Comprehensive UPLC-MS/MS Method for Quantifying Four Key Intestinal Permeability Markers in Caco-2 Models
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioanalytical Method Validation
2.1.1. Selectivity, Linearity, Carryover, Precision and Accuracy
2.1.2. Recovery, Matrix Effect, Stability and Robustness
2.2. TEER Results and Monolayer Integrity
2.3. Cell Viability and Cytotoxicity Assessment
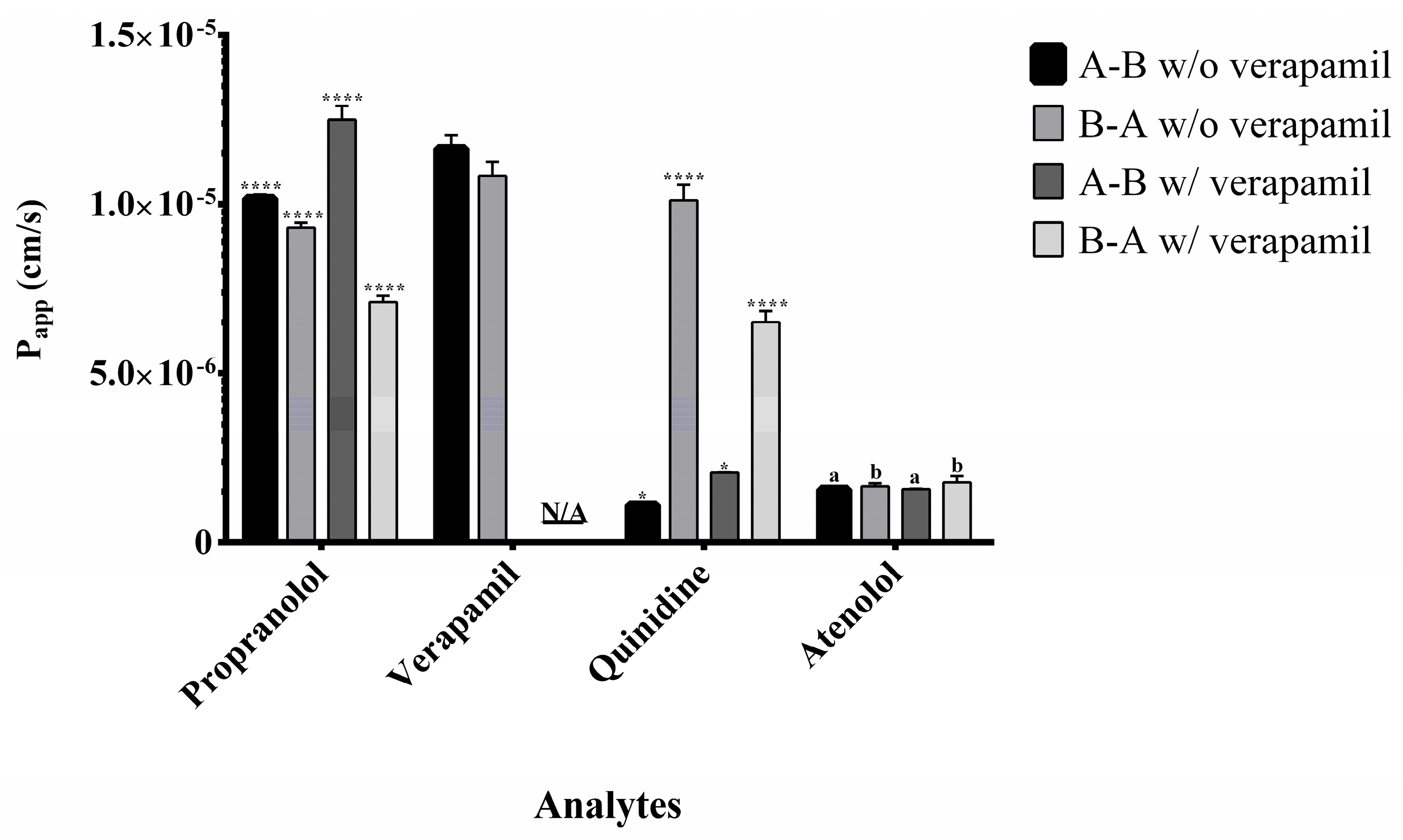
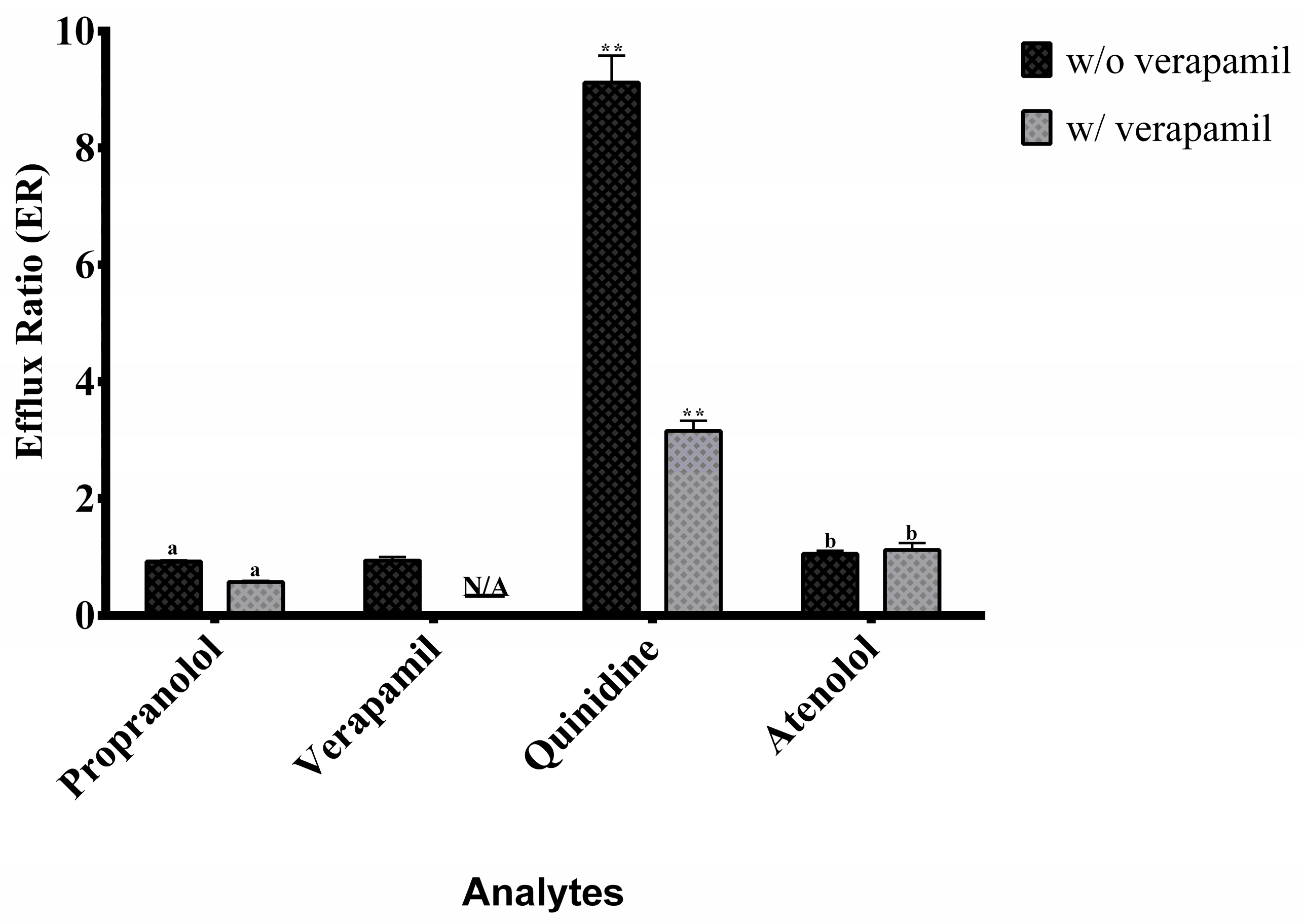
2.4. Application to Permeability Assessment and Efflux Ratio Results
3. Materials and Methods
3.1. Reagents and Equipment
3.2. Instrumental Analytical Conditions
3.3. Bioanalytical Method Validation
3.3.1. Selectivity
3.3.2. Linearity
3.3.3. Carryover Analysis
3.3.4. Precision and Accuracy
3.3.5. Recovery and Matrix Effects
3.3.6. Stability
3.3.7. Robustness
3.4. Cytotoxicity Assays
3.5. Caco-2 Cell Permeability Assay
3.6. Preparation of Samples
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| ANOVA | Analysis of Variance |
| A→B | Apical to Basolateral |
| B→A | Basolateral to Apical |
| BCS | Biopharmaceutics Classification System |
| CAPES | Coordination for the Improvement of Higher Education Personnel |
| CV | Coefficient of Variation |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| EMA | European Medicines Agency |
| ESI | Electrospray Ionization |
| ER | Efflux Ratio |
| FAPESP | São Paulo Research Foundation |
| FBS | Fetal Bovine Serum |
| FDA | Food and Drug Administration |
| HBSS | Hank’s Balanced Salt Solution |
| HEPES | N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) |
| INT | Iodonitrotetrazolium |
| IS | Internal Standard |
| LDH | Lactate Dehydrogenase |
| LOD | Limit of Detection |
| LLOQ | Lower Limit of Quantification |
| MF | Matrix Factor |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| MRM | Multiple Reaction Monitoring |
| NEAA | Non-Essential Amino Acids |
| N/A | Not Applicable |
| PBS | Phosphate-Buffered Saline |
| Papp | Apparent Permeability Coefficient |
| P-gp | P-glycoprotein |
| QC | Quality Control |
| RE | Relative Error |
| SPE | Solid-Phase Extraction |
| TEER | Transepithelial Electrical Resistance |
| ULOQ | Upper Limit of Quantification |
| UPLC-MS/MS | Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry |
| WHO | World Health Organization |
References
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 Cell Line Standardization with Pharmaceutical Requirements and In Vitro Model Suitability for Permeability Assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Yoshida, H.; Tomita, N.; Sato, Y. Comparison of in Vitro Screening Methods for Evaluating the Effects of Pharmaceutical Excipients on Membrane Permeability. Int. J. Pharm. 2024, 665, 124727. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH M9: Biopharmaceutics Classification System-Based Biowaivers. Available online: https://www.ema.europa.eu/en/ich-m9-biopharmaceutics-classification-system-based-biowaivers-scientific-guideline (accessed on 21 July 2025).
- Food and Drug Administration M9 Biopharmaceutics Classification System-Based Biowaivers: Guidance for Industry; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/media/148472/download (accessed on 21 July 2025).
- World Health Organization. WHO Expert Committee on Specifications for Pharmaceutical Preparations: Fortieth Report; WHO Technical Report Series No. 937; World Health Organization: Geneva, Switzerland, 2006; Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards-for-pharmaceuticals/expert-committee-on-specifications-for-pharmaceutical-preparations (accessed on 24 July 2025).
- Boonnop, R.; Meetam, P.; Siangjong, L.; Tuchinda, P.; Thongphasuk, P.; Soodvilai, S.; Soodvilai, S. Black Ginger Extract and Its Active Compound, 5,7-Dimethoxyflavone, Increase Intestinal Drug Absorption via Efflux Drug Transporter Inhibitions. Drug Metab. Pharmacokinet. 2023, 50, 100500. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System: Guidance for Industry; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2017. Available online: https://digirepo.nlm.nih.gov/master/borndig/101720038/UCM070246.pdf (accessed on 24 July 2025).
- Dahlgren, D.; Lennernäs, H. Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of Drug Permeability and Prediction of Drug Absorption in Caco-2 Monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Zheng, Y.; Benet, L.Z.; Okochi, H.; Chen, X. pH Dependent but Not P-Gp Dependent Bidirectional Transport Study of S-Propranolol: The Importance of Passive Diffusion. Pharm. Res. 2015, 32, 2516–2526. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Faassen, F.; Vogel, G.; Spanings, H.; Vromans, H. Caco-2 Permeability, P-Glycoprotein Transport Ratios and Brain Penetration of Heterocyclic Drugs. Int. J. Pharm. 2003, 263, 113–122. [Google Scholar] [CrossRef]
- Anderle, P.; Niederer, E.; Rubas, W.; Hilgendorf, C.; Spahn-Langguth, H.; Wunderli-Allenspach, H.; Merkle, H.P.; Langguth, P. P-Glycoprotein (P-Gp) Mediated Efflux in Caco-2 Cell Monolayers: The Influence of Culturing Conditions and Drug Exposure on P-Gp Expression Levels. J. Pharm. Sci. 1998, 87, 757–762. [Google Scholar] [CrossRef]
- Patil, A.G.; D’Souza, R.; Dixit, N.; Damre, A. Validation of Quinidine as a Probe Substrate for the in Vitro P-Gp Inhibition Assay in Caco-2 Cell Monolayer. Eur. J. Drug Metab. Pharmacokinet. 2011, 36, 115–119. [Google Scholar] [CrossRef]
- Venkatesh, G.; Ramanathan, S.; Mansor, S.M.; Nair, N.K.; Sattar, M.A.; Croft, S.L.; Navaratnam, V. Development and Validation of RP-HPLC-UV Method for Simultaneous Determination of Buparvaquone, Atenolol, Propranolol, Quinidine and Verapamil: A Tool for the Standardization of Rat in Situ Intestinal Permeability Studies. J. Pharm. Biomed. Anal. 2007, 43, 1546–1551. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Jia, J.; Liu, Y.; Pan, C.; Yu, C.; Cai, Y.; Ren, J. Simultaneous Determination of Ten Antiarrhythmic Drugs and a Metabolite in Human Plasma by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2007, 847, 174–181. [Google Scholar] [CrossRef]
- Van Domburg, B.; Van Der Nagel, B.C.H.; Andrews, L.M.; Koch, B.C.P. High-Throughput Quantification of Ten Antiarrhythmic Drugs in Human Plasma Using UPLC-MS/MS. J. Chromatogr. B 2019, 1129, 121793. [Google Scholar] [CrossRef]
- Varma, M.V.S.; Panchagnula, R. pH-Dependent Functional Activity of P-Glycoprotein in Limiting Intestinal Absorption of Protic Drugs: Kinetic Analysis of Quinidine Efflux In Situ. J. Pharm. Sci. 2005, 94, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry; US Department of Health and Human Services: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 24 July 2025).
- Ötles, S.; Kartal, C. Solid-Phase Extraction (SPE): Principles and Applications in Food Samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Einspanier, R.; Schoen, J. Transepithelial Electrical Resistance (TEER): A Functional Parameter to Monitor the Quality of Oviduct Epithelial Cells Cultured on Filter Supports. Histochem. Cell Biol. 2015, 144, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In Vitro Models Replicating the Human Intestinal Epithelium for Absorption and Metabolism Studies: A Systematic Review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Okur, N.Ü.; Çağlar, E.Ş.; Kaynak, M.S.; Diril, M.; Özcan, S.; Karasulu, H.Y. Enhancing Oral Bioavailability of Domperidone Maleate: Formulation, In Vitro Permeability Evaluation In-Caco-2 Cell Monolayers and In Situ Rat Intestinal Permeability Studies. Curr. Drug Deliv. 2024, 21, 1010–1023. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Ong, W.S.Y.; Subelzu, N.; Gleeson, J.P. Validation of a Caco-2 Microfluidic Chip Model for Predicting Intestinal Absorption of BCS Class I-IV Drugs. Int. J. Pharm. 2024, 656, 124089. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, Y.-B.; Yang, X.-W.; Wu, X.-W.; Yang, Y.-F.; Xu, W.; Wan, M.-Q.; Gong, Y.; Liu, N.-F.; Zhang, P. Biological Analysis of Constituents in Spatholobi caulis by UFLC-MS/MS: Enhanced Quantification and Application to Permeability Properties Study in Caco-2 Cell Monolayer Model. J. Pharm. Biomed. Anal. 2023, 226, 115235. [Google Scholar] [CrossRef]
- Yang, B.; Li, Y.; Shi, W.; Liu, Y.; Kan, Y.; Chen, J.; Hu, J.; Pang, W. Use of Fluorescent 2-AB to Explore the Bidirectional Transport Mechanism of Pseudostellaria heterophylla Polysaccharides across Caco-2 Cells. Molecules 2022, 27, 3192. [Google Scholar] [CrossRef]
- Ren, S.; Liu, J.; Xue, Y.; Zhang, M.; Liu, Q.; Xu, J.; Zhang, Z.; Song, R. Comparative Permeability of Three Saikosaponins and Corresponding Saikogenins in Caco-2 Model by a Validated UHPLC-MS/MS Method. J. Pharm. Anal. 2021, 11, 435–443. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, H.R.; Shim, S.M. Determination of S-Methyl-L-Methionine (SMM) from Brassicaceae Family Vegetables and Characterization of the Intestinal Transport of SMM by Caco-2 Cells. J. Food Sci. 2017, 82, 36–43. [Google Scholar] [CrossRef]
- Spinks, C.B.; Zidan, A.S.; Khan, M.A.; Habib, M.J.; Faustino, P.J. Pharmaceutical Characterization of Novel Tenofovir Liposomal Formulations for Enhanced Oral Drug Delivery: In Vitro Pharmaceutics and Caco-2 Permeability Investigations. Clin. Pharmacol. 2017, 9, 29–38. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Borrás-Linares, I.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Further Exploring the Absorption and Enterocyte Metabolism of Quercetin Forms in the Caco-2 Model Using Nano-LC-TOF-MS. Electrophoresis 2016, 37, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Fukuuchi, T.; Kobayashi, M.; Yamaoka, N.; Kaneko, K. Evaluation of Cellular Purine Transport and Metabolism in the Caco-2 Cell Using Comprehensive High-Performance Liquid Chromatography Method for Analysis of Purines. Nucleosides Nucleotides Nucleic Acids 2016, 35, 663–669. [Google Scholar] [CrossRef]
- Logoyda, L.; Herasymiuk, M.; Popovych, D.; Pidruchna, S.; Hlushok, V.; Herasymiuk, N.; Zarivna, N. HPLC MS/MS Method Development for the Quantitative Determination of Verapamil Hydrochloride from Caco-2 Cell Monolayers. Pharmacia 2020, 67, 63–69. [Google Scholar] [CrossRef]
- Yost, R.; Enke, C. Selected Ion Fragmentation with a Tandem Quadrupole Mass Spectrometer. J. Am. Chem. Soc. 1978, 100, 2274–2275. [Google Scholar] [CrossRef]
- Li, F.; Du, P.; Yang, W.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the Seeds of Plantago asiatica L. Alleviates Nonylphenol Induced Intestinal Barrier Injury by Regulating Tight Junctions in Human Caco-2 Cell Line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef]
- Roche Diagnostics GmbH. Cytotoxicity Detection Kit (LDH): For the Quantitative Determination of Lactate Dehydrogenase Released from Damaged Cells; Roche Diagnostics GmbH: Mannheim, Germany, 2020; Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/167/504/11644793001.pdf (accessed on 24 July 2025).
- Hiebl, B.; Peters, S.; Gemeinhardt, O.; Niehues, S.M.; Jung, F. Impact of Serum in Cell Culture Media on in Vitro Lactate Dehydrogenase (LDH) Release Determination. J. Clin. Biochem. 2017, 3, 9–13. [Google Scholar] [CrossRef]
- Li, B.; Wu, J.; Li, H.; Jiang, Z.; Zhou, X.; Xu, C.; Ding, N.; Zha, J.; He, W. In Vitro and In Vivo Approaches to Determine Intestinal Epithelial Cell Permeability. J. Vis. Exp. 2018, 140, 57032. [Google Scholar] [CrossRef]
- Ude, V.; Brown, D.; Viale, L.; Kanase, N.; Stone, V.; Johnston, H. Impact of Copper Oxide Nanomaterials on Differentiated and Undifferentiated Caco-2 Intestinal Epithelial Cells; Assessment of Cytotoxicity, Barrier Integrity, Cytokine Production and Nanomaterial Penetration. Part. Fibre. Toxicol. 2017, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of Active Flavonoids, Based on Cytotoxicity and Lipophilicity: An Evaluation Using the Blood–Brain Barrier Cell and Caco-2 Cell Models. Toxicol. In Vitro 2014, 28, 388–396. [Google Scholar] [CrossRef] [PubMed]

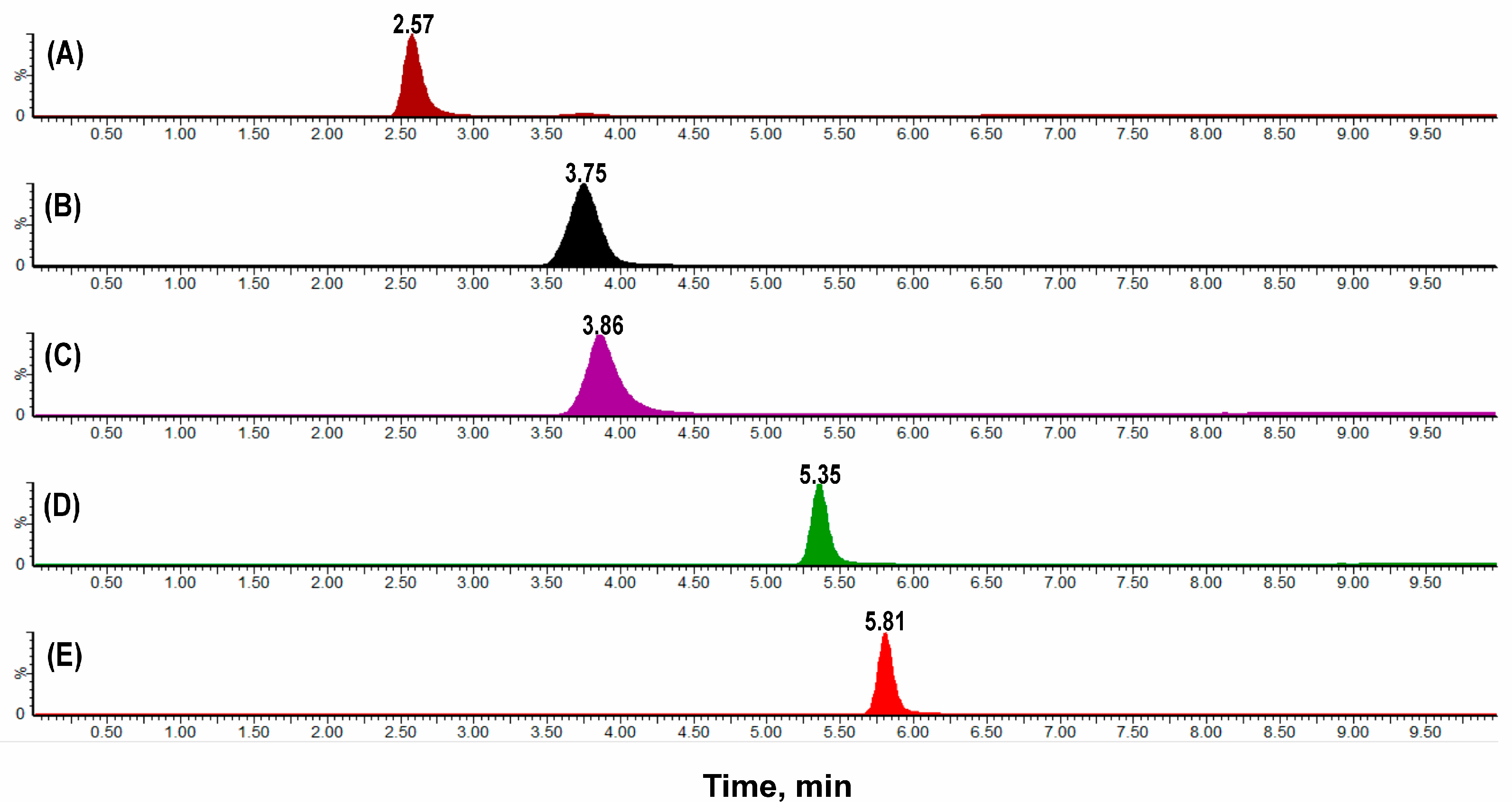
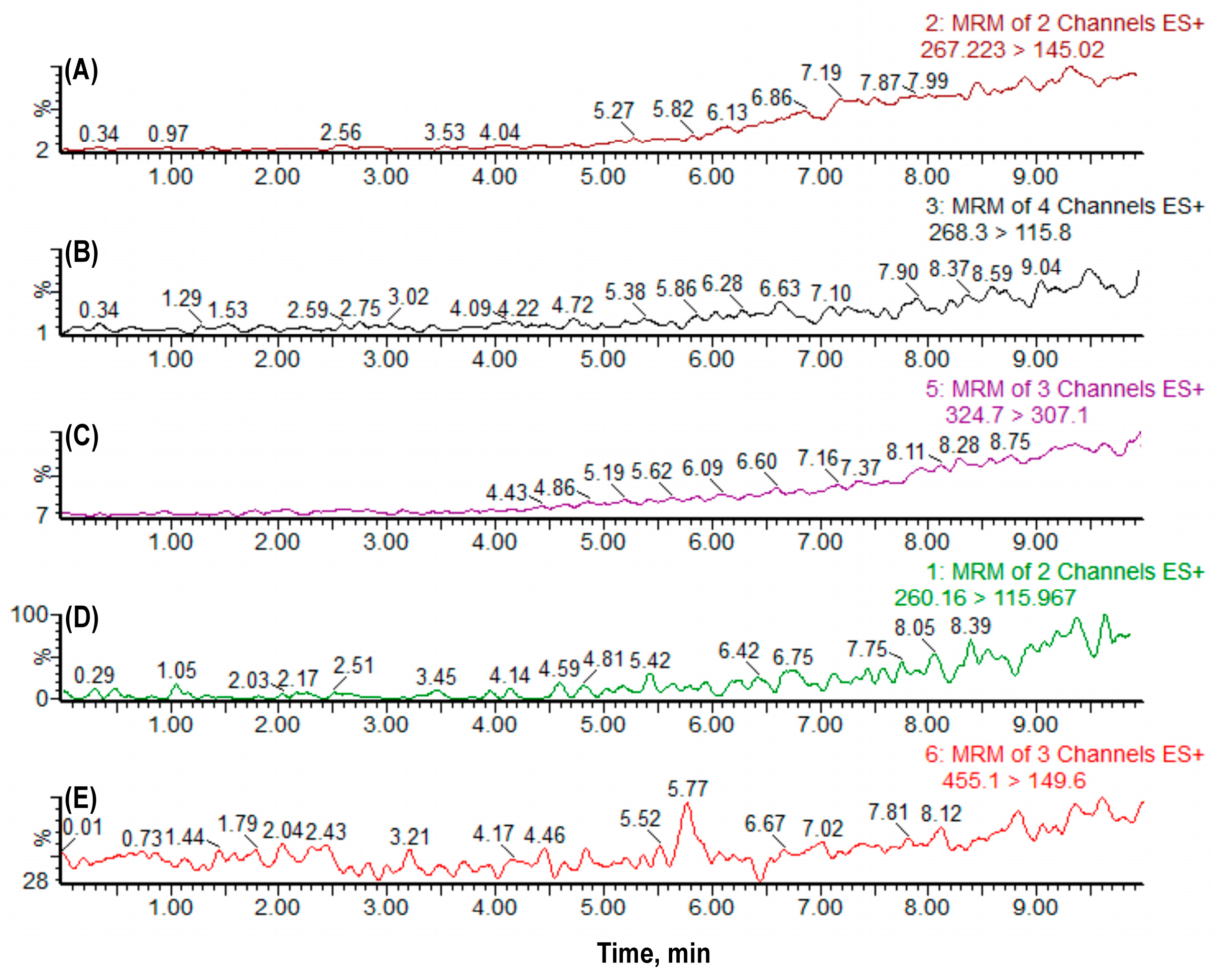
| Analyte | Regression Equation | Coefficients of Determination | Lack of Fit (p-Value) | Normality (p-Value) | LOD/LLOQ * (ng/mL) |
|---|---|---|---|---|---|
| Atenolol | Y = 4.7817x − 0.0809 | 0.995 | 0.091 | 0.790 | 10/25.4 |
| Quinidine | Y = 4.2705x − 0.00861 | 0.999 | 0.766 | 0.049 | 2/12.7 |
| Propranolol | Y = 2.4184x − 0.04191 | 0.999 | 0.334 | 0.678 | 1/25.4 |
| Verapamil | Y = 5.498x + 8.632 | 1.000 | 0.069 | 0.081 | 1/600 |
| Analyte | Level | Intra-Day Precision (CV%) | Inter-Day Precision (CV%) | Intra-Day Accuracy (RE%) | Inter-Day Accuracy (RE%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | ||||
| Atenolol | LLOQ | 4.87 | 7.34 | 1.61 | 5.26 | −0.53 | −13.10 | −16.69 | −19.44 |
| Low | 1.65 | 1.92 | 15.10 | 2.58 | −6.91 | 6.69 | −6.20 | −8.33 | |
| Mid | 2.87 | 0.42 | 12.95 | 3.53 | 4.98 | 1.10 | −4.34 | 11.80 | |
| High | 2.37 | 0.31 | 15.08 | 1.62 | −3.26 | −0.62 | −9.51 | −14.82 | |
| Quinidine | LLOQ | 1.86 | 4.00 | 0.42 | 1.05 | 0.02 | −14.13 | 15.93 | −14.41 |
| Low | 1.19 | 0.71 | 0.84 | 3.56 | −0.44 | 7.80 | 12.17 | −4.78 | |
| Mid | 1.31 | 0.24 | 3.55 | 1.67 | 3.92 | −2.21 | 2.17 | 0.24 | |
| High | 0.73 | 0.67 | 1.39 | 1.19 | −13.22 | −13.97 | −1.94 | −6.40 | |
| Propranolol | LLOQ | 6.59 | 6.97 | 9.81 | 2.26 | −5.27 | −17.41 | −8.50 | −4.58 |
| Low | 3.09 | 1.75 | 8.87 | 2.20 | −2.27 | 7.00 | −3.54 | 1.21 | |
| Mid | 4.01 | 0.07 | 13.41 | 4.09 | 5.07 | −2.21 | −10.62 | 1.44 | |
| High | 0.34 | 0.96 | 13.01 | 7.25 | −1.38 | −11.32 | −8.21 | 11.62 | |
| Verapamil | LLOQ | 1.55 | 7.23 | 1.73 | 4.89 | −4.07 | 0.94 | 9.51 | 15.69 |
| Low | 6.42 | 1.29 | 3.36 | 0.39 | 0.50 | 5.95 | −6.18 | 4.82 | |
| Mid | 0.34 | 1.94 | 0.71 | 0.63 | 0.86 | 0.04 | −8.07 | −8.53 | |
| High | 1.73 | 0.57 | 4.56 | 3.69 | 5.52 | 7.61 | −4.25 | 2.12 | |
| Analyte | Level | IS-Normalized MF (n = 6) | Mean IS-Normalized MF | CV (%) |
|---|---|---|---|---|
| Atenolol | Low | 0.98, 0.89, 0.92, 1.04, 0.89, 0.91 | 0.94 | 6.47 |
| High | 1.04, 1.06, 1.04, 1.04, 1.06, 1.08 | 1.05 | 1.37 | |
| Quinidine | Low | 0.99, 1.03, 0.96, 1.06, 0.96, 0.96 | 0.99 | 4.55 |
| High | 1.05, 1.05, 1.07, 1.08, 1.08, 1.07 | 1.07 | 1.38 | |
| Propranolol | Low | 0.98, 0.93, 0.89, 1.02, 0.86, 0.95 | 0.94 | 6.02 |
| High | 1.20, 1.13, 1.14, 1.17, 1.21, 1.12 | 1.16 | 3.20 | |
| Verapamil | Low | 0.90, 0.90, 0.91, 1.04, 0.91, 0.96 | 0.94 | 6.14 |
| High | 0.97, 1.11, 1.13, 0.97, 1.11, 1.13 | 1.07 | 7.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, L.S.d.; Crevelin, E.J.; Moraes, L.A.B.d.; Jacometti Cardoso Furtado, N.A. Comprehensive UPLC-MS/MS Method for Quantifying Four Key Intestinal Permeability Markers in Caco-2 Models. Molecules 2025, 30, 3477. https://doi.org/10.3390/molecules30173477
Araújo LSd, Crevelin EJ, Moraes LABd, Jacometti Cardoso Furtado NA. Comprehensive UPLC-MS/MS Method for Quantifying Four Key Intestinal Permeability Markers in Caco-2 Models. Molecules. 2025; 30(17):3477. https://doi.org/10.3390/molecules30173477
Chicago/Turabian StyleAraújo, Luciana Silva de, Eduardo José Crevelin, Luiz Alberto Beraldo de Moraes, and Niege Araçari Jacometti Cardoso Furtado. 2025. "Comprehensive UPLC-MS/MS Method for Quantifying Four Key Intestinal Permeability Markers in Caco-2 Models" Molecules 30, no. 17: 3477. https://doi.org/10.3390/molecules30173477
APA StyleAraújo, L. S. d., Crevelin, E. J., Moraes, L. A. B. d., & Jacometti Cardoso Furtado, N. A. (2025). Comprehensive UPLC-MS/MS Method for Quantifying Four Key Intestinal Permeability Markers in Caco-2 Models. Molecules, 30(17), 3477. https://doi.org/10.3390/molecules30173477








