Investigation of Removing Basic Yellow 28 and Basic Blue 3 Dyes from Water Using Mulberry Leaves (Morus nigra L.) and Assessment of Ultrasonic Effects
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Instrumentation
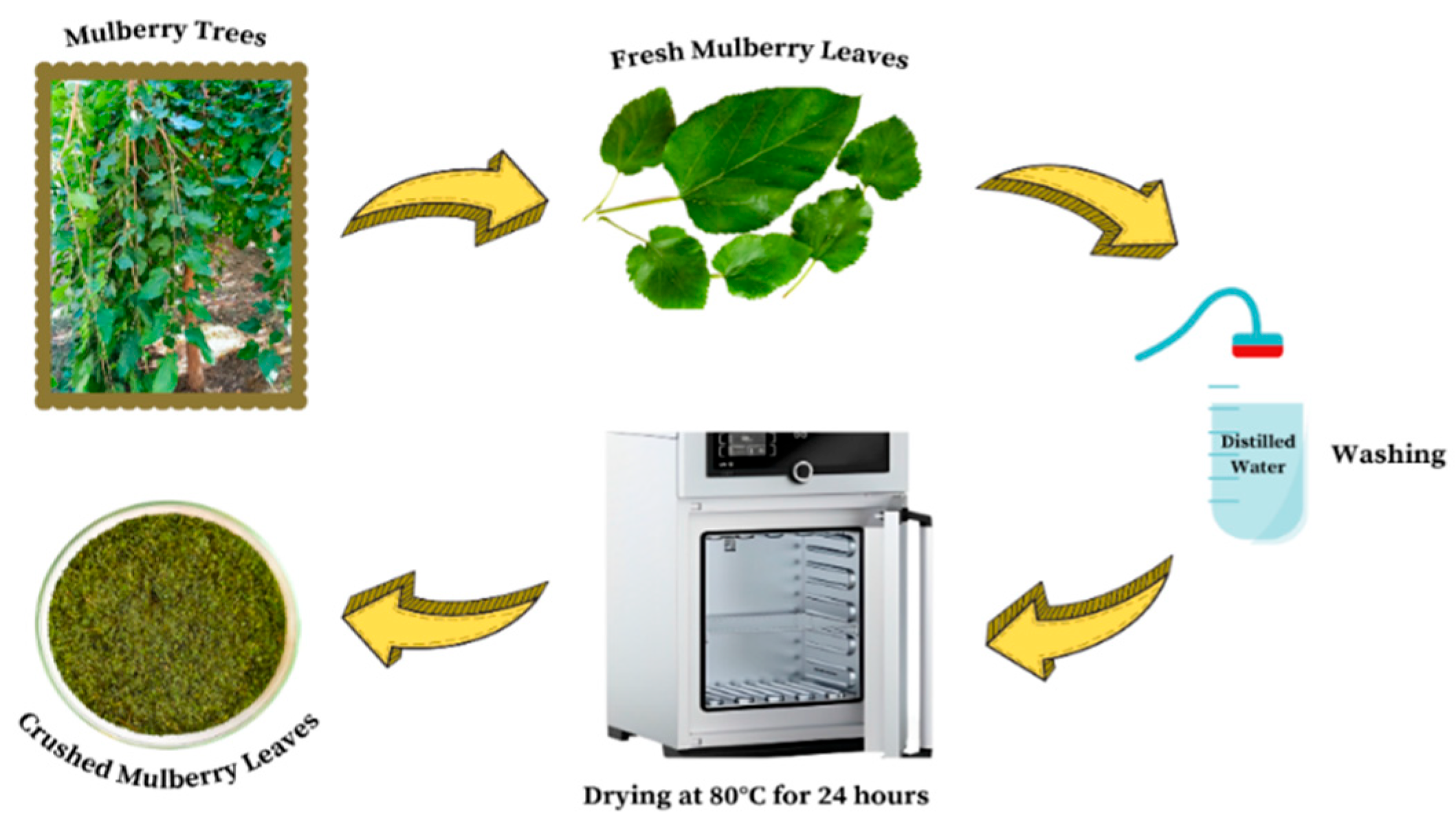
2.3. Preparation of the Adsorbent
2.4. Adsorption Process of BY28 and BB3
2.5. Adsorption Isotherms
3. Results and Discussion
3.1. Characterization of Mulberry Leaf Adsorbent Before and After Adsorption
3.1.1. SEM Analysis
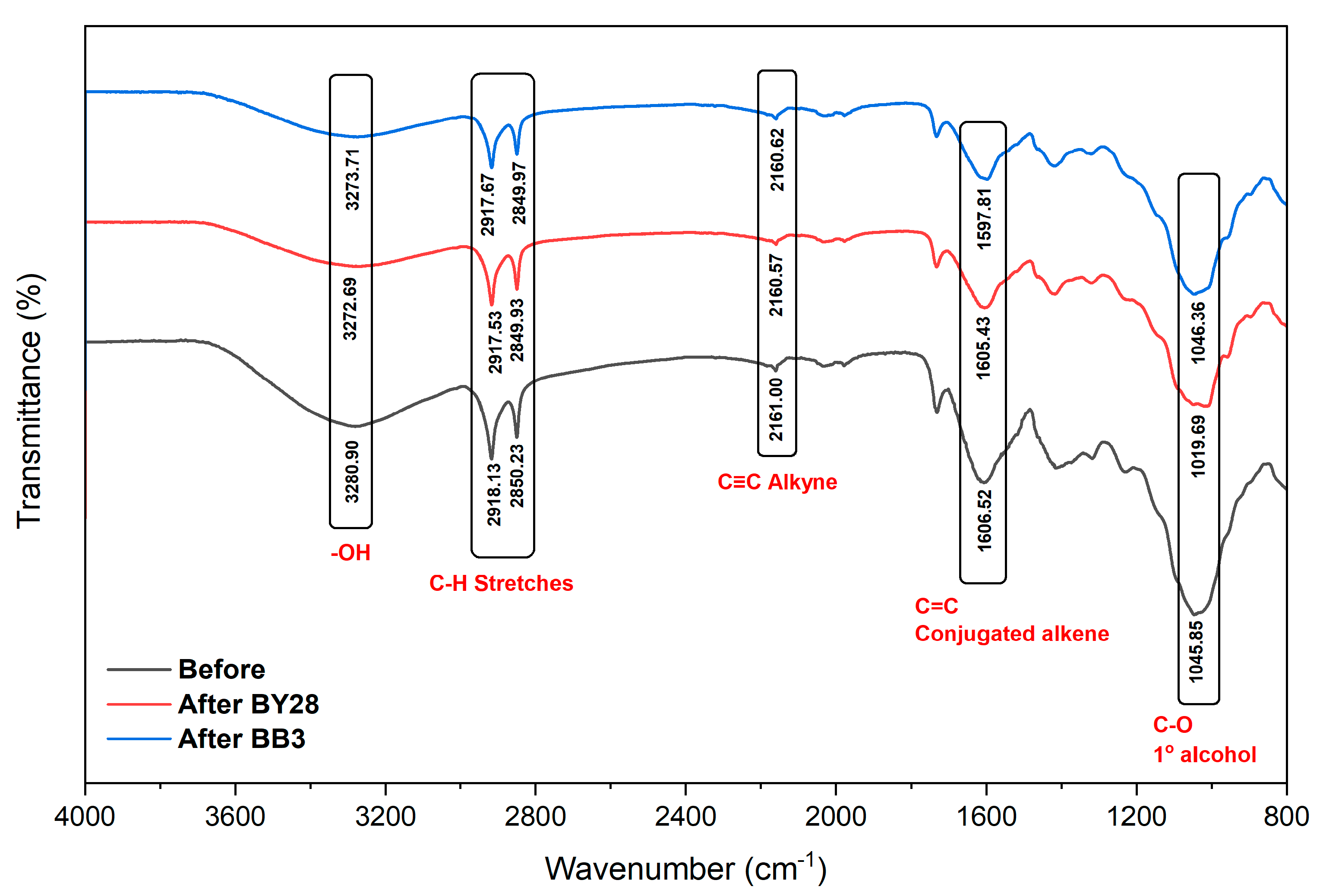
3.1.2. FTIR Analysis
3.1.3. XRD Analysis
3.1.4. BET Analysis
3.2. Determination of pH Point of Zero Charge
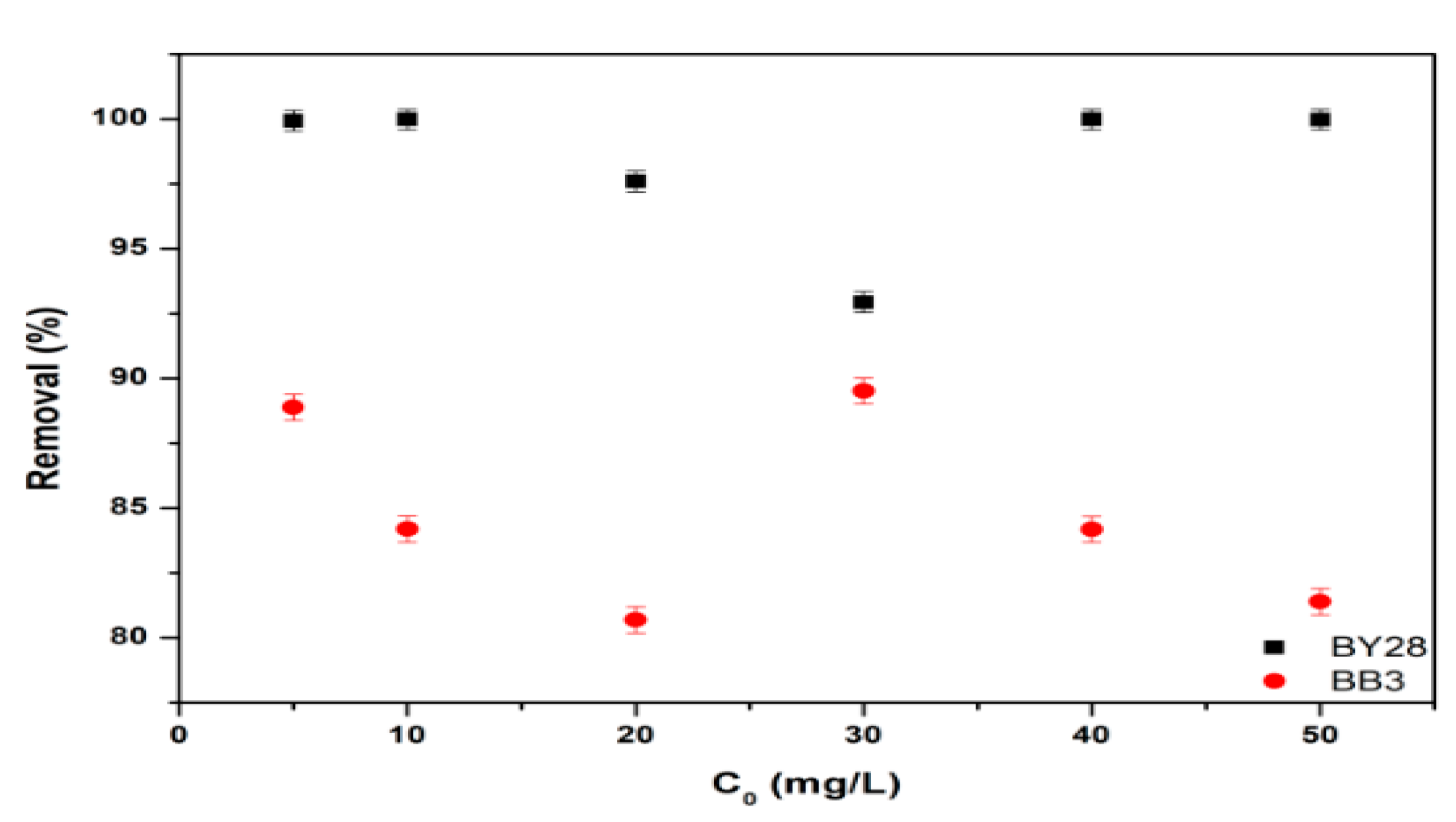
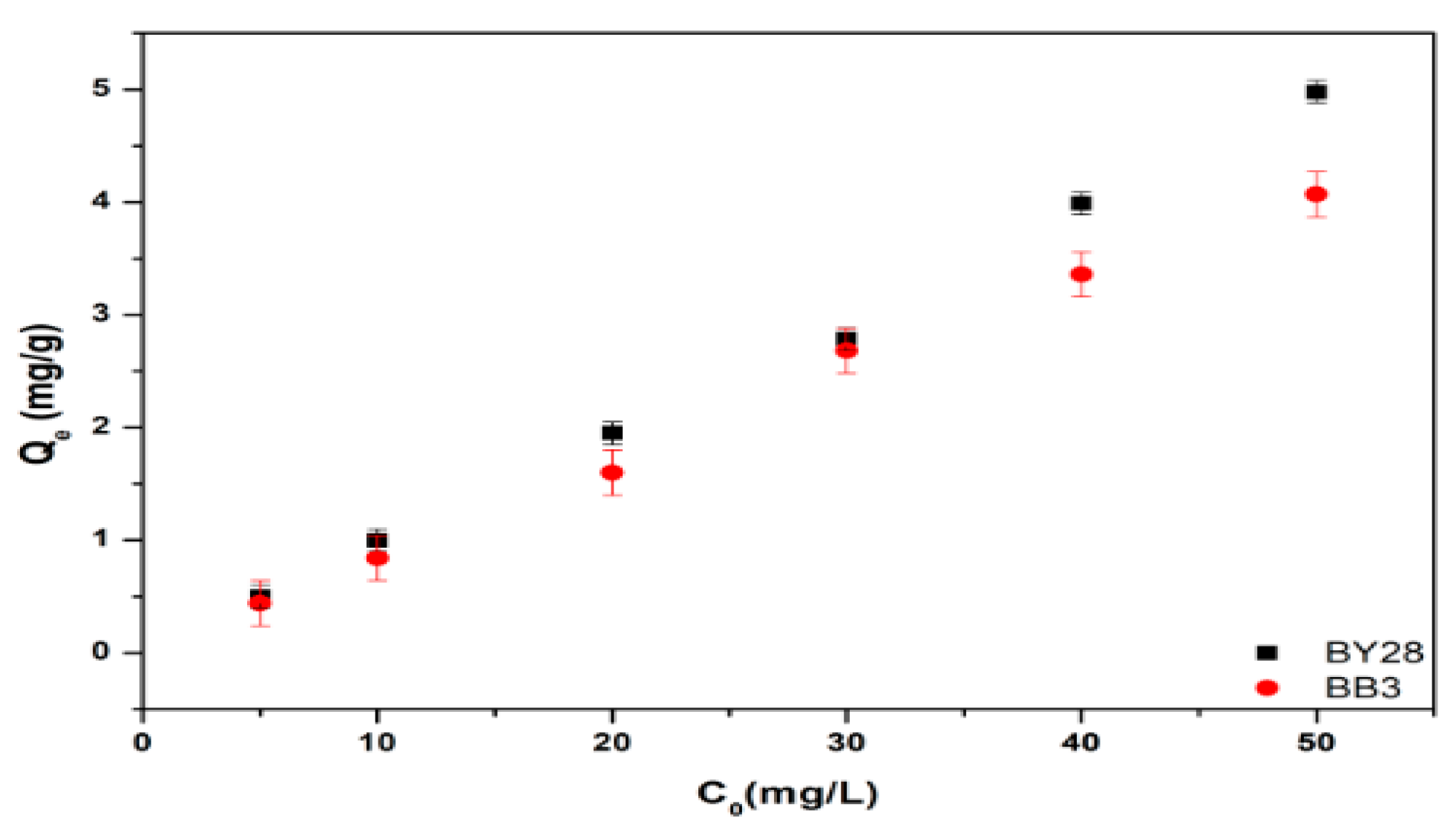
3.3. Effect of Initial Dye Concentration on Adsorption of BY28 and BB3
3.4. The Effect of Varying Amounts of Adsorbent on the Adsorption of BY28 and BB3
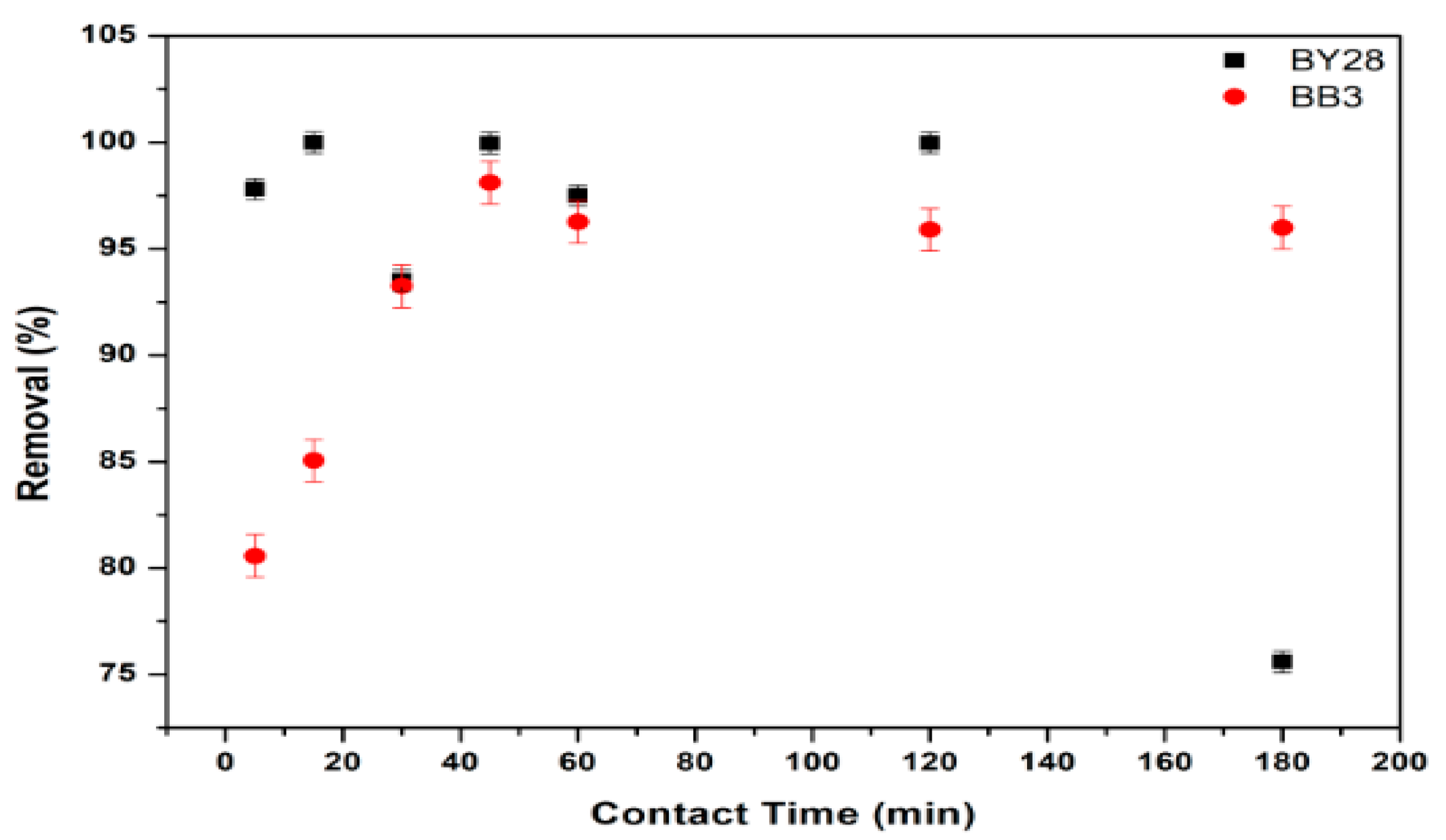
3.5. Effect of Contact Time on Adsorption of BY28 and BB3
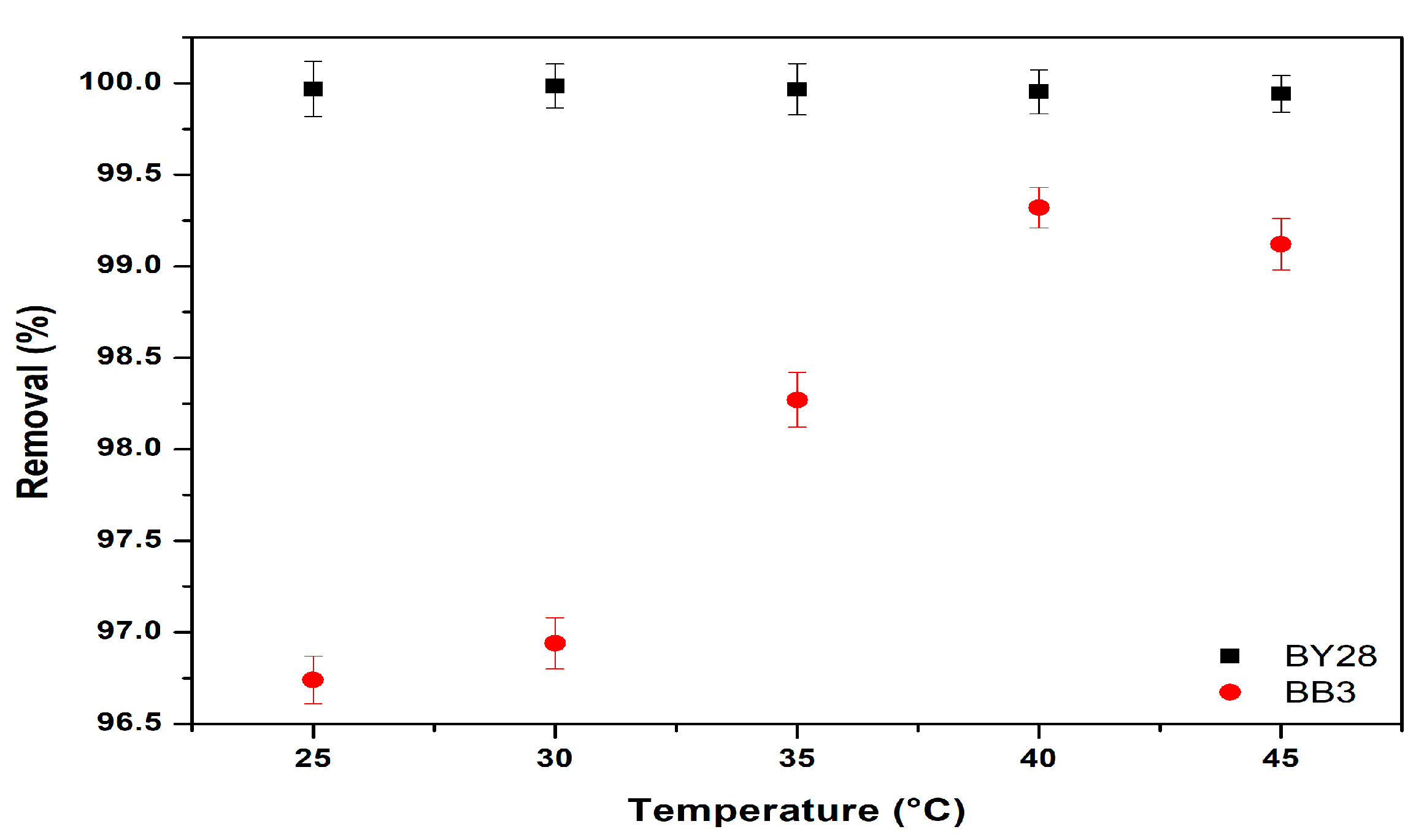
3.6. Effect of Temperature on Adsorption of BY28 and BB3
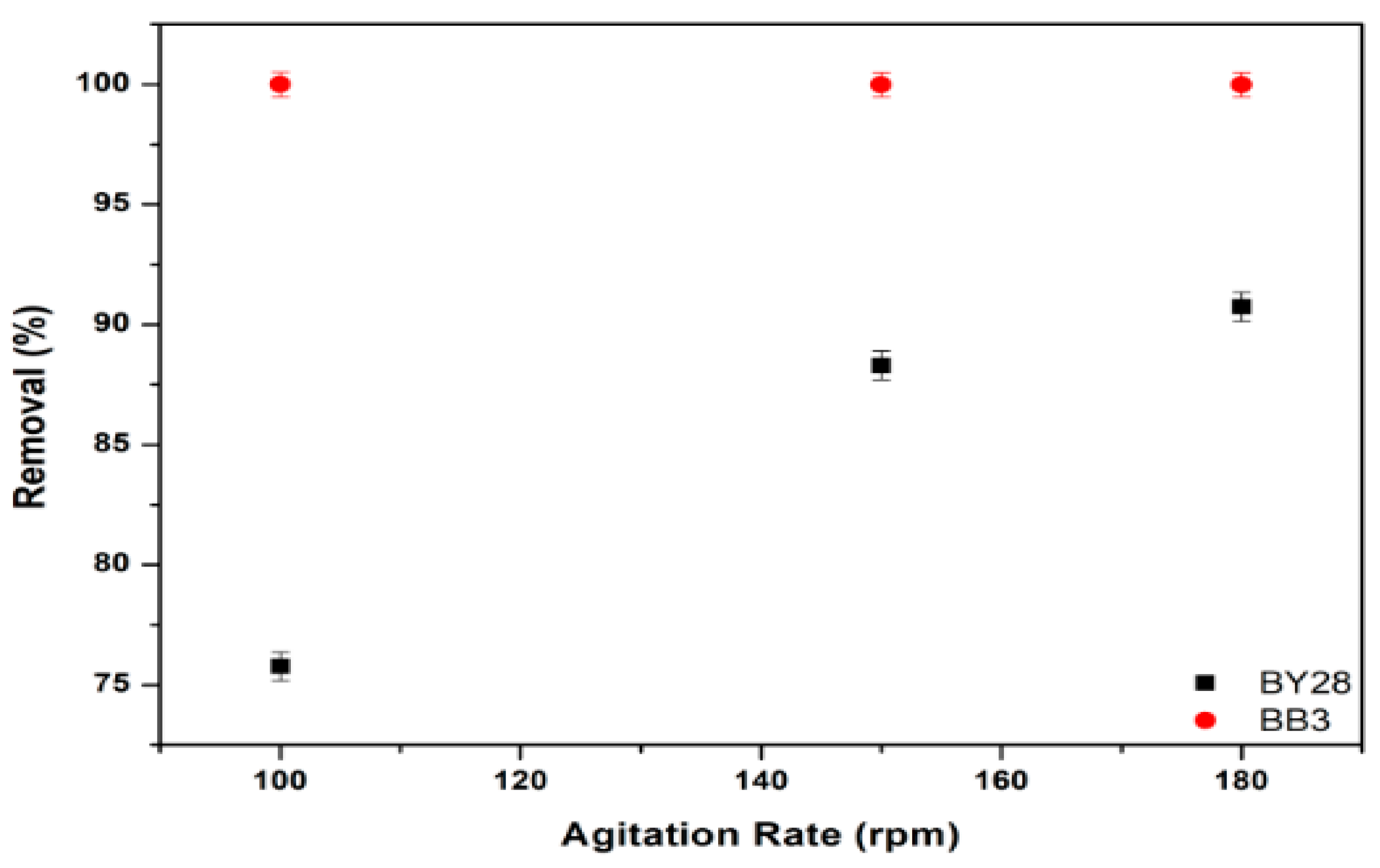
3.7. Effect of Stirring Speed on Adsorption of BY28 and BB3
3.8. Effect of pH on Adsorption of BY28 and BB3
3.9. Ultrasonic Water Bath Shaker Experiment
3.10. Use of Real Water Sources Experiments
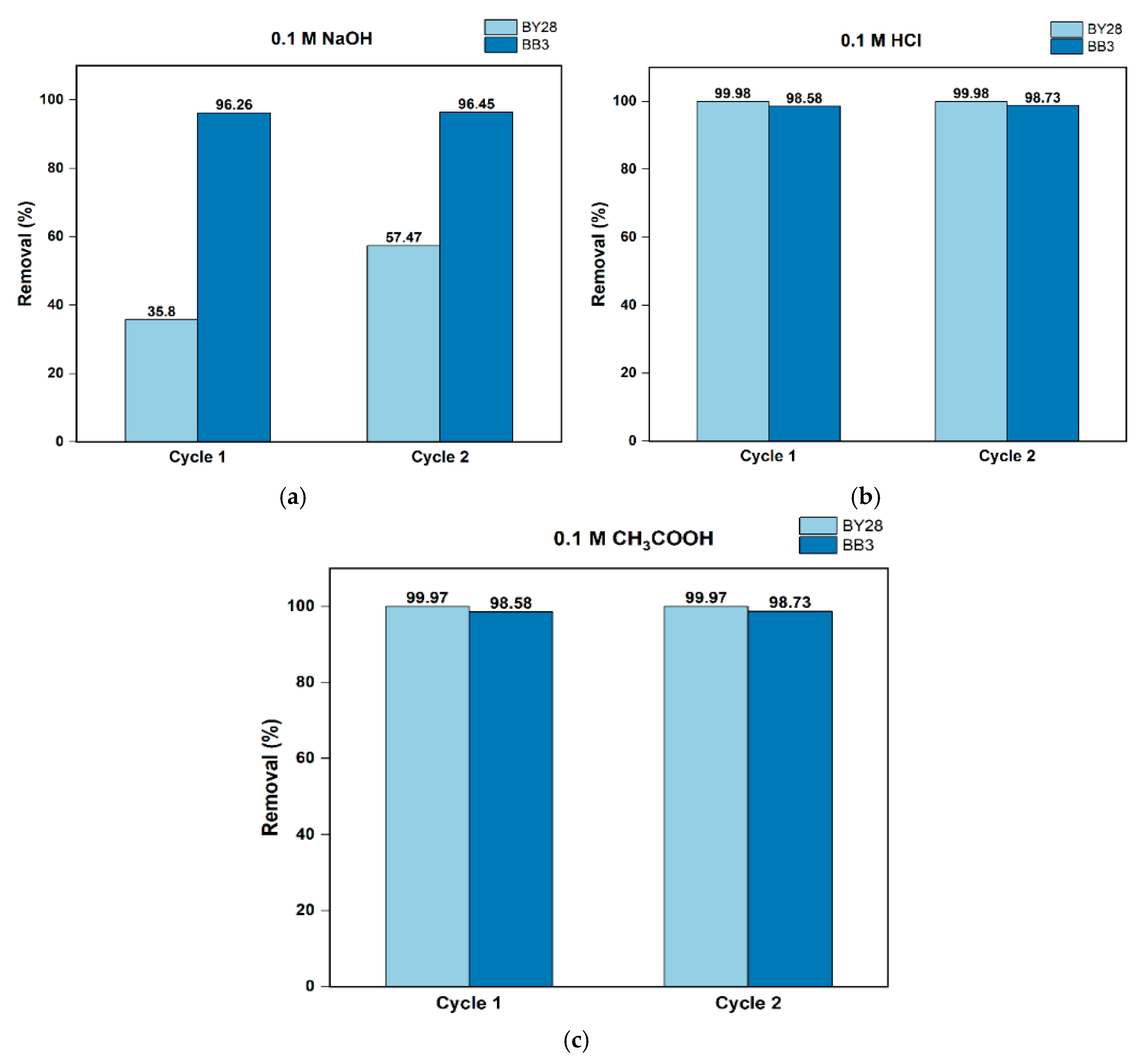
3.11. Regeneration and Reusability of Adsorbent
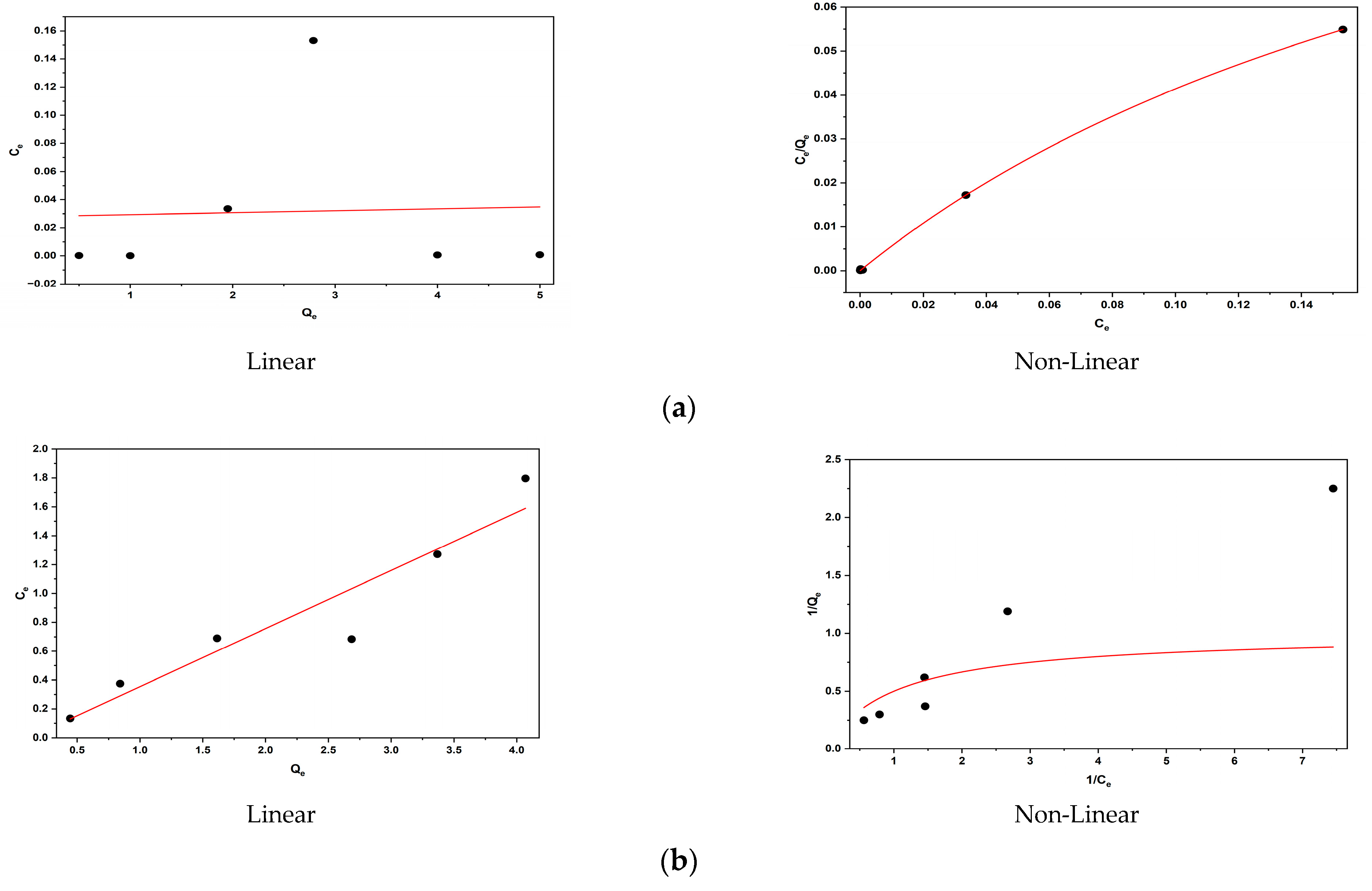
3.12. Application of Adsorption Isotherms
3.13. Comparative Research of Adsorption Capacity for BY28 and BB3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, I. Water Treatment by Adsorption Columns: Evaluation at Ground Level. Sep. Purif. Rev. 2013, 43, 175–205. [Google Scholar] [CrossRef]
- Gürses, A.; Güneş, K.; Şahin, E.; Açıkyıldız, M. Investigation of the removal kinetics, thermodynamics and adsorption mechanism of anionic textile dye, Remazol Red RB, with powder pumice, a sustainable adsorbent from waste water. Front. Chem. 2023, 11, 1156577. [Google Scholar] [CrossRef] [PubMed]
- Mushahary, N.; Sarkar, A.; Das, B.; Rokhum, S.L.; Basumatary, S. A facile and green synthesis of corn cob-based graphene oxide and its modification with corn cob-K2CO3 for efficient removal of methylene blue dye: Adsorption mechanism, isotherm, and kinetic studies. J. Indian Chem. Soc. 2024, 101, 101409. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Wen, Y.; Xie, Z.; Xue, S.; Zhao, M.; Liu, T.; Shi, W. Acylhydrazone-functionalized starch for efficient removal of hazardous dyes, heavy metal ion, and sulfides from wastewater: Adsorption behavior and mechanism analysis. Int. J. Biol. Macromol. 2024, 279, 135461. [Google Scholar] [CrossRef]
- Ahmed, S.; Ferdous, Z.; Zohra, F.T. Prospective application of eco-friendly banana peel reduced graphene oxide (BRGO) for aqueous Cr (VI) and acid dye adsorption: A waste utilization approach. Bioresour. Technol. Rep. 2024, 27, 101936. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid. Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Abbas, A.H.; Aljaberi, F.Y. Utilization of Agricultural Waste as Low-Cost Adsorbents in Dye Wastewater Treatment: Kinetic and Thermodynamic Study. IOP Conf. Ser. Earth Environ. Sci. 2023, 1215, 012055. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Tecirli, E.Ş.; Akgün, K.; Çağlak, A.; Erkan, H.S.; Engi, G.O. Treatment of textile wastewater in combined granular activated carbonmembrane bioreactor (GAC-MBR). Sigma J. Eng. Nat. Sci. 2024, 42, 1490–1499. [Google Scholar] [CrossRef]
- Ullah, B.; Alam, S.; Shah, L.A.; Zahoor, M.; Umar, M.N.; Ullah, R.; Ali, E.A. Synthesis, characterization and adsorption studies of hydroxy ethyl cellulose grafted polyacrylic acid hydrogels; using basic yellow-28 as model dye. Desalination Water Treat. 2024, 318, 100360. [Google Scholar] [CrossRef]
- Mahmoud, H.R.; Ibrahim, S.M.; El-Molla, S.A. Textile dye removal from aqueous solutions using cheap MgO nanomaterials: Adsorption kinetics, isotherm studies and thermodynamics. Adv. Powder Technol. 2016, 27, 223–231. [Google Scholar] [CrossRef]
- Li, L.; Ye, M.; Gan, X.; Xiao, T.; Zhu, Z. Development of membrane separation technology and membrane-based biore-actor in wastewater treatment: Conventional membrane and dynamic membrane. Desalination Water Treat. 2023, 304, 36–46. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Mamba, G.; Mamba, B.B. A facile synthesis approach for GO-ZnO/PES ultrafiltration mixed matrix photocatalytic membranes for dye removal in water: Leveraging the synergy between photocatalysis and membrane filtration. J. Environ. Chem. Eng. 2023, 11, 110065. [Google Scholar] [CrossRef]
- Mousa, H.M.; Sayed, M.M.; Mohamed, I.M.A.; El-Sadek, M.S.A.; Nasr, E.A.; Mohamed, M.A.; Taha, M. Engineering of Multifunctional Nanocomposite Membranes for Wastewater Treatment: Oil/Water Separation and Dye Degradation. Membranes 2023, 13, 810. [Google Scholar] [CrossRef]
- Habib, N.R.; Taddesse, A.M.; Sainz, R.; Sánchez-Sánchez, M.; Diaz, I. Copper-loaded sustainable MOFs as photocatalysts for dye removal. J. Photochem. Photobiol. A Chem. 2024, 456, 115842. [Google Scholar] [CrossRef]
- Dang, T.; Mai, T.; Truong, M.; Dao, L.; Nguyen, T. Optimization of the Photochemical Degradation of Textile Dye Industrial Wastewaters. ASEAN J. Sci. Technol. Dev. 2016, 33, 10–17. [Google Scholar] [CrossRef]
- Das, M.; Ghatak, A.; Ray, P.G.; Stachewicz, U. Advancements in ZnO-based photocatalysts for effective rhodamine dye removal from water. Sustain. Mater. Technol. 2024, 42, e01138. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Aggour, Y.A.; Elaassar, M.R.; Beghet, G.E.; Atta, R.R. Development of nano-crosslinked polyacrylonitrile ions exchanger particles for dye removal: Kinetic, isotherm, and thermodynamic studies. Desalination Water Treat. 2020, 175, 293–303. [Google Scholar] [CrossRef]
- Türk, F.N.; Çiftçi, H.; Arslanoğlu, H. Removal of Basic Yellow 51 Dye by Using Ion Exchange Resin Obtained by Modification of Byproduct Sugar Beet Pulp. Sugar Tech 2022, 25, 569–579. [Google Scholar] [CrossRef]
- Greluk, M.; Hubicki, Z. Efficient removal of Acid Orange 7 dye from water using the strongly basic anion exchange resin Amberlite IRA-958. Desalination 2011, 278, 219–226. [Google Scholar] [CrossRef]
- Abbas, M.J.; Mohamed, R.; Al-Sahari, M.; Al-Gheethi, A.; Daud, A.M.M. Optimizing FeCl3 in coagulation-flocculation treatment of dye wastes. Songklanakarin J. Sci. Technol. 2021, 43, 1094–1102. [Google Scholar]
- Muniasamy, S.K.; Alobaid, A.A.; Warad, I.; Ravindiran, G. Removal of Brilliant Green dye in aqueous solution using synthetic coagulation and flocculation technique. Desalination Water Treat. 2023, 314, 231–240. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Adimule, V.M.; Jayaprakash, G.K.; Pandith, A. Electrochemical oxidation of methylene blue dye in wastewater using mechanically alloyed high entropy alloy modified carbon paste electrode using cyclic voltammetry. Mater. Res. Express 2023, 10, 054003. [Google Scholar] [CrossRef]
- Shalapy, A.E.; Molouk, A.F.S.; Awad, F.S.; Khalifa, M.E.; Abdallah, A.B. Flexible imprinted electrochemical sensing platform for ultra-trace detection of indigo carmine dye in coloured food additive for candy and ice cream. Inorg. Chem. Commun. 2025, 174, 113951. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Arslanoğlu, H.; Keskin, Z.S.; Mehmeti, V.; El Messaoudi, N. Biosorption of rhodamine B and sunset yellow dyes on cross-linked chitosan-alginate biocomposite beads: Experimental and theoretical studies. Int. J. Biol. Macromol. 2025, 298, 139264. [Google Scholar] [CrossRef]
- Adeleke, A.O.; Omar, R.; Katibi, K.K.; Dele-Afolabi, T.T.; Ahmad, A.; Quazim, J.O.; Amusa, A.A.; Alshammari, M.B. Process optimization of superior biosorption capacity of biogenic oyster shells nanoparticles for Congo red and Bromothymol blue dyes removal from aqueous solution: Response surface methodology, equilibrium isotherm, kinetic, and reusability studies. Alex. Eng. J. 2024, 92, 11–23. [Google Scholar] [CrossRef]
- Venkatesan, A.; Srividhya, B.; Alajmi, A.; Sadeq, A.M.; Chava, R.K.; Habila, M.A.; Kumar, D.S.; Guganathan, L.; Ragupathy, S. High adsorption capacities of rhodamine B dye by activated carbon synthesized from cotton stalks agricultural waste by chemical activation. Ceram. Int. 2025, 51, 13345–13354. [Google Scholar] [CrossRef]
- Tong, J.; Li, G.; Wei, J.; Li, C. Functionalized spent coffee grounds by citric acid and polydopamine for eliminating cationic dyes from wastewater: Investigation on recyclability, salt resistance and adsorption mechanism. Ind. Crops Prod. 2024, 223, 120198. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Anjum, M.N.; Ali, A.; Ijaz, R.; Iqbal, S.; Al-Salahi, R.; Abuelizz, H.A.; Delattre, C. Water pollution remediation: Synthesis, characterization and adsorption studies of poly(o-anisidine)/MWCNTs nanocomposite for the elimination of direct yellow 26 dye. Kuwait J. Sci. 2025, 52, 100368. [Google Scholar] [CrossRef]
- Diksha, K.; Bhavanam, A.; Giribabu, D. Lignin and black liquor based composite hydrogels for the enhanced adsorption of malachite green dye from aqueous solutions: Kinetics, rheology and isotherm studies. Int. J. Biol. Macromol. 2025, 296, 139613. [Google Scholar] [CrossRef]
- Ali, I. The quest for active carbon adsorbent substitutes: Inexpensive adsorbents for toxic metal ions removal from wastewater. Sep. Purif. Rev. 2010, 39, 95–171. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Budak, T.B. The Investigation Of Basic Yellow 28 Adsorption by Using Different Carbon Material. Eur. J. Res. Dev. 2022, 2, 106–114. [Google Scholar] [CrossRef]
- Danazumi, K.; Sani, A.M.; Usman, A.K.; Uba, I.M.; Shehu, U. THERMODYNAMIC ADSORPTION STUDIES OF ACTIVATED CARBON FROM COCONUT SHELL USING METHYL ORANGE DYE. Fudma J. Sci. 2024, 8, 196–203. [Google Scholar] [CrossRef]
- Bakhsh, E.M.; Bilal, M.; Ali, M.; Ali, J.; Wahab, A.; Akhtar, K.; Fagieh, T.M.; Danish, E.Y.; Asiri, A.M.; Khan, S.B. Synthesis of Activated Carbon from Trachycarpus fortunei Seeds for the Removal of Cationic and Anionic Dyes. Materials 2022, 15, 1986. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: Optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef]
- El-Shafey, S.; Salama, A.; Abouzeid, R. Nanocomposite of cellulose nanofibers/alumina for effective adsorption of brilliant blue and ethyl orange dyes: Equilibrium, kinetic and mechanism studies. Desalination Water Treat. 2024, 321, 100875. [Google Scholar] [CrossRef]
- Sibhat, W.T.; Ayele, H.S.; Atlabachew, M.; Mohammed, K.S.; Aragaw, B.A.; Abebaw, B.; Ayele, D.T. Effect of Ethiopian kaolin treatment on the performance of adsorptive removal of methylene blue dye. Results Chem. 2025, 13, 102027. [Google Scholar] [CrossRef]
- El-Habacha, M.; Lagdali, S.; Dabagh, A.; Mahmoudy, G.; Assouani, A.; Benjelloun, M.; Miyah, Y.; Iaich, S.; Chiban, M.; Zerbet, M. High efficiency of treated-phengite clay by sodium hydroxide for the Congo red dye adsorption: Optimization, cost estimation, and mechanism study. Environ. Res. 2024, 259, 119542. [Google Scholar] [CrossRef]
- Ritter, M.T.; Lobo-Recio, M.Á.; Padilla, I.; Nagel-Hassemer, M.E.; Romero, M.; López-Delgado, A. Adsorption of Safranine-T dye using a waste-based zeolite: Optimization, kinetic and isothermal study. J. Ind. Eng. Chem. 2024, 136, 177–187. [Google Scholar] [CrossRef]
- Yaqub, A.; Zahid, M.; Nisa, M.U.; Iqbal, T.; Shah, K.H.; Shah, N.S.; Draz, M.U. Sustainable removal of methylene blue dye from textile effluents by magnetized Tea waste and Peanut shells. Chem. Eng. Sci. 2024, 299, 120498. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, J.; Cao, P.; Xu, R.; Wang, W.; Ma, C.; Guo, Y.; Chen, Y. A novel strategy for preparing high-performance, low-cost biomass charcoal for dye adsorption using aquatic agricultural waste lotus stem fibers. Ind. Crops Prod. 2024, 214, 118594. [Google Scholar] [CrossRef]
- Vakili, A.; Zinatizadeh, A.; Rahimi, Z.; Zinadini, S.; Mohammadi, P.; Azizi, S.; Karami, A.; Abdulgader, M. The impact of activation temperature and time on the characteristics and performance of agricultural waste-based activated carbons for removing dye and residual COD from wastewater. J. Clean. Prod. 2022, 382, 134899. [Google Scholar] [CrossRef]
- Lim, L.B.L.; Priyantha, N.; Latip, S.A.A.; Lu, Y.C.; Mahadi, A.H. Converting Hylocereus undatus (white dragon fruit) peel waste into a useful potential adsorbent for the removal of toxic Congo red dye. Desalination Water Treat. 2020, 185, 307–317. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Ssouni, S.; Iaich, S.; El-Habacha, M.; Lagdali, S.; Saka, K.; Iboustaten, E.M.; Addi, A.A.; Lairini, S.; et al. Capparis spinosa L waste activated carbon as an efficient adsorbent for crystal violet toxic dye removal: Modeling, optimization by experimental design, and ecological analysis. Chin. J. Chem. Eng. 2024, 71, 283–302. [Google Scholar] [CrossRef]
- Siraorarnroj, S.; Kaewtrakulchai, N.; Fuji, M.; Eiad-Ua, A. High performance nanoporous carbon from mulberry leaves (Morus alba L.) residues via microwave treatment assisted hydrothermal-carbonization for methyl orange adsorption: Kinetic, equilibrium and thermodynamic studies. Materialia 2022, 21, 101288. [Google Scholar] [CrossRef]
- Poguberović, S.S.; Krčmar, D.M.; Maletić, S.P.; Kónya, Z.; Pilipović, D.D.T.; Kerkez, D.V.; Rončević, S.D. Removal of As(III) and Cr(VI) from aqueous solutions using ‘green’ zero-valent iron nanoparticles produced by oak, mulberry and cherry leaf extracts. Ecol. Eng. 2016, 90, 42–49. [Google Scholar] [CrossRef]
- Mangood, A.H.; Abdelfattah, I.; El-Saied, F.A.; Mansour, M.Z. Removal of heavy metals from polluted aqueous media using berry leaf. Int. J. Environ. Anal. Chem. 2021, 103, 4450–4466. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, R.; Yan, X.; Jia, C.; Jiang, S.; Long, T. Mulberry for environmental protection. Pak. J. Bot. 2017, 49, 781–788. [Google Scholar]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef] [PubMed]
- Núñez, S.; Millán-Laleona, A.; Cano-Lou, J.; Corella, A.; Moliner, C.; Cásedas, G.; Maggi, F.; López, V.; Gómez-Rincón, C. Black mulberry (Morus nigra L.) prevents deleterious effects of excess glucose in obese C. elegans decreasing lipofuscin accumulation and ROS production. Heliyon 2025, 11, e41898. [Google Scholar] [CrossRef]
- Chen, S.; Xi, M.; Gao, F.; Li, M.; Dong, T.; Geng, Z.; Liu, C.; Huang, F.; Wang, J.; Li, X.; et al. Evaluation of mulberry leaves’ hypoglycemic properties and hypoglycemic mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef]
- Vukmirović, S.; Ilić, V.; Tadić, V.; Čapo, I.; Pavlović, N.; Tomas, A.; Kusturica, M.P.; Tomić, N.; Maksimović, S.; Stilinović, N. Comprehensive Analysis of Antioxidant and Hepatoprotective Properties of Morus nigra L. Antioxidants 2023, 12, 382. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Wesolowski, M.; Viapiana, A. Morus alba L. and Morus nigra L. Leaves as a Promising Food Source of Phenolic Compounds with Antioxidant Activity. Plant Foods Hum. Nutr. 2021, 76, 458–465. [Google Scholar] [CrossRef]
- Cui, X.; Yang, Y.; Zhang, M.; Liu, S.; Wang, H.; Jiao, F.; Bao, L.; Lin, Z.; Wei, X.; Qian, W.; et al. Transcriptomics and metabolomics analysis reveal the anti-oxidation and immune boosting effects of mulberry leaves in growing mutton sheep. Front. Immunol. 2023, 13, 1088850. [Google Scholar] [CrossRef]
- Sakar, E.; Ercisli, S.; Marc, R.A.; Gulen, H.; Assouguem, A.; Ullah, R.; Shahat, A.A.; Bari, A.; Farah, A. Black mulberry (Morus nigra L.) fruits: As a medicinal plant rich in human health-promoting compounds. Open Chem. 2023, 21, 20220323. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, K.L.; Zhang, J.; Li, Y.; Zhang, H.; Wang, Y. Purification of Flavonoids from Mulberry Leaves via High-Speed Counter-Current Chromatography. Processes 2019, 7, 91. [Google Scholar] [CrossRef]
- Duran, C.; Ozeken, S.T.; Camoglu, A.Y.; Ozdes, D. Enhancement of adsorptive removal efficiency of an anionic dye from aqueous solutions using carboxylic acid-modified mulberry leaves: Artificial neural network modeling, isotherm, and kinetics evaluation. J. Water Health 2023, 21, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, T.A.; Alene, A.N. A comparative study of acidic, basic, and reactive dyes adsorption from aqueous solution onto kaolin adsorbent: Effect of operating parameters, isotherms, kinetics, and thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; El-Sayyad, G.S.; Saad, F.A.; Shah, R.K.; Abdelrahman, E.A. Efficient removal of basic yellow 28 dye from water using facilely synthesized ZnO and Mg3B2O6 nanostructures. Sci. Rep. 2024, 14, 26181. [Google Scholar] [CrossRef] [PubMed]
- Taşar, Ş.; Kaya, F.; Özer, A. Adsorption of CI Basic Blue 3 Dye Molecules from Aqueous Media by Sulfuric Acid-Activated Montmorillonite Mineral. J. Turk. Chem. Soc. Sect. B Chem. Eng. 2017, 1, 1–16. [Google Scholar]
- Wanyonyi, W.C.; Onyari, J.M.; Shiundu, P.M. Adsorption of Congo Red Dye from Aqueous Solutions Using Roots of Eichhornia Crassipes: Kinetic and Equilibrium Studies. Energy Procedia 2014, 50, 862–869. [Google Scholar] [CrossRef]
- Regti, A.; Laamari, M.R.; Stiriba, S.E.; El Haddad, M. Potential use of activated carbon derived from Persea species under alkaline conditions for removing cationic dye from wastewaters. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 24, 10–18. [Google Scholar] [CrossRef]
- Khan, F.A.; Farooqui, M. Removal of methylene blue dye from aqueous solutions onto Morus nigra L. (mulberry tree) leaves powder and its biochar—Equilibrium, kinetic and thermodynamic study. Int. J. Environ. Anal. Chem. 2022, 104, 4364–4383. [Google Scholar] [CrossRef]
- Tunçeli, A.; Ulaş, A.; Acar, O.; Türker, A.R. Adsorption isotherms, kinetic and thermodynamic studies on cadmium and lead ions from water solutions using Amberlyst 15 resin. Turk. J. Chem. 2021, 46, 193–205. [Google Scholar] [CrossRef]
- Amini, M.; Younesi, H.; Bahramifar, N. Biosorption of U(VI) from Aqueous Solution by Chlorella vulgaris: Equilibrium, Kinetic, and Thermodynamic Studies. J. Environ. Eng. 2013, 139, 410–421. [Google Scholar] [CrossRef]
- Zavala-Flores, E.; Flores-López, L.Z.; Alonso-Nuñez, G.; Espinoza-Gómez, H. Removal and adsorption kinetics of methylene blue dye by pristine cotton husk bracts (Gossypium hirsutum L.) from agroindustrial waste. Ind. Crops Prod. 2023, 209, 117947. [Google Scholar] [CrossRef]
- Setiawan, A.; Kusumawati, T.A.; Ramadani, T.A.; Wang, Y.F.; Kusumawardani, A.; Dermawan, D. Composite of activated carbon-hydroxyapatite from corn (Zea mays L.) cob and catfish (Pangasius sp.) bones with H3PO4 activation for methylene blue dye adsorption. Inorg. Chem. Commun. 2024, 167, 112744. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Tsoutsa, E.K.; Katsoyiannis, I.A.; Kyzas, G.Z. Simultaneous removal of anionic and cationic dyes on quaternary mixtures by adsorption onto banana, orange and pomegranate peels. Colloids Surfaces A: Physicochem. Eng. Asp. 2024, 685, 133176. [Google Scholar] [CrossRef]
- Gaur, J.; Vikrant, K.; Kim, K.-H.; Kumar, S.; Pal, M.; Badru, R.; Masand, S.; Momoh, J. Photocatalytic degradation of Congo red dye using zinc oxide nanoparticles prepared using Carica papaya leaf extract. Mater. Today Sustain. 2023, 22, 100339. [Google Scholar] [CrossRef]
- Raji, Y.; Nadi, A.; Mechnou, I.; Saadouni, M.; Cherkaoui, O.; Zyade, S. High adsorption capacities of crystal violet dye by low-cost activated carbon prepared from Moroccan Moringa oleifera wastes: Characterization, adsorption and mechanism study. Diam. Relat. Mater. 2023, 135, 109834. [Google Scholar] [CrossRef]
- Yu, S.; Bo, J.; Fengli, L.; Jiegang, L. Structure and fractal characteristic of micro- and meso-pores in low, middle-rank tectonic deformed coals by CO2 and N2 adsorption. Microporous Mesoporous Mater. 2017, 253, 191–202. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Pan, M.; Shi, Y. Nano-Scale Pore Structure and Its Multi-Fractal Characteristics of Tight Sandstone by N2 Adsorption/Desorption Analyses: A Case Study of Shihezi Formation from the Sulige Gas Filed, Ordos Basin, China. Minerals 2020, 10, 377. [Google Scholar] [CrossRef]
- Neusatz Guilhen, S.; Watanabe, T.; Tieko Silva, T.; Rovani, S.; Takehiro Marumo, J.; Alberto Soares Tenório, J.; Mašek, O.; Goulart de Araujo, L. Role of Point of Zero Charge in the Adsorption of Cationic Textile Dye on Standard Biochars from Aqueous Solutions: Selection Criteria and Performance Assessment. Recent Prog. Mater. 2022, 4, 10. [Google Scholar] [CrossRef]
- Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, M.T. Cationic Dye Adsorption on Hydrochars of Winery and Citrus Juice Industries Residues: Performance, Mechanism, and Thermodynamics. Energies 2020, 13, 4686. [Google Scholar] [CrossRef]
- Chuah, A.L.; Rashid, S.A.; Tumin, N.D.; Chuah, A.L.; Zawani, Z. Adsorption of Copper from Aqueous Solution by Elais Guineensis Kernel Activated Carbon. 2008. Available online: https://www.researchgate.net/publication/49593906 (accessed on 8 August 2025).
- Ileri, B. Sono-Assisted Adsorption of Acid Violet 7 and Basic Violet 10 Dyes from Aqueous Solutions: Evaluation of Isotherm and Kinetic Parameters. Available online: https://www.eeer.org/journal/view.php?doi=10.4491/eer.2020.287 (accessed on 7 August 2025).
- Ouahabi, I.E.; Slimani, R.; Hachoumi, I.; Anouar, F.; Taoufik, N.; Elmchaouri, A.; Lazar, S. Adsorption of a Cationic Dye (Yellow Basic 28) onto the Calcined Mussel Shells: Kinetics, Isotherm and Thermodynamic Parameters. 2018. Available online: www.medjchem.com (accessed on 8 August 2025).
- Mutunga, M.F.; Wanyonyi, W.C.; Ongera, G. Utilization of Macadamia seed husks as a low-cost sorbent for removing cationic dye (basic blue 3 dye) from aqueous solution. Environ. Chem. Ecotoxicol. 2020, 2, 194–200. [Google Scholar] [CrossRef]
- Budak, T.B. Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent. Molecules 2023, 28, 6639. [Google Scholar] [CrossRef]
- Bencheqroun, Z.; El Mrabet, I.; Nawdali, M.; Benali, M.; Zaitan, H. Adsorption removal of cationic dyes from aqueous solutions by raw and chemically activated cedar sawdust. Desalination Water Treat. 2021, 240, 177–190. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.U.H.A.; Bilal, S.; Rahman, G. Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Tan, Y.P.; Abdullah, A.H.; Ong, S.T. Removal of basic blue 3 and reactive orange 16 by adsorption onto quartenized sugar cane bagasse. Malays. J. Anal. Sci. 2009, 13, 185–193. Available online: http://www.ukm.my/mjas/v13_n2/Wong.pdf (accessed on 22 March 2023).
- Cao, H.; Zhang, Q.; Zhang, C. Substituent effect on hole mobility of conjugated polymers in photovoltaic cells. Asian J. Chem. 2013, 25, 2177–2181. [Google Scholar] [CrossRef]
- Ong, S.T.; Tan, S.Y.; Khoo, E.C.; Lee, S.L.; Ha, S.T. Equilibrium studies for Basic blue 3 adsorption onto durian peel (Durio zibethinus Murray). Desalination Water Treat. 2012, 45, 161–169. [Google Scholar] [CrossRef]










| Concentration (mg/L) | Adsorbent Dosage (g) | Agitation Speed (rpm) | Time (min) | Temperature (°C) | pH | |
|---|---|---|---|---|---|---|
| BY28 | 10 | 1.5 | 180 | 15 | 30 | 7 |
| BB3 | 30 | 1.5 | 100 | 45 | 40 | 10 |
| BY28–River water | Y = 0.035X + 0.007 | R2 = 0.997 |
| BY28–Tap water | Y = 0.022X + 0.051 | R2 = 0.995 |
| BY28–Distilled water | Y = 0.014X + 0.042 | R2 = 0.999 |
| BB3–River water | Y = 0.042X + 0.071 | R2 = 0.999 |
| BB3–Tap water | Y = 0.031X + 0.026 | R2 = 0.998 |
| BB3–Distilled water | Y = 0.023X + 0.056 | R2 = 0.997 |
| Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|
| KL (mg/L) | qmax (mg/g) | R2 | Kf (mg/L) | 1/n | R2 | |
| Linear Isotherm | ||||||
| BY28 | 0.050 | 714.29 | 0.002 | 3.118 | 0.034 | 0.027 |
| BB3 | −8.235 | 2.483 | 0.903 | 2.696 | 0.871 | 0.951 |
| Non-linear Isotherm | ||||||
| BY28 | 4.099 | 0.142 | 0.999 | 3.131 | 0.034 | 0.028 |
| BB3 | 0.062 | 7.192 | 0.973 | 1 | 1 | −0.422 |
| Adsorbent | Adsorption Capacities (mg g−1) | Adsorption Model | References | |
|---|---|---|---|---|
| BY28 | BB3 | |||
| Mulberry leaves (Morus nigra L.) | 0.14 | 10.30 | Langmuir | Present study |
| Ethiopian kaolinite clay | 5.71 | - | Langmuir | [60] |
| Macadamia seed husks | - | 1.40 | Langmuir–Freundlich | [80] |
| Silybum marianum (SLM) Stem-Natural | 36.54 | 13.96 | Langmuir | [81] |
| Raw cedar sawdust | - | 47.62 | Langmuir | [82] |
| Mussel shells | 93.45 | - | Freundlich | [79] |
| Polyaniline/Magnetite (Fe3O4) Composites | - | 78.13 | Langmuir | [83] |
| Quarternized sugarcane bagasse | - | 37.59 | Freundlich | [84] |
| Activated carbon | 769.23 | - | Langmuir | [85] |
| Natural Durian Peel | - | 49.50 | Langmuir–Freundlich | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardieka, A.M.; Börklü Budak, T. Investigation of Removing Basic Yellow 28 and Basic Blue 3 Dyes from Water Using Mulberry Leaves (Morus nigra L.) and Assessment of Ultrasonic Effects. Molecules 2025, 30, 3539. https://doi.org/10.3390/molecules30173539
Hardieka AM, Börklü Budak T. Investigation of Removing Basic Yellow 28 and Basic Blue 3 Dyes from Water Using Mulberry Leaves (Morus nigra L.) and Assessment of Ultrasonic Effects. Molecules. 2025; 30(17):3539. https://doi.org/10.3390/molecules30173539
Chicago/Turabian StyleHardieka, Adella Myori, and Türkan Börklü Budak. 2025. "Investigation of Removing Basic Yellow 28 and Basic Blue 3 Dyes from Water Using Mulberry Leaves (Morus nigra L.) and Assessment of Ultrasonic Effects" Molecules 30, no. 17: 3539. https://doi.org/10.3390/molecules30173539
APA StyleHardieka, A. M., & Börklü Budak, T. (2025). Investigation of Removing Basic Yellow 28 and Basic Blue 3 Dyes from Water Using Mulberry Leaves (Morus nigra L.) and Assessment of Ultrasonic Effects. Molecules, 30(17), 3539. https://doi.org/10.3390/molecules30173539







