Unraveling the Molecular Composition and Reactivity Differentiation of Algae- and Macrophyte-Derived Dissolved Organic Matter in Plateau Lakes: Insights from Optical Properties and High Resolution Mass Spectrometry Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Distribution of Macrophytes and Algae
2.2. Spectral Properties of Macrophytes and Algae
2.2.1. UV-Vis Absorption Spectral Parameters Analysis
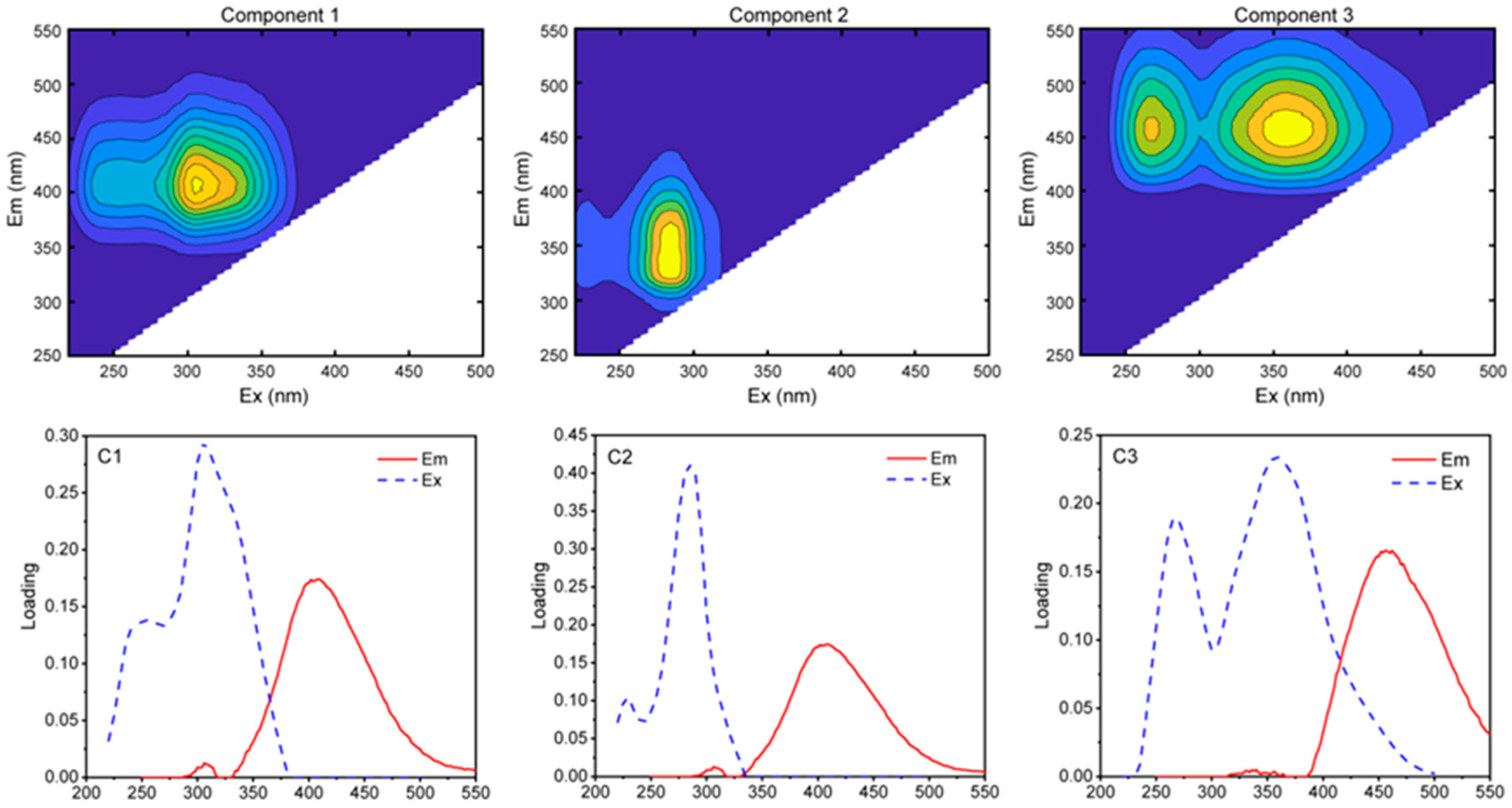
2.2.2. Three-Dimensional EEMs Fluorescence Spectrum Analysis
2.3. FT-ICR MS and Orbitrap MS Analysis of Bulk-DOM
2.3.1. Mass Spectral Parameters Analysis of Bulk-DOM
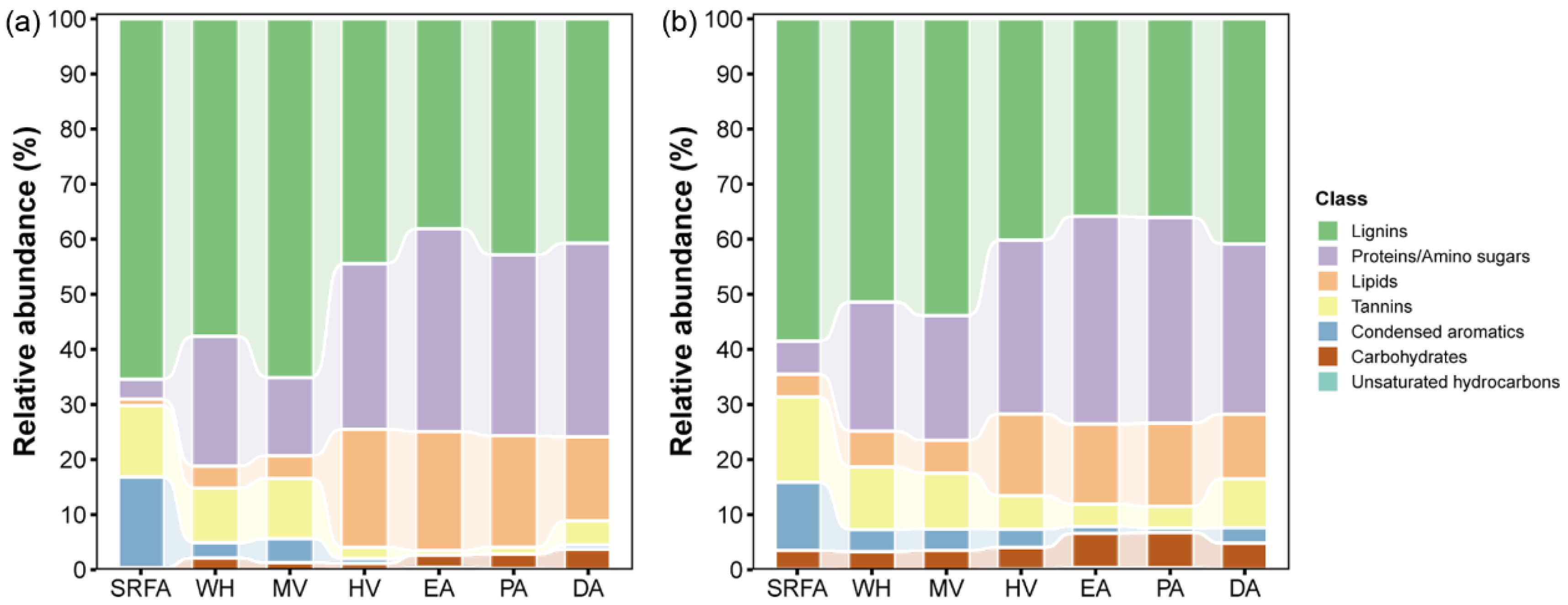
2.3.2. Molecular Composition of Bulk-DOM
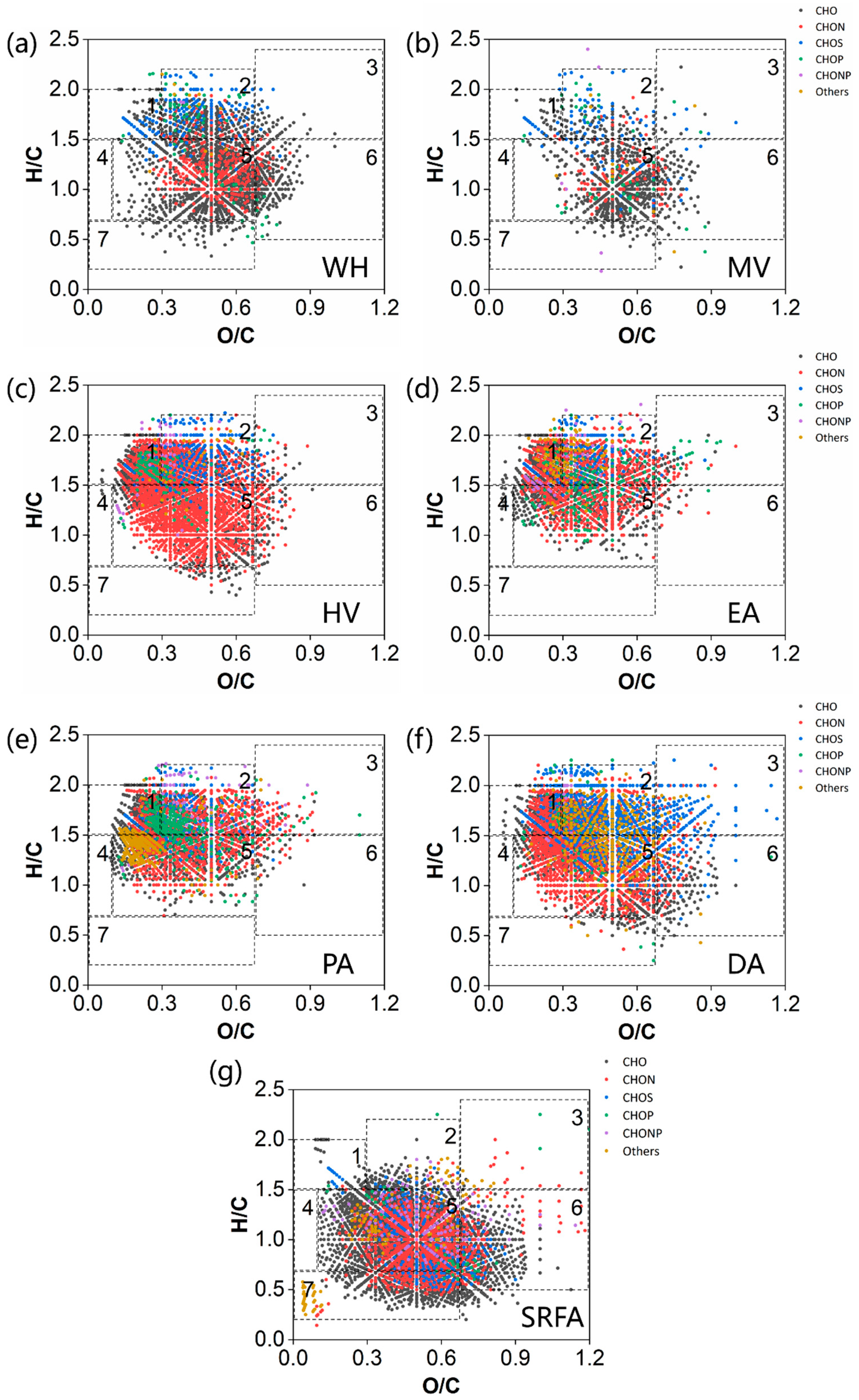
2.3.3. Van Krevelen Plots of Bulk-DOM in Macrophytes and Algae
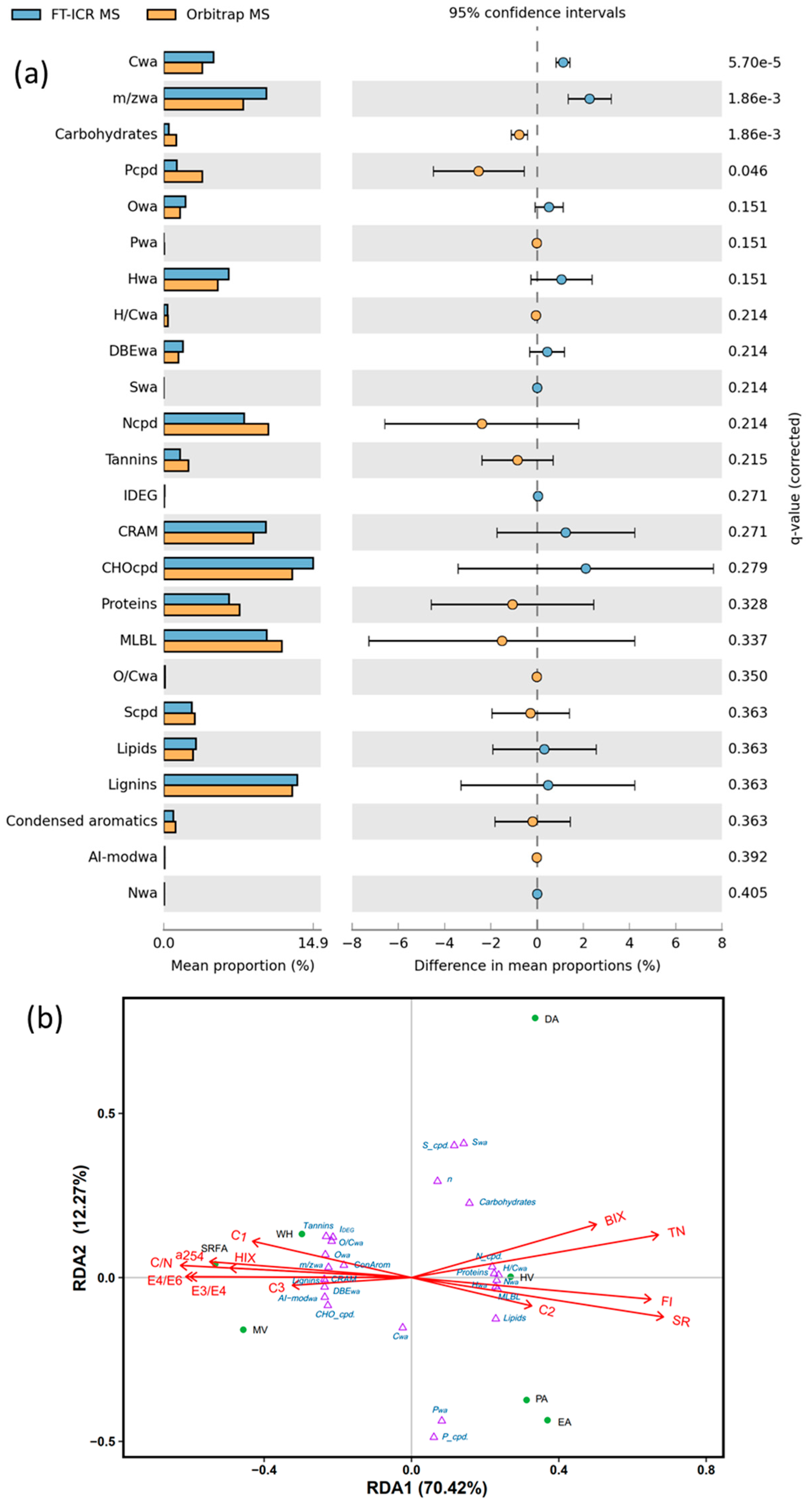
2.4. Comparative Analysis Between FT-ICR MS and Orbitrap MS
2.5. Characterization of LMW-DOM by Orbitrap MS Analysis
2.6. Environmental Implications
3. Materials and Methods
3.1. Sample Collection and Preparation
3.2. Organic Element Analysis
3.3. DOM Extraction
3.4. Optical Spectroscopy Analysis
3.5. Mass Spectrometry Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, C.; Yi, Y.; He, D.; Cai, R.; Chen, C.; Shi, Q. Molecular composition of dissolved organic matter across diverse ecosystems: Preliminary implications for biogeochemical cycling. J. Environ. Manag. 2023, 344, 118559. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Kothawala, D.N.; Dittmar, T.; Tranvik, L.J. Persistence of dissolved organic matter in lakes related to its molecular characteristics. Nat. Geosci. 2015, 8, 454–457. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, X.; Zhi, G.; Zhang, Y.; Sun, X.; Jiao, L.; Deng, W.; Zhou, H.; Shao, Z. Responses of dissolved organic matter (DOM) characteristics in eutrophic lake to water diversion from external watershed. Environ. Pollut. 2022, 312, 119992. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Li, Y.; Zhang, J.; Ma, C.; Weng, N.; Gao, X.; Wu, F.; Huo, S. Strong associations between dissolved organic matter and microbial communities in the sediments of Qinghai-Tibetan Plateau lakes depend on salinity. Sci. Total Environ. 2024, 926, 171857. [Google Scholar] [CrossRef]
- Liu, H.; Tu, Y.-n.; Lei, Y.; Zhou, D.; Zhao, Q.; Li, Y.; Pan, W. Photochemistry of plateau lake-derived dissolved organic matter: Reactive species generation and effects on 17β-estradiol photodegradation. J. Hazard. Mater. 2024, 473, 134615. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, T.; Chen, W.; Zheng, G.; Shum, C.; Yang, K.; Piao, S.; Sheng, Y.; Yi, S.; Li, J. Regional differences of lake evolution across China during 1960s–2015 and its natural and anthropogenic causes. Remote Sens. Environ. 2019, 221, 386–404. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Guo, M.; Li, F.; Yang, K.; Liu, X. Dissolved organic matters with low molecular weight fractions exhibit high photochemical potential for reactive oxygen formation. Chemosphere 2022, 305, 135542. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; He, Z.Q.; Tang, Z.; Liu, L.Z.; Hou, J.W.; Li, T.T.; Zhang, Y.H.; Shi, Q.; Giesy, J.P.; Wu, F.C. Linking the molecular composition of autochthonous dissolved organic matter to source identification for freshwater lake ecosystems by combination of optical spectroscopy and FT-ICR-MS analysis. Sci. Total Environ. 2020, 703, 134764. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Zhang, R.Y.; Huang, G.P.; Yuan, H.J.; Wang, L.Y.; Xu, S.X. Characterization of Low-Molecular-Weight Dissolved Organic Matter Using Optional Dialysis and Orbitrap Mass Spectrometry. Molecules 2024, 29, 3370. [Google Scholar] [CrossRef]
- Herzsprung, P.; Hertkorn, N.; Von Tümpling, W.; Harir, M.; Friese, K.; Schmitt-Kopplin, P. Molecular formula assignment for dissolved organic matter (DOM) using high-field FT-ICR-MS: Chemical perspective and validation of sulphur-rich organic components (CHOS) in pit lake samples. Anal. Bioanal. Chem. 2016, 408, 2461–2469. [Google Scholar] [CrossRef]
- Brailsford, F.L.; Glanville, H.C.; Marshall, M.R.; Yates, C.A.; Owen, A.T.; Golyshin, P.N.; Johnes, P.J.; Jones, D.L. Land cover and nutrient enrichment regulates low-molecular weight dissolved organic matter turnover in freshwater ecosystems. Limnol. Oceanogr. 2021, 66, 2979–2987. [Google Scholar] [CrossRef]
- Wen, Z.; Shang, Y.; Song, K.; Liu, G.; Hou, J.; Lyu, L.; Tao, H.; Li, S.; He, C.; Shi, Q. Composition of dissolved organic matter (DOM) in lakes responds to the trophic state and phytoplankton community succession. Water Res. 2022, 224, 119073. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Runyu, Z.; Liying, W.; Zhi, Z.; Haijun, Y. Multidimensional features and sources apportionment of dissolved organic matter from Endmember Samples in the Karst Area. Acta Sci. Circumstantiae 2022, 42, 425–437. [Google Scholar]
- Guo, M.; Yu, M.; Wang, X.; Xiao, N.; Huguet, A.; Zhang, Y.; Liu, G. Deciphering the link between particulate organic matter molecular composition and lake eutrophication by FT-ICR MS analysis. Water Res. 2024, 272, 122936. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gao, A.; He, C.; Shi, Q.; Hou, Z.; Zhao, H.Z. Using ESI FT-ICR MS to Characterize Dissolved Organic Matter in Salt Lakes with Different Salinity. Environ. Sci. Technol. 2020, 54, 12929–12937. [Google Scholar] [CrossRef]
- Pan, B.; Liu, S.; Wang, Y.; Li, D.; Li, M. FT-ICR-MS combined with fluorescent spectroscopy reveals the driving mechanism of the spatial variation in molecular composition of DOM in 22 plateau lakes. Environ. Res. 2023, 232, 116272. [Google Scholar] [CrossRef]
- Remucal, C.K.; Cory, R.M.; Sander, M.; McNeill, K. Low Molecular Weight Components in an Aquatic Humic Substance As Characterized by Membrane Dialysis and Orbitrap Mass Spectrometry. Environ. Sci. Technol. 2012, 46, 9350–9359. [Google Scholar] [CrossRef]
- He, W.; Bai, Z.L.; Li, Y.L.; Liu, W.X.; He, Q.S.; Yang, C.; Xu, F.L. Advances in the characteristics analysis and source identification of the dissolved organic matter. Acta Sci. Circumstantiae 2016, 36, 359–372. [Google Scholar]
- Justine, M.F.; Yang, W.Q.; Wu, F.Z.; Tan, B.; Khan, M.N.; Li, Z.J. Dissolved organic matter in soils varies across a chronosequence of Pinus massoniana plantations. Ecosphere 2017, 8, e01764. [Google Scholar] [CrossRef]
- Li, H.; Miller, T.; Lu, J.; Goel, R. Nitrogen fixation contribution to nitrogen cycling during cyanobacterial blooms in Utah Lake. Chemosphere 2022, 302, 134784. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhao, T.H.; Zhu, Y.R.; Qu, X.X.; He, Z.Q.; Giesy, J.P.; Meng, W. Molecular characterization of macrophyte-derived dissolved organic matters and their implications for lakes. Sci. Total Environ. 2018, 616, 602–613. [Google Scholar] [CrossRef]
- Derrien, M.; Brogi, S.R.; Gonçalves-Araujo, R. Characterization of aquatic organic matter: Assessment, perspectives and research priorities. Water Res. 2019, 163, 114908. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhou, L.; Zhou, Y.Q.; Zhang, L.Q.; Yao, X.L.; Shi, K.; Jeppesen, E.; Yu, Q.; Zhu, W.N. Chromophoric dissolved organic matter in inland waters: Present knowledge and future challenges. Sci. Total Environ. 2021, 759, 143550. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Deng, H.; Du, Y.; Jin, H. Absorption features of chromophoric dissolved organic matter (CDOM) and tracing implication for dissolved organic carbon (DOC) in Changjiang Estuary, China. Biogeosci. Discuss. 2013, 10, 12217–12250. [Google Scholar]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Liu, S.; Hou, J.; Suo, C.; Chen, J.; Liu, X.; Fu, R.; Wu, F. Molecular-level composition of dissolved organic matter in distinct trophic states in Chinese lakes: Implications for eutrophic lake management and the global carbon cycle. Water Res. 2022, 217, 118438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Liu, X.H.; Wang, M.Z.; Qin, B.Q. Compositional differences of chromophoric dissolved organic matter derived from phytoplankton and macrophytes. Org. Geochem. 2013, 55, 26–37. [Google Scholar] [CrossRef]
- Bai, L.; Cao, C.; Wang, C.; Wang, C.; Zhang, H.; Jiang, H. Roles of phytoplankton-and macrophyte-derived dissolved organic matter in sulfamethazine adsorption on goethite. Environ. Pollut. 2017, 230, 87–95. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Guillemette, F.; Podgorski, D.C.; Aiken, G.R.; Butler, K.D.; Spencer, R.G.M. Unifying Concepts Linking Dissolved Organic Matter Composition to Persistence in Aquatic Ecosystems. Environ. Sci. Technol. 2018, 52, 2538–2548. [Google Scholar] [CrossRef]
- Yang, L.; Hur, J. Critical evaluation of spectroscopic indices for organic matter source tracing via end member mixing analysis based on two contrasting sources. Water Res. 2014, 59, 80–89. [Google Scholar] [CrossRef]
- Huguet, A.; Roux-de Balmann, H.; Parlanti, E. Fluorescence spectroscopy applied to the optimisation of a desalting step by electrodialysis for the characterisation of marine organic matter. J. Membr. Sci. 2009, 326, 186–196. [Google Scholar] [CrossRef]
- Hawkes, J.A.; Dittmar, T.; Patriarca, C.; Tranvik, L.; Bergquist, J. Evaluation of the Orbitrap Mass Spectrometer for the Molecular Fingerprinting Analysis of Natural Dissolved Organic Matter. Anal. Chem. 2016, 88, 7698–7704. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.A.; D’Andrilli, J.; Agar, J.N.; Barrow, M.P.; Berg, S.M.; Catalán, N.; Chen, H.; Chu, R.K.; Cole, R.B.; Dittmar, T. An international laboratory comparison of dissolved organic matter composition by high resolution mass spectrometry: Are we getting the same answer? Limnol. Oceanogr. Methods 2020, 18, 235–258. [Google Scholar] [CrossRef]
- Pan, Q.; Zhuo, X.C.; He, C.; Zhang, Y.H.; Shi, Q. Validation and Evaluation of High-Resolution Orbitrap Mass Spectrometry on Molecular Characterization of Dissolved Organic Matter. ACS Omega 2020, 5, 5372–5379. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Roth, V.-N.; Dittmar, T.; Gleixner, G. Molecular signals of heterogeneous terrestrial environments identified in dissolved organic matter: A comparative analysis of orbitrap and ion cyclotron resonance mass spectrometers. Front. Earth Sci. 2018, 6, 138. [Google Scholar] [CrossRef]
- Yuthawong, V.; Kasuga, I.; Kurisu, F.; Furumai, H. Comparison of low molecular weight dissolved organic matter compositions in Lake Inba and Kashima River by Orbitrap mass spectrometry. J. Water Environ. Technol. 2017, 15, 12–21. [Google Scholar] [CrossRef]
- Sapci, Z.; Ustun, E.B. Heavy metal uptakes by Myriophyllum verticillatum from two environmental matrices: The water and the sediment. Int. J. Phytoremediation 2015, 17, 290–297. [Google Scholar] [CrossRef]
- Carniatto, N.; Fugi, R.; Thomaz, S.M.; Cunha, E.R. The invasive submerged macrophyte Hydrilla verticillata as a foraging habitat for small-sized fish. Nat. Conserv. 2014, 12, 30–35. [Google Scholar] [CrossRef]
- Mangal, V.; Stock, N.L.; Guéguen, C. Molecular characterization of phytoplankton dissolved organic matter (DOM) and sulfur components using high resolution Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 1891–1900. [Google Scholar] [CrossRef]
- Suo, C.; Zhao, W.; Liu, S.; Fu, R.; Ren, Y.; Qiu, Y.; Zhang, Y.; He, Z.; Xing, B.; Wu, F. Molecular-level insight into the origin-dependent adsorption fractionation of dissolved organic matter on ferrihydrite in aquatic environment: Implications for carbon sink in eutrophic lakes. Chem. Eng. J. 2024, 494, 152960. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, H.; Hong, Z.; Fu, G.; Zheng, Y.; Li, Z.; Cui, F. Photo-reactivity and photo-transformation of algal dissolved organic matter unraveled by optical spectroscopy and high-resolution mass spectrometry analysis. Environ. Sci. Technol. 2022, 56, 13439–13448. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, C.; Shan, B.Q. Stability of sedimentary organic matter: Insights from molecular and redox analyses. Environ. Sci. Ecotechnol. 2024, 22, 100470. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Du, H.; Xu, H. Comparison in UV-induced photodegradation properties of dissolved organic matters with different origins. Chemosphere 2021, 280, 130633. [Google Scholar] [CrossRef]
- Hertkorn, N.; Frommberger, M.; Witt, M.; Koch, B.P.; Schmitt-Kopplin, P.; Perdue, E.M. Natural organic matter and the event horizon of mass spectrometry. Anal. Chem. 2008, 80, 8908–8919. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Kusakabe, T.; Takaoka, M. Characterization and differentiation of dissolved organic matter in leachate derived from an old Japanese landfill site through Orbitrap mass spectrometry. J. Mater. Cycles Waste Manag. 2024, 26, 2138–2151. [Google Scholar] [CrossRef]
- Patriarca, C.; Bergquist, J.; Sjöberg, P.J.; Tranvik, L.; Hawkes, J.A. Online HPLC-ESI-HRMS method for the analysis and comparison of different dissolved organic matter samples. Environ. Sci. Technol. 2018, 52, 2091–2099. [Google Scholar] [CrossRef]
- Tfaily, M.M.; Chu, R.K.; Tolić, N.; Roscioli, K.M.; Anderton, C.R.; Paša-Tolić, L.; Robinson, E.W.; Hess, N.J. Advanced solvent based methods for molecular characterization of soil organic matter by high-resolution mass spectrometry. Anal. Chem. 2015, 87, 5206–5215. [Google Scholar] [CrossRef]
- Cao, F.; Medeiros, P.M.; Miller, W.L. Optical characterization of dissolved organic matter in the Amazon River plume and the Adjacent Ocean: Examining the relative role of mixing, photochemistry, and microbial alterations. Mar. Chem. 2016, 186, 178–188. [Google Scholar] [CrossRef]
- Wagner, S.; Riedel, T.; Niggemann, J.; Vahatalo, A.V.; Dittmar, T.; Jaffé, R. Linking the molecular signature of heteroatomic dissolved organic matter to watershed characteristics in world rivers. Environ. Sci. Technol. 2015, 49, 13798–13806. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Hu, C.; Chow, A.T.; Karanfil, T. Molecular Alterations of Algal Organic Matter in Oxidation Processes: Implications to the Formation of Disinfection Products. ACS EsT Water 2024, 4, 5890–5901. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, X.; Peng, Y.; Wang, G.; Liu, H.; Jin, Q.; Jia, R.; Ma, J.; Kinouchi, T.; Wang, G. Response of sulfate concentration to eutrophication on spatio-temporal scale in freshwater lakes. Sci. Total Environ. 2024, 953, 176142. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Tfaily, M.M.; Wilmoth, J.L.; Toor, G.S. Molecular characterization of dissolved organic nitrogen and phosphorus in agricultural runoff and surface waters. Water Res. 2022, 222, 118859. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; He, C.; Klaproth, K.; Merder, J.; Li, P.; Qi, Y.; Fu, P.; Li, S.; Dittmar, T.; Shi, Q. Will various interpretation strategies of the same ultrahigh-resolution mass spectrometry data tell different biogeochemical stories? A first assessment based on natural aquatic dissolved organic matter. Limnol. Oceanogr. Methods 2023, 21, 320–333. [Google Scholar] [CrossRef]
- Osborne, D.M.; Podgorski, D.C.; Bronk, D.A.; Roberts, Q.; Sipler, R.E.; Austin, D.; Bays, J.S.; Cooper, W.T. Molecular-level characterization of reactive and refractory dissolved natural organic nitrogen compounds by atmospheric pressure photoionization coupled to Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 851–858. [Google Scholar] [CrossRef]
- Pan, J.; Fu, X.; Wang, C.; Song, N.; Lv, X.; Xu, H. Adsorption and molecular weight fractionation of dissolved organic matters with different origins on colloidal surface. Chemosphere 2020, 261, 127774. [Google Scholar] [CrossRef]
- Bridoux, M.C.; Malandain, H.; Leprince, F.; Progent, F.; Machuron-Mandard, X. Quantitative analysis of phosphoric acid esters in aqueous samples by isotope dilution stir-bar sorptive extraction combined with direct analysis in real time (DART)-Orbitrap mass spectrometry. Anal. Chim. Acta 2015, 869, 1–10. [Google Scholar] [CrossRef]
- De Brabandere, H.; Forsgard, N.; Israelsson, L.; Petterson, J.; Rydin, E.; Waldebäck, M.; Sjöberg, P.J. Screening for organic phosphorus compounds in aquatic sediments by liquid chromatography coupled to ICP-AES and ESI-MS/MS. Anal. Chem. 2008, 80, 6689–6697. [Google Scholar] [CrossRef]
- Phungsai, P.; Kurisu, F.; Kasuga, I.; Furumai, H. Molecular characterization of low molecular weight dissolved organic matter in water reclamation processes using Orbitrap mass spectrometry. Water Res. 2016, 100, 526–536. [Google Scholar] [CrossRef]
- Suo, C.; Zhao, W.; Liu, S.; Ren, Y.; Zhang, Y.; Qiu, Y.; Wu, F. Molecular insight into algae-derived dissolved organic matters via Fourier-transform ion cyclotron resonance mass spectrometry: Effects of pretreatment methods and electrospray ionization modes. J. Hazard. Mater. 2024, 480, 136220. [Google Scholar] [CrossRef]
- Cooper, W.T.; Llewelyn, J.M.; Bennett, G.L.; Salters, V.J. Mass spectrometry of natural organic phosphorus. Talanta 2005, 66, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Affolter, C.; Pretsch, E.; Bhuhlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Brooker, M.R.; Longnecker, K.; Kujawinski, E.B.; Evert, M.H.; Mouser, P.J. Discrete Organic Phosphorus Signatures are Evident in Pollutant Sources within a Lake Erie Tributary. Environ. Sci. Technol. 2018, 52, 6771–6779. [Google Scholar] [CrossRef] [PubMed]
- Sleighter, R.L.; Hatcher, P.G. The application of electrospray ionization coupled to ultrahigh resolution mass spectrometry for the molecular characterization of natural organic matter. J. Mass Spectrom. 2007, 42, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jung, J.; Lee, Y.K.; Hur, J. Surface accumulation of low molecular weight dissolved organic matter in surface waters and horizontal off-shelf spreading of nutrients and humic-like fluorescence in the Chukchi Sea of the Arctic Ocean. Sci. Total Environ. 2018, 639, 624–632. [Google Scholar] [CrossRef]
- Brailsford, F.; Glanville, H.; Marshall, M.; Golyshin, P.; Johnes, P.; Yates, C.A.; Owen, A.; Jones, D.L. Microbial use of low molecular weight DOM in filtered and unfiltered freshwater: Role of ultra-small microorganisms and implications for water quality monitoring. Sci. Total Environ. 2017, 598, 377–384. [Google Scholar] [CrossRef]
- Ni, Z.; Huang, D.; Xiao, M.; Liu, X.; Wang, S. Molecular weight driving bioavailability and intrinsic degradation mechanisms of dissolved organic phosphorus in lake sediment. Water Res. 2022, 210, 117951. [Google Scholar] [CrossRef]
- Shao, M.Y.; Liu, Z.H.; Sun, H.L.; He, H.B.; Li, Q.; Zeng, S.B.; Yan, J.Y.; Fang, Y.; He, Q.F.; Liu, H.L.; et al. Multi-tracer evidence of hydrology and primary production controls on dissolved organic matter composition and stability in the semi-arid aquatic continuum. Geochim. Cosmochim. Acta 2024, 384, 80–92. [Google Scholar] [CrossRef]
- Qian, C.; Wang, L.F.; Chen, W.; Wang, Y.S.; Liu, X.Y.; Jiang, H.; Yu, H.Q. Fluorescence Approach for the Determination of Fluorescent Dissolved Organic Matter. Anal. Chem. 2017, 89, 4264–4271. [Google Scholar] [CrossRef]
- Merder, J.; Freund, J.A.; Feudel, U.; Hansen, C.T.; Hawkes, J.A.; Jacob, B.; Klaproth, K.; Niggemann, J.; Noriega-Ortega, B.E.; Osterholz, H.; et al. ICBM-OCEAN: Processing Ultrahigh-Resolution Mass Spectrometry Data of Complex Molecular Mixtures. Anal. Chem. 2020, 92, 6832–6838. [Google Scholar] [CrossRef]
- He, D.; Li, P.; He, C.; Wang, Y.; Shi, Q. Eutrophication and watershed characteristics shape changes in dissolved organic matter chemistry along two river-estuarine transects. Water Res. 2022, 214, 118196. [Google Scholar] [CrossRef]
- Guo, M.L.; Li, X.L.; Wang, Y.; Zhang, Y.L.; Fu, Q.L.; Huguet, A.; Liu, G.L. New insights into the mechanism of phosphate release during particulate organic matter photodegradation based on optical and molecular signatures. Water Res. 2023, 236, 119954. [Google Scholar] [CrossRef]

| Sample | m/zwa | n | Cwa | Hwa | Owa | Nwa | Swa | Pwa | H/Cwa | O/Cwa | DBEwa | AI-Modwa | NOSCwa | IDEG | CRAM (%) | MLBL (%) | CHO Cpd. (%) | N Cpd. (%) | S Cpd. (%) | P Cpd. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WH | 395.94 | 3177 | 17.35 | 18.65 | 10.45 | 0.03 | 0.03 | 0.01 | 1.14 | 0.61 | 8.54 | 0.31 | 0.15 | 0.40 | 42.78 | 29.78 | 78.06 | 12.78 | 6.70 | 2.90 |
| MV | 414.33 | 1514 | 18.89 | 17.95 | 10.44 | 0.02 | 0.03 | 0.04 | 1.02 | 0.56 | 10.44 | 0.40 | 0.16 | 0.84 | 52.18 | 19.75 | 77.74 | 9.51 | 8.98 | 5.02 |
| HV | 376.32 | 6831 | 19.45 | 30.29 | 6.57 | 0.36 | 0.05 | 0.01 | 1.61 | 0.35 | 4.99 | 0.12 | −0.80 | 0.10 | 34.36 | 52.72 | 41.17 | 49.90 | 7.28 | 2.77 |
| EA | 358.82 | 3949 | 18.77 | 29.26 | 5.96 | 0.33 | 0.06 | 0.06 | 1.62 | 0.32 | 4.84 | 0.13 | −0.87 | 0.07 | 29.41 | 60.72 | 44.87 | 40.59 | 9.57 | 9.01 |
| PA | 375.67 | 5157 | 19.53 | 29.24 | 6.46 | 0.34 | 0.06 | 0.06 | 1.55 | 0.34 | 5.61 | 0.16 | −0.77 | 0.06 | 30.91 | 55.87 | 45.32 | 41.17 | 11.19 | 9.04 |
| DA | 372.20 | 7817 | 18.48 | 28.39 | 6.92 | 0.33 | 0.19 | 0.01 | 1.59 | 0.39 | 4.96 | 0.11 | −0.68 | 0.34 | 30.74 | 54.27 | 36.04 | 43.26 | 25.06 | 1.62 |
| SRFA | 437.41 | 7759 | 20.52 | 20.02 | 10.57 | 0.05 | 0.02 | 0.01 | 1.03 | 0.52 | 11.04 | 0.41 | 0.07 | 0.68 | 50.64 | 5.21 | 73.00 | 20.08 | 6.64 | 4.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, R.; Yuan, H.; Wang, L.; Xu, S. Unraveling the Molecular Composition and Reactivity Differentiation of Algae- and Macrophyte-Derived Dissolved Organic Matter in Plateau Lakes: Insights from Optical Properties and High Resolution Mass Spectrometry Characterization. Molecules 2025, 30, 3510. https://doi.org/10.3390/molecules30173510
Li Q, Zhang R, Yuan H, Wang L, Xu S. Unraveling the Molecular Composition and Reactivity Differentiation of Algae- and Macrophyte-Derived Dissolved Organic Matter in Plateau Lakes: Insights from Optical Properties and High Resolution Mass Spectrometry Characterization. Molecules. 2025; 30(17):3510. https://doi.org/10.3390/molecules30173510
Chicago/Turabian StyleLi, Qiuxing, Runyu Zhang, Haijun Yuan, Liying Wang, and Shuxia Xu. 2025. "Unraveling the Molecular Composition and Reactivity Differentiation of Algae- and Macrophyte-Derived Dissolved Organic Matter in Plateau Lakes: Insights from Optical Properties and High Resolution Mass Spectrometry Characterization" Molecules 30, no. 17: 3510. https://doi.org/10.3390/molecules30173510
APA StyleLi, Q., Zhang, R., Yuan, H., Wang, L., & Xu, S. (2025). Unraveling the Molecular Composition and Reactivity Differentiation of Algae- and Macrophyte-Derived Dissolved Organic Matter in Plateau Lakes: Insights from Optical Properties and High Resolution Mass Spectrometry Characterization. Molecules, 30(17), 3510. https://doi.org/10.3390/molecules30173510






