.02%) | 619,904 (0.01%) |
| 12 | 3-p-Coumaroylquinic acid | 19.20 | 339.1068 | 920,529 (0.02%) | 7,626,802 (0.17%) |
| 13 | N-methyl-2,4-dihydroxy-3-phenylquinoline | 19.37 | 252.0863 | 14,777,481 (0.30%) | 67,508,138 (1.46%) |
| 14 | 1,2,6-Trigalloylglucose | 19.53 | 635.0864 | 107,962,646 (2.16%) | 3,546,009 (0.08%) |
| 15 | Methyl chlorogenate | 19.59 | 369.1172 | 2,114,685 (0.04%) | 13,688,373 (0.30%) |
| 16 | 1,6-bis-O-galloyl-β-D-glucose | 19.70 | 483.0758 | 251,605,789 (5.04%) | 8,383,446 (0.18%) |
| 17 | Myricetin-3-rutinoside | 19.75 | 627.1544 | 98,083,034 (1.90%) | 17,940,256 (0.39%) |
| 18 | 8-O-Acetylharpagide | 19.78 | 429.1359 | 12,707,623 (0.25%) | 39,216,825 (0.85%) |
| 19 | β-D-Glucosiduronic acid, 2-(3,4-dihydroxyphenyl) -5-hydroxy-6-methoxy-4-oxo-4H-1-benzopyran-7-yl | 20.16 | 395.0939 | 27,823,579 (0.56%) | 12,271,382 (0.27%) |
| 20 | 2′-O-galloylhyperin | 20.17 | 617.1126 | 256,823,533 (5.14%) | 11,358,492 (0.25%) |
| 21 | Myricetin 3-galactoside | 20.35 | 481.0964 | 143,761,867 (2.88%) | 10,531,074 (0.23%) |
| 22 | Peltatoside (quercetin-3-O-arabinoglucoside) | 20.59 | 597.1439 | 252,993,403 (5.07%) | 101,001,688 (2.19%) |
| 23 | Sayaendoside | 20.62 | 439.1569 | 294,250,466 (5.89%) | 305,140,167 (6.62%) |
| 24 | Palatinose | 20.84 | 325.0910 | 2,161,600 (0.04%) | 734,945 (0.02%) |
| 25 | Hydroxysafflor yellow A | 21.00 | 611.1595 | 423,917,474 (8.49%) | 372,294,712 (8.07%) |
| 26 | Hyperoside | 21.01 | 465.1015 | 569,496,692 (11.41%) | 230,351,300 (5.00%) |
| 27 | β-D-Glucosyl 2-phenylethyl-6-O-β-D-xylopyranoside | 21.14 | 439.1565 | 2,054,691 (0.04%) | 11,365,070 (0.25%) |
| 28 | Rutin | 21.15 | 611.1592 | 182,945,024 (3.66%) | 211,206,590 (4.58%) |
| 29 | N-acetyltryptophan | 21.25 | 247.1071 | 1,860,401 (0.04%) | 31,686,457 (0.69%) |
| 30 | (E,E)-3-Methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienoic acid | 21.44 | 443.1894 | 2,362,093 (0.05%) | 44,427,156 (0.96%) |
| 31 | Isovitexin | 21.47 | 433.1119 | 11,963,439 (0.24%) | 18,199,468 (0.39%) |
| 32 | Quercetin | 21.62 | 303.0493 | 9,263,420 (0.19%) | 6,573,430 (0.14%) |
| 33 | Naringenin-7-O-β-D-glucoside | 21.63 | 435.1274 | 22,373,699 (0.45%) | 138,909,348 (3.01%) |
| 34 | 6′′-O-L-arabinopyranosyl astragalin | 21.77 | 581.1489 | 2,508,029 (0.05%) | 40,684,936 (0.88%) |
| 35 | kaempferol-3-O-rutinoside | 21.78 | 595.1647 | 103,900,471 (2.08%) | 118,492,531 (2.57%) |
| 36 | 1,2,3,6-tetragalloylglucose | 21.86 | 787.0966 | 231,807,467 (4.64%) | 4,140,064 (0.09%) |
| 37 | Luteolin-7,3′-di-O-β-D-glucoside | 22.12 | 609.1444 | 0 (0%) | 193,827 (0.04%) |
| 38 | Pulvinic acid | 22.15 | 307.0447 | 5,145,102 (0.10%) | 34,943,156 (0.76%) |
| 39 | Luteolin-7-O-β-D-glucoside | 22.16 | 449.1066 | 263,284,078 (5.27%) | 213,769,931 (4.64%) |
| 40 | 3’-Methoxyquercetin-3-O-rutinoside | 22.33 | 625.1745 | 2,807,934 (0.06%) | 32,042,186 (0.69%) |
| 41 | 7-O-Neohesperidosyl chrysoeriol | 22.38 | 609.1804 | 0 (0%) | 196,837 (0.04%) |
| 42 | Roseoside | 22.43 | 387.2001 | 6,909,671 (0.14%) | 18,017,930 (0.39%) |
| 43 | 1,3-Dihydroxyanthraquinone | 22.59 | 239.0190 | 25,438,392 (0.51%) | 47,110,390 (1.02%) |
| 44 | 8,8-dimethyl-2-phenylpyrano[2,3-f]chromen-4-one | 22.65 | 305.1125 | 272,436 (0.01%) | 11,677,199 (0.25%) |
| 45 | β-Penta-O-galloyl-glucose | 22.91 | 939.1073 | 53,919,155 (1.08%) | 0 (0%) |
| 46 | Subaphyllin | 23.01 | 314.1379 | 0 (0%) | 870,665 (0.02%) |
| 47 | Tiliroside | 23.04 | 593.1490 | 485,708 (0.01%) | 723,658 (0.02%) |
| 48 | Myricetin-3-O-β-D-galactoside 6′′-O-gallate | 23.18 | 631.0919 | 118,510,736 (2.37%) | 8,806,699 (0.19%) |
| 49 | L-phenylalanine butyl ester | 23.27 | 222.1483 | 332,365,866 (6.66%) | 90,019,584 (1.95%) |
| 50 | Byzantionoside B | 23.72 | 373.2196 | 13,907,102 (0.28%) | 92,036,025 (2.00%) |
| 51 | (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[4-(hydroxymethyl)-1-propan-2-ylcyclohex-3-en-1-yl]oxyoxane-3,4,5-triol | 23.85 | 355.1718 | 268,591,559 (5.38%) | 327,376,033 (7.10%) |
| 52 | Diosmin | 24.36 | 607.1649 | 0 (0%) | 273,383 (0.01%) |
| 53 | Nematophin | 24.66 | 273.1648 | 3,474,799 (0.07%) | 1,634,480 (0.04%) |
| 54 | Luteolin | 24.68 | 287.0543 | 4,595,397 (0.09%) | 9,688,778 (0.21%) |
| 55 | 4′-Methylisoscutellarein-7-[2-O-(6-O-acetyl-β-D-allopyranosyl)-β-D-glucoside | 25.03 | 667.1860 | 3,509,386 (0.07%) | 178,674,894 (3.88%) |
| 56 | 9-(2,3-dihydroxypropoxy)-9-oxononanoic acid | 25.22 | 261.1336 | 0 (0%) | 582,638 (0.01%) |
| 57 | Apigenin-7-O-β-D-glucoside | 25.36 | 431.0966 | 1,876,149 (0.04%) | 1,578,292 (0.03%) |
| 58 | Myricitrin | 25.85 | 463.0863 | 324,708,952 (6.50%) | 108,056,798 (2.34%) |
| 59 | Geranyl-6-O-α-L-arabinofuranosyl-O-β-D glucoside | 25.96 | 471.2197 | 18,719,776 (0.37%) | 83,466,864 (1.81%) |
| 60 | (R)-Linalyl-β-vicianoside | 26.20 | 471.2194 | 69,919,724 (1.40%) | 250,642,158 (5.44%) |
| 61 | Naringenin | 26.47 | 271.0604 | 61,307,962 (1.23%) | 114,285,140 (2.48%) |
| 62 | 8,3′,4′-trihydroxyflavone-7-O-(6′-O-p-coumaroyl)-β-D-glucoside | 26.89 | 593.1493 | 117,608,434 (2.36%) | 216,792,607 (4.70%) |
| 63 | 2-butanone,4-[3-(β-D-glucopyranosyloxy)-4-hydroxy-2,6,6-trimethyl-1-cyclohexen-1-yl] | 26.92 | 371.2043 | 4,451,363 (0.09%) | 2,517,190 (0.05%) |
| 64 | 6,8-dihydroxy-3-(10-hydroxyundecyl)-3,4-dihydroisochromen-1-one | 26.98 | 351.2133 | 38,345,199 (0.77%) | 71,191,586 (1.54%) |
| 65 | Narcissin | 27.23 | 623.1595 | 19,122,741 (0.38%) | 33,449,19 (0.73%) |
| 66 | Lauryldiethanolamide | N,N-bis(2-hydroxyethyl)dodecanamide | 27.88 | 288.2525 | 450,500 (0.01%) | 49,395,923 (1.07%) |
| 67 | Phytosphingosine | 28.69 | 318.2994 | 0 (0%) | 450,011 (0.01%) |
| 68 | Afzelin | 28.77 | 431.0968 | 23,368,330 (0.47%) | 27,836,625 (0.60%) |
| 69 | Emodin | 28.85 | 269.0447 | 10,100,304 (0.20%) | 7,322,516 (0.16%) |
| 70 | 2′,4′,6′-Trihydroxydihydrochalcone | 30.78 | 257.0811 | 204,060 (0.004%) | 0 (0%) |
| 71 | Tetrahydroxyflavone | 31.12 | 285.0394 | 14,040,475 (0.28%) | 183,589,163 (3.98%) |
| 72 | Pyrenophorol | 31.41 | 311.1673 | 2,104,127 (0.04%) | 2,137,819 (0.05%) |
| IC50 Values (μg/mL) | ||
|---|---|---|
| Cell Type | BESD | BF1SD |
| PC3 | 196.40 ± 6.20 | 281.10 ± 6.30 |
| MDA-MB-231 | 182.40 ± 8.10 | 273.10 ± 3.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchouka, R.L.; Kabouche, Z.; Defondaumière, M.; Debiossat, M.; Ghezzi, C.; Riou, L.; Taha, T.H.; Boufahja, F.; Bendif, H.; Garzoli, S. LC-HRMS/MS-Guided Profiling and Biological Evaluation of Stachys duriaei Extracts: Anticancer and Vasorelaxant Mechanisms via Apoptosis and Endothelium-Dependent Pathways. Molecules 2025, 30, 3570. https://doi.org/10.3390/molecules30173570
Bouchouka RL, Kabouche Z, Defondaumière M, Debiossat M, Ghezzi C, Riou L, Taha TH, Boufahja F, Bendif H, Garzoli S. LC-HRMS/MS-Guided Profiling and Biological Evaluation of Stachys duriaei Extracts: Anticancer and Vasorelaxant Mechanisms via Apoptosis and Endothelium-Dependent Pathways. Molecules. 2025; 30(17):3570. https://doi.org/10.3390/molecules30173570
Chicago/Turabian StyleBouchouka, Racha Lydia, Zahia Kabouche, Marie Defondaumière, Marlène Debiossat, Catherine Ghezzi, Laurent Riou, Tarek H. Taha, Fehmi Boufahja, Hamdi Bendif, and Stefania Garzoli. 2025. "LC-HRMS/MS-Guided Profiling and Biological Evaluation of Stachys duriaei Extracts: Anticancer and Vasorelaxant Mechanisms via Apoptosis and Endothelium-Dependent Pathways" Molecules 30, no. 17: 3570. https://doi.org/10.3390/molecules30173570
APA StyleBouchouka, R. L., Kabouche, Z., Defondaumière, M., Debiossat, M., Ghezzi, C., Riou, L., Taha, T. H., Boufahja, F., Bendif, H., & Garzoli, S. (2025). LC-HRMS/MS-Guided Profiling and Biological Evaluation of Stachys duriaei Extracts: Anticancer and Vasorelaxant Mechanisms via Apoptosis and Endothelium-Dependent Pathways. Molecules, 30(17), 3570. https://doi.org/10.3390/molecules30173570







